Abstract
Background
To evaluate the effect of the cumulative number of ovulatory cycles and its contributing components on the risk of breast cancer among BRCA mutation carriers.
Methods
We conducted a matched case-control study on 2,854 pairs of women with a BRCA1 or BRCA2 mutation. Conditional logistic regression was used to estimate the association between the number of ovulatory cycles and various exposures and the risk of breast cancer. Information from a subset of these women enrolled in a prospective cohort study was used to calculate age-specific breast cancer rates.
Results
The annual risk of breast cancer decreased with the number of ovulatory cycles experienced (ρ = −0.69; P = 0.03). Age at menarche and duration of breastfeeding were inversely related with risk of breast cancer among BRCA1 (P-trend < 0.0001) but not among BRCA2 (P-trend ≥ 0.28) mutation carriers. The reduction in breast cancer risk associated with surgical menopause (OR = 0.52; 95%CI 0.40–0.66; P-trend < 0.0001) was greater than that associated with natural menopause (OR = 0.81; 95%CI 0.62–1.07; P-trend = 0.14). There was a highly significant reduction in breast cancer risk among women who had an oophorectomy after natural menopause (OR = 0.13; 95%CI 0.02–0.54; P = 0.006).
Conclusions
These data challenge the hypothesis that breast cancer risk can be predicted by the lifetime number of ovulatory cycles in women with a BRCA mutation. Both pre- and post-menopausal oophorectomy protect against breast cancer.
Impact
Understanding the basis for the protective effect of oophorectomy has important implications for chemoprevention.
Keywords: BRCA1, ovulatory cycles, breast cancer, oophorectomy
Introduction
A woman's reproductive history is important in determining her risk of developing breast cancer (1). Factors which increase risk include early age at menarche, nulliparity and late age at menopause. In contrast, breastfeeding, parity and premenopausal oophorectomy confer protection. It has been proposed that early menarche and late menopause increase cancer risk via their effects on the lifetime number of ovulatory cycles (e.g., by increasing the duration of exposure to and the cumulative dose of ovarian hormones)(2). The protective effects of pregnancy and breastfeeding on breast cancer risk may act through differentiation of terminal breast lobules (3) or through modifying endogenous hormones.
The role of reproductive factors on breast cancer risk is unclear for women with a BRCA1 or BRCA2 mutation. A late age at menarche (4–6), breastfeeding (7), and high parity (8, 9) have been reported to decrease the risk in BRCA1 carriers, but age at first birth does not appear to influence risk (10). The importance of reproductive factors in the etiology of BRCA2-associated cancers is less clear (7, 8, 11). Surgical bilateral oophorectomy is often recommended to women with either type of mutation and has been found to reduce the risks of both breast and ovarian cancer (12). It has been assumed that the protective effect of oophorectomy on breast cancer risk is a consequence of reducing exposure to endogenous estrogens. If oophorectomy acts through modulating the number of ovulatory cycles, then we would not expect surgical menopause and natural menopause to have similar effects. Also, we would not expect an oophorectomy in postmenopausal women to reduce the risk of breast cancer.
The extent to which these reproductive factors act through a common pathway by influencing the cumulative number of ovulatory cycles – and exposure to endogenous ovarian hormones – is of interest and may inform cancer prevention strategies. We undertook a case-control study to evaluate the effect of the cumulative number of ovulatory cycles and each of the contributing components on the risk of developing breast cancer among women with a BRCA1 or BRCA2 mutation. We asked if there is protection associated with an oophorectomy if it took place after a woman had entered menopause.
Materials and Methods
Study Population and Design
Eligible study subjects were identified among 70 participating centers in 12 countries. These women sought testing for BRCA1 and BRCA2 mutations because of a personal or family history of breast and/or ovarian cancer. All study subjects (with the exception of some of those from the University of Utah and the University of California Irvine) received genetic counselling. The institutional review boards of the host institutions approved the study. All subjects provided written informed consent. Mutation detection was performed using a range of techniques, but all nucleotide sequences were confirmed by direct sequencing of DNA. A woman was eligible for the current study when the molecular analysis established that she was a carrier of a deleterious mutation in the BRCA1 or BRCA2 gene.
All study subjects completed a baseline questionnaire at the individual center at the time of a clinic appointment or at their home at a later date. The questionnaire requested information on family and personal history of cancer, reproductive and medical histories, including preventive oophorectomy and mastectomy. Detailed information regarding ages at menarche and menopause, cause of menopause, pregnancy, breastfeeding, and hormone use was also queried. In total, 12,106 women were entered in the database and were eligible for inclusion in the studies.
Cohort Study
A subset of these women (n = 2,125, from 40 participating centers) were also enrolled in a prospective cohort study of BRCA mutation carriers and completed biennial follow-up questionnaires. These were either mailed to each study participant to complete and return, or were administered over the phone by a genetic counsellor or research assistant at each center. A woman was eligible for inclusion in the cohort study if she was between 25 and 75 years old at the time of completion of the baseline questionnaire, she had completed at least one follow-up questionnaire and she did not have a mastectomy or a known diagnosis of breast, ovarian or other cancer at the time of completion of the baseline questionnaire. Of 2,647 women who were eligible, 522 women for whom follow-up information was not available were excluded. Information on incident breast cancers was collected and pathology records were reviewed.
Case-Control Study
Information on cancer status was available for a total of 12,106 women who carried a BRCA1 or BRCA2 mutation. Case subjects were women with a diagnosis of invasive breast cancer. Control subjects were women who never had breast cancer and who were also carriers of a mutation in BRCA1 or BRCA2. Potential subjects were excluded if they had been diagnosed with ovarian cancer (n = 1,243) if information on menopausal status or age at menopause was missing (n = 1,685) or if other pertinent information was missing (n = 201). After exclusions, there was a total of 8,977 eligible women, including 3,914 women with breast cancer (potential case subjects) and 5,063 women without breast cancer (potential controls). A single control subject was selected for each case subject, matched according to mutation in the same gene (BRCA1 or BRCA2), year of birth (within one year), and country of residence. A control was eligible to be matched to a given case if the date of interview or date of prophylactic mastectomy in the matched control occurred at or after the year of breast cancer diagnosis of the case. In total, 2,854 matched sets were identified.
Menopause was classified as either: 1) natural, 2) medication-induced, 3) surgical (e.g., bilateral salpingo-oophorectomy defined as both ovaries having been surgically removed), 4) radiation-induced, or 5) other/unknown. Women who reported medication-induced menopause as a consequence of the breast cancer diagnosis were coded as premenopausal at diagnosis. Only those oophorectomies that took place prior to the diagnosis of breast cancer (i.e., in different calendar years) were considered as exposures. Similarly, only oophorectomies that took place prior to the age of diagnosis of breast cancer in the matched case was considered to be an exposure for the control. The cumulative number of ovulatory cycles for each woman at a given age was estimated by using the following equations: 1) if premenopausal: ovulatory cycles = 12 * [(current age – age at menarche – years of oral contraceptive use – parity * 0.77 – years of breastfeeding)]; 2) if postmenopausal, current age was replaced with age at menopause.
We also conducted a sub-analysis to evaluate risk factors for postmenopausal breast cancer. For this analysis, we only included women who had experienced a natural menopause and who did not have breast cancer prior to menopause. We excluded 6,799 women who did not undergo natural menopause. After exclusions, there was a total of 1,153 eligible women, including 527 women with breast cancer (potential case subjects) and 626 women without breast cancer (potential controls). Similar matching criteria were used as listed above (mutation in the same gene, year of birth, country of residence). Women were also matched on age at menopause (within one year). In total, 203 matched sets were identified.
Statistical Analysis
Cohort Study
The purpose of the cohort study was to calculate age-specific breast cancer rates and to correlate these with the cumulative number of ovulatory cycles. There were 178 incident cancers detected in the 2,125 women under follow-up. For each interval, the number of observed cancers was divided by the total number of person-years at risk contributed by members of the cohort to that interval. Cumulative ovulatory cycles were calculated for each woman in the cohort for each age interval. The Pearson correlation coefficient (ρ) was used to estimate the correlation coefficient between cumulative ovulatory cycles and breast cancer risk.
Case-Control Study
A matched case-control analysis was performed to evaluate associations between various reproductive and surgical exposures and the risk of breast cancer. The distributions of continuous and categorical variables between cases and controls were compared using the Student's t-test and chi-square test, respectively. Conditional logistic regression was used to estimate the univariate odds ratios (OR) and 95% confidence intervals (CI) for breast cancer associated with various exposures. Only exposures in the control that took place before the date of diagnosis in the matched cases were considered in the analysis. A multivariate analysis was carried out to control for potential confounders. All analyses were performed using the SAS statistical package, version 9.1.3 (SAS Institute, Cary, NC, USA). All P values were based on two-sided tests and were considered statistically significant if P ≤ 0.05.
Results
Cohort Study
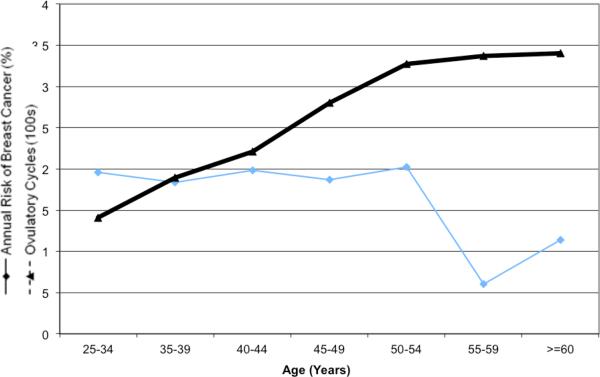
After a mean follow-up of 3.3 years, 178 new breast cancers were diagnosed in the cohort of 2,125 women (annual rate 1.7%; 95%CI 1.4% to 1.9%)(data not shown). For members of the cohort, we calculated the mean number of ovulatory cycles achieved at the beginning of each five-year interval. Figure 1 shows the average annual risk of breast cancer plotted against the mean number of cumulative ovulatory cycles for women in the cohort at the beginning of the age group. As expected, the number of ovulatory cycles increased steadily over a woman's reproductive years, followed by a plateau as the women achieved menopause. In contrast, the observed annual risk of breast cancer was uniform between ages 25 and 54 (between 1.8% and 2.0%), declined to 0.6% between ages 55 to 59 and then rose slightly to 1.1% after age 60 (data not shown). There was a statistically significant inverse correlation between the number of ovulatory cycles experienced and the annual risk of breast cancer (ρ = −0.69; P = 0.03).
Figure 1.
Breast cancer incidence and cumulative number of ovulatory cycles.
Case-Control Study
A total of 2,854 matched case-control pairs was identified (2,055 pairs with BRCA1 and 799 pairs with BRCA2 mutations). The characteristics of the cases and controls are presented in Table 1. On average, the controls experienced more ovulatory cycles than the cases (mean 242.9 vs. 248.0, but the difference was not significant (P = 0.07).
Table 1.
Baseline characteristics of breast cancer cases and controls with BRCA1 and BRCA2 mutations.
| Characteristic | Controls (n = 2,854) | Cases (n = 2,854) | P |
|---|---|---|---|
| Date of birth, mean (range) | 1957.1 (1911–82) | 1957.0 (1911–83) | 0.71 |
| Age at interview, mean (range | 47.1 (21–85) | 46.8 (18–86) | 0.19 |
| Age at diagnosis, mean (range) | n/a1 | 40.4 (19–76) | |
| Mutation, n (%) | |||
| BRCA1 | 2055 (72.0%) | ||
| BRCA2 | 799 (28.0%) | matched | |
| Country of residence, n (%) | |||
| United States | 992 (34.8%) | ||
| Canada | 804 (28.2%) | ||
| Israel | 99 (3.5%) | ||
| Poland | 724 (25.4%) | ||
| Other Countries | 235 (8.2%) | matched | |
| Parity, n (%) | |||
| Parous | 2241(78.5%) | 2286 (80.1%) | |
| Nulliparous | 613 (21.5%) | 568 (19.9%) | 0.14 |
| Mean (range) | 1.81(0–8) | 1.77 (0–8) | 0.30 |
| Months breastfed, mean (range) | 9.6 (0–147) | 7.5 (0–102) | <0.0001 |
| Age at menarche, mean (range) | 13.03(8–27) | 12.91(8–30) | 0.005 |
| Menopausal, n (%) | |||
| No | 2361 (82.7%) | 2446 (85.7%) | 0.002 |
| Yes | 493 (17.3%) | 408 (14.3%) | |
| Age at menopause, mean (range) | 44.2 (12–58) | 45.3(24–59) | 0.01 |
| Age at natural menopause, mean (range) | 47.90 (33–58) | 48.05 (33–59) | 0.71 |
| Age at surgical menopause, mean (range)2 | 40.2 (12–55) | 41.0 (25–54) | 0.27 |
| Oral contraceptive use, n (%) | |||
| Never | 1042 (36.7%) | 1002 (35.4%) | |
| Ever | 1796 (63.3%) | 1832 (64.6%) | 0.29 |
| Years used, mean (SD) | 3.91 (0–29) | 4.14 (0.28) | 0.11 |
| Cumulative ovulatory cycles, mean (range)3 | 242.9 (0–578) | 248.0 (0–532) | 0.07 |
n/a = not applicable.
Among women who had surgical menopause (n = 123 cases and n = 213 controls).
Estimated among subjects that had no information missing for the components needed to calculate cumulative ovulatory cycles (n = 2,518 cases and n = 2,567 controls).
only among women who had information available for all the necessary variables (see Methods for individual components used to estimate ovulatory cycles).
Next, we evaluated the associations between the individual components used to estimate the cumulative number of ovulatory cycles and breast cancer risk (Table 2). There was a significant inverse association between increasing age at menarche and breast cancer risk among BRCA1 mutation carriers (P – trend ≤ 0.0001), but not among BRCA2 mutation carriers (P – trend = 0.28). Compared to women whose age at menarche was ≤ 11 years, BRCA1 carriers with menarche at or after 15 years old had a 42% decrease in the risk of breast cancer (OR = 0.58; 95% CI 0.45–0.74). Similarly, there was a significant inverse relationship between duration of breastfeeding and breast cancer risk among women with a BRCA1 mutation (P – trend < 0.0001) but not among those with a BRCA2 mutation (P – trend = 0.68). Increasing parity was not a risk factor for carriers of either mutation (P – trend = 0.11 and 0.49 for BRCA1 and BRCA2, respectively). In the unadjusted analysis, parity (vs. nulliparity) was not a risk factor for breast cancer for BRCA1 carriers (OR = 1.04; 95%CI 0.87–1.25); however, after adjusting for age at menarche, breastfeeding and oral contraceptive use, we found a modest and significant increase in breast cancer risk for parous versus nulliparous women (OR = 1.36; 95%CI 1.11–1.68). Parity was also a risk factor for BRCA2 mutation carriers (OR = 1.52; 95%CI 1.12–2.06). We found no significant relationship between ever use of an oral contraceptive and breast cancer risk in either subgroup (P ≥ 0.45).
Table 2.
Relationship between various reproductive factors and risk of breast cancer among BRCA1 and BRCA2 mutation carriers.
| BRCA1 (n = 1,847 pairs) | P | BRCA2 (n = 714 pairs) | P | |
|---|---|---|---|---|
| OR (95%CI) | OR (95%CI) | |||
| Age at menarche (years)1 | ||||
| ≤ 11 | 1.00 (reference) | 1.00 (reference) | ||
| 12 | 0.66 (0.52–0.84) | 0.0005 | 0.85(0.61–1.20) | 0.36 |
| 13 | 0.74 (0.59–0.92) | 0.007 | 0.84(0.60–1.16) | 0.28 |
| 14 | 0.65 (0.51–0.84) | 0.0002 | 0.98(0.65–1.46) | 0.90 |
| ≥ 15 | 0.58 (0.45–0.74) | <0.0001 | 1.27(0.82–1.96) | 0.28 |
| Trend | 0.91 (0.86–0.96) | <0.0001 | 1.05(0.96–1.15) | 0.28 |
| Parity (per birth)2 | ||||
| Nulliparous | 1.00 (reference) | 1.00 (reference) | ||
| 1 | 1.36 (1.03–1.80) | 0.03 | 1.57 (1.03–2.40) | 0.04 |
| 2 | 1.39 (1.07–1.82) | 0.01 | 1.46 (1.00–2.14) | 0.05 |
| 3 | 1.44 (1.06–1.95) | 0.02 | 1.45 (0.94–2.22) | 0.09 |
| ≥ 4 | 1.19 (0.82–1.74) | 0.37 | 0.94 (0.53–1.67) | 0.84 |
| Trend | 1.07 (0.99–1.15) | 0.11 | 1.04 (0.93–1.16) | 0.49 |
| Breastfeeding (years)3 | ||||
| 0 | 1.00 (reference) | 1.00 (reference) | ||
| ≤ 1 | 0.81 (0.66–1.00) | 0.05 | 1.03 (0.76–1.40) | 0.85 |
| 1 – ≤ 2 | 0.65 (0.50–0.85) | 0.001 | 1.04 (0.70–1.53) | 0.86 |
| 2 – ≤ 3 | 0.51 (0.35–0.75) | 0.0006 | 1.33 (0.76–2.32) | 0.31 |
| > 3 | 0.45 (0.30–0.68) | 0.0002 | 1.02 (0.56–1.88) | 0.94 |
| Trend | 0.82 (0.75–0.89) | <0.0001 | 1.01 (0.95–1.08) | 0.68 |
| OC use (years)4 | ||||
| 0 | 1.00 (reference) | 1.00 (reference) | ||
| ≤ 1 | 1.15 (0.90–1.47) | 0.27 | 1.01 (0.71–1.46) | 0.93 |
| 1 – ≤ 2 | 0.89 (0.65–1.22) | 0.47 | 1.15 (0.72–1.84) | 0.56 |
| 2 – ≤ 3 | 0.92 (0.66–1.27) | 0.60 | 0.90 (0.55–1.46) | 0.66 |
| > 3 | 1.10 (0.82–1.34) | 0.32 | 1.03 (0.77–1.37) | 0.86 |
| Trend | 1.02 (0.97–1.07) | 0.45 | 1.04 (0.92–1.17) | 0.54 |
Only includes women who had no missing data for age at menarche (n = 2,561).
Estimate adjusted for parity, breastfeeding and oral contraceptive use.
Estimate adjusted for age at menarche, breastfeeding and oral contraceptive use.
Estimate adjusted for age at menarche, parity and oral contraceptive use.
Estimate adjusted for age at menarche, breastfeeding and parity.
We evaluated the relationships between the different causes of menopause and the risk of breast cancer in BRCA1 and BRCA2 carriers combined as compared to premenopausal women (Table 3). A similar number of cases and controls reported natural menopause; however, cases were much less likely to have had undergone surgical menopause than controls. Natural menopause was associated with a modest and non-significant decrease in breast cancer risk (OR = 0.81; 95% CI 0.62–1.07; P = 0.14); however, women who underwent surgical menopause had a 48% decrease in the risk of developing breast cancer (OR = 0.52; 95% CI 0.40–0.66; P < 0.0001). For every age group, surgical menopause conferred a stronger degree of protection than menopause per se (Table 4).
Table 3.
Association between cause of menopause and risk of breast cancer.
| Exposure | Controls (n = 2,854) | Cases (n = 2,854) | OR (95%CI)1 | P |
|---|---|---|---|---|
| Menopausal | ||||
| No, n (%) | 2361 (82.7%) | 2446 (85.7%) | 1.00 (reference) | |
| Yes, n (%) | 493 (17.3%) | 408 (14.3%) | 0.65 (0.53– 0.79) | <0.0001 |
| Cause of menopause, n (%) | ||||
| Natural | 260 (53%) | 262 (64%) | 0.81 (0.62–1.07) | 0.14 |
| Medication-induced | 8 (1.6%) | 12 (3%) | 1.38 (0.56–3.40) | 0.48 |
| Surgical (i.e., oophorectomy) | 213 (43%) | 123 (30%) | 0.52 (0.40–0.66) | <0.0001 |
| Radiation-induced | 4 (0.8%) | 2 (1%) | 0.48 (0.09–2.64) | 0.40 |
| Other/unknown | 8 (1.6%) | 9 (2%) | 1.05 (0.44–2.72) | 0.93 |
Estimates are univariate odds ratios.
Table 4.
Breast cancer risk, menopausal status and history of bilateral oophorectomy, by age at diagnosis.
| Age at diagnosis | Pairs (n) | Menopausal | Menopausal1 | P | Oophorectomy | Oophorectomy | P |
|---|---|---|---|---|---|---|---|
| % | OR (95%CI)2 | % | OR (95%CI) | ||||
| <=30 | 276 | 0.4 | n/a3 | 0.2 | n/a3 | ||
| 31–35 | 567 | 1.5 | 1.43 (0.54–2.74) | 0.47 | 0.5 | 1.00 (0.20–4.96) | 1.00 |
| 36–40 | 711 | 4.9 | 0.62 (0.37–102) | 0.06 | 3.0 | 0.23 (0.10–0.51) | 0.0004 |
| 41–45 | 607 | 9.7 | 0.75 (0.52–1.09) | 0.13 | 4.5 | 0.36 (0.20–0.66) | 0.001 |
| 46–50 | 384 | 25.0 | 0.55 (0.39–0.79) | 0.001 | 9.1 | 0.35 (0.20–0.60) | 0.0002 |
| >=50 | 309 | 81.4 | 0.53 (0.33–1.88) | 0.01 | 17.0 | 0.46 (0.29–0.72) | 0.0006 |
| all | 2854 | 15.8 | 0.65 (0.53–0.79) | <0.0001 | 4.9 | 0.38 (0.29–0.50) | <0.0001 |
Menopausal includes natural, surgical, medication- and radiation-induced menopause, cases and controls combined.
Estimates are univariate odds ratios.
n/a = estimates could not be generated because there were too few individuals that were menopausal (n = 2) or had an oophorectomy (n = 1) for age at diagnosis ≤ 30.
Because of this unexpected finding, we evaluated the effect of oophorectomy on the risk of breast cancer in postmenopausal women. There were 1,159 BRCA mutation carriers who experienced natural menopause and who did not have breast cancer prior to menopause. Using the same matching criteria, we generated 203 matched pairs (146 BRCA1 and 57 BRCA2 mutation carriers) (data not shown). There was a significant reduction in breast cancer risk among women who had an oophorectomy after menopause (univariate OR = 0.13; 95%CI 0.02–0.54; P = 0.006) (Table 5).
Table 5.
Association between various reproductive, hormonal and surgical exposures and risk of postmenopausal breast cancer among women who underwent natural menopause.
| Exposure | Univariate OR (95%CI) | P | Multivariate OR (95%CI) | P |
|---|---|---|---|---|
| Oophorectomy, ever/never | 0.13 (0.02–0.54) | 0.006 | 0.13 (0.03–0.57)1 | 0.007 |
| Parity, per birth | 0.97 (0.83–1.13) | 0.70 | 0.97 (0.83–1.13)2 | 0.69 |
| Breastfeeding, per month4 | 0.99 (0.97–1.01) | 0.16 | ||
| Oral contraceptive use, ever/never | 0.77 (0.46–1.27) | 0.30 | 0.89 (0.52–1.50)3 | 0.66 |
| Age at menarche, per year4 | 0.91 (0.81–1.03) | 0.14 |
Estimate adjusted for parity (per birth) and oral contraceptive use (ever/never).
Estimate adjusted for oophorectomy (ever/never) and oral contraceptive use (ever/never).
Estimate adjusted for oophorectomy (ever/never) and parity (per birth).
Two of the three cases that had an oophorectomy had missing data on breastfeeding and one case had missing data on age at menarche and thus were excluded from the multivariate model for these exposures.
Discussion
It was proposed by Henderson et al. in 1985 that breast cancer incidence rates closely parallel the lifetime number of ovulatory cycles, in support of the hypothesis that endogenous estrogen and progesterone are important etiologic factors (2). We show here that among BRCA mutation carriers, the cumulative number of ovulatory cycles is not associated with risk. The mean number of ovulatory cycles for the controls was in fact greater (248) than it was for the cases (243), opposite to what we would expect if risk was positively associated with the number of cycles. The negative association between ovulatory cycles achieved and breast cancer risk is a reflection of the declining risk with age. Nevertheless, a number of reproductive factors are important in BRCA1 carriers including age at menarche, breastfeeding and oophorectomy, while only oophorectomy was protective in BRCA2 carriers.
The diminution of risk associated with a delay of menarche by one year is approximately 9% and is greater than the proportional diminution in the lifetime number of ovulatory cycles by delaying ovulation by one year (approximately 2% – 3%). For each year of delay in the age of menarche beyond age 11, we observed a decline of 9% in the risk of breast cancer among BRCA1 carriers. Our data more closely fits a model in which the lifetime risk is proportional to the length of time from menarche to the end of breast development (approximately age 18). This suggests that endogenous or exogenous exposures during this period may be hazardous. It is of interest that the effect of a delayed menarche persists into the postmenopausal period. Similar to our previous report, we observed a significant inverse relationship between age at menarche and breast cancer risk in BRCA1, but not BRCA2 mutation carriers (4). Other studies found no relationship between age at menarche and breast cancer risk in carriers of either mutation (6, 11, 13).
In our multivariate analyses, we found an increased risk for parous compared to nulliparous women with a BRCA1 or BRCA2 mutation; this is opposite to what we would expect if ovulatory cycles per se were a risk factor. In an earlier publication by our group, multiparity (≥ 4 children) protected against BRCA1- but increased the risk of BRCA2 – associated breast cancers (8). These data extend that of our earlier publications, which included approximately one-half of the women enrolled in the current study (4, 7). Two other studies found no association of breast cancer with increasing parity (11, 14).
We also confirmed that breastfeeding for more than one year reduces risk among BRCA1, but not among BRCA2 mutation carriers (7). Another group found no association with breastfeeding (15).
The magnitude of the protective effect associated with an oophorectomy reported here is similar to what has been previously reported for BRCA mutation carriers (16). Of particular interest is our observation that the reduction in risk associated with surgical menopause is much stronger than that associated with natural menopause. This suggests that hormones that continue to be secreted by the postmenopausal ovary have a cancer-promoting effect. Hormonal changes in postmenopausal women include a substantial decrease in circulating levels of estradiol and estrone, but only a small decline in androgen synthesis by the ovaries and adrenal glands (17). Thus, androgens may influence cancer risk directly by increasing cellular growth and proliferation or indirectly through the aromatization to estrogens (18). It is not yet clear which mechanism is responsible for the effect observed here and both possibilities should be studied.
In the general population, studies have consistently shown protective effects of both natural and surgical menopause and subsequent breast cancer risk. In the collaborative reanalysis of 51 epidemiologic studies, the authors reported that the relationship between age at menopause and risk was similar for women who experienced natural menopause and for women who had surgical menopause (19). They estimated a 2.9% and 2.4% increase in the risk of breast cancer for each year of delay of menopause by surgical and natural menopause, respectively. Other groups have shown that the protective effect of early surgical menopause is stronger than that for natural menopause (20, 21).
Interestingly, we observed that the protective effect of an oophorectomy persists after a woman experiences natural menopause. The stronger effect of surgical menopause than natural menopause may be due to the reduction in circulating androgen levels in oophorectomized women. Oophorectomy was associated with an 87% reduction in the risk of postmenopausal breast cancer among women who experienced natural menopause, however the number of women in this subgroup was small and the confidence limits were wide.
The postmenopausal ovary secretes testosterone and androstenedione, which can be converted to estradiol and estrone by aromatisation in the breast and other tissues (22). In the general population, there is epidemiologic evidence to support an etiologic role of androgens for postmenopausal breast cancer (23). It is also important to determine if androgen has a direct effect on breast cancer risk in BRCA1 mutation carriers (i.e., without conversion to estrogen). In favour of the androgenic hypothesis, hormone replacement therapy with estrogen alone, or with a combination of estrogen and progesterone, does not seem to counteract the protective effect of oophorectomy in BRCA1 carriers (24, 25). Interestingly, one paper reported that aromatase expression is increased in the breast adipose and ovarian tissue of BRCA1 mutation carriers, compared with non-carrier controls (26). We reported that BRCA1 carrier women experienced a decline in sexual functioning with oophorectomy, even if the oophorectomy was done after menopause (27); presumably, due to diminishing circulating androgens. These data support a model whereby a small amount of circulating estrogen is necessary to promote breast carcinogenesis in BRCA1 mutation carriers. The reduction in estrogen exposure associated with a postmenopausal oophorectomy is sufficient to reduce the risk. We speculate that a further reduction in estrogen levels by the use of an aromatase inhibitor could further reduce the risk. Studies directly quantifying levels of androgens, estrogens and aromatase activity in the both the pre- and postmenopausal breast tissue will help elucidate a role of endogenous hormones.
In summary, our data challenges the prevailing hypothesis that the risk of breast cancer in a woman can be predicted by her lifetime number of ovulatory cycles, at least in women with a BRCA mutation. Reproductive risk factors, such as age at menarche and breastfeeding are important in BRCA1 carriers, but it is likely that these act through mechanisms other than through affecting endogenous hormone levels (e.g., expanding the breast stem cell population or inducing terminal differentiation in ductal cells). The observation that a postmenopausal oophorectomy protects against breast cancer suggests that circulating hormone levels in women after menopause are important in predicting risk. This is consistent with either a direct carcinogenic activity of ovarian androgens or through aromatisation of androgen to estrogen. It is important to distinguish between these two possibilities because of the different implications and options for chemoprevention.
Supplementary Material
Acknowledgements
We would like to acknowledge the study coordinators Adriana Valentini, Marcia Llacuachaqui, and Alejandra Ragone, as well as the students and staff Jennifer Ng, Kristi De Buono, Kate Bisnaire, Dina Nikitina, Anneli Loo, Bita Khorram, Dina Gordon, Courtney May, Michelle Jones, Jose Miguel Lozano, who helped with the data collection and data entry.
Supported by the Canadian Cancer Society Research Initiative and an NIH grant (R01 CA 74415). Joanne Kotsopoulos is the recipient of a Cancer Care Ontario Research Chair in Population Studies. Steven Narod is the recipient of a Canada Research Chair tier I.
References
- 1.Bernstein L. Epidemiology of endocrine-related risk factors for breast cancer. J Mammary Gland Biol Neoplasia. 2002;7:3–15. doi: 10.1023/a:1015714305420. [DOI] [PubMed] [Google Scholar]
- 2.Henderson BE, Ross RK, Judd HL, Krailo MD, Pike MC. Do regular ovulatory cycles increase breast cancer risk? Cancer. 1985;56:1206–8. doi: 10.1002/1097-0142(19850901)56:5<1206::aid-cncr2820560541>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 3.Russo J, Rivera R, Russo IH. Influence of age and parity on the development of the human breast. Breast Cancer Res Treat. 1992;23:211–8. doi: 10.1007/BF01833517. [DOI] [PubMed] [Google Scholar]
- 4.Kotsopoulos J, Lubinski J, Lynch HT, Neuhausen SL, Ghadirian P, Isaacs C, et al. Age at menarche and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Cancer Causes Control. 2005;16:667–74. doi: 10.1007/s10552-005-1724-1. [DOI] [PubMed] [Google Scholar]
- 5.Gronwald J, Byrski T, Huzarski T, Cybulski C, Sun P, Tulman A, et al. Influence of selected lifestyle factors on breast and ovarian cancer risk in BRCA1 mutation carriers from Poland. Breast Cancer Res Treat. 2006;95:105–9. doi: 10.1007/s10549-005-9051-5. [DOI] [PubMed] [Google Scholar]
- 6.Chang-Claude J, Becher H, Eby N, Bastert G, Wahrendorf J, Hamann U. Modifying effect of reproductive risk factors on the age at onset of breast cancer for German BRCA1 mutation carriers. J Cancer Res Clin Oncol. 1997;123:272–9. doi: 10.1007/BF01208638. [DOI] [PubMed] [Google Scholar]
- 7.Jernstrom H, Lubinski J, Lynch HT, Ghadirian P, Neuhausen S, Isaacs C, et al. Breast-feeding and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2004;96:1094–8. doi: 10.1093/jnci/djh211. [DOI] [PubMed] [Google Scholar]
- 8.Cullinane CA, Lubinski J, Neuhausen SL, Ghadirian P, Lynch HT, Isaacs C, et al. Effect of pregnancy as a risk factor for breast cancer in BRCA1/BRCA2 mutation carriers. Int J Cancer. 2005;117:988–9. doi: 10.1002/ijc.21273. [DOI] [PubMed] [Google Scholar]
- 9.Moorman PG, Iversen ES, Marcom PK, Marks JR, Wang F, Lee E, et al. Evaluation of established breast cancer risk factors as modifiers of BRCA1 or BRCA2: a multi-center case-only analysis. Breast Cancer Res Treat. 2010;124:441–51. doi: 10.1007/s10549-010-0842-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotsopoulos J, Lubinski J, Lynch HT, Klijn J, Ghadirian P, Neuhausen SL, et al. Age at first birth and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat. 2007;105:221–8. doi: 10.1007/s10549-006-9441-3. [DOI] [PubMed] [Google Scholar]
- 11.Tryggvadottir L, Olafsdottir EJ, Gudlaugsdottir S, Thorlacius S, Jonasson JG, Tulinius H, et al. BRCA2 mutation carriers, reproductive factors and breast cancer risk. Breast Cancer Res. 2003;5:R121–8. doi: 10.1186/bcr619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101:80–7. doi: 10.1093/jnci/djn442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang-Claude J, Andrieu N, Rookus M, Brohet R, Antoniou AC, Peock S, et al. Age at menarche and menopause and breast cancer risk in the International BRCA1/2 Carrier Cohort Study. Cancer Epidemiol Biomarkers Prev. 2007;16:740–6. doi: 10.1158/1055-9965.EPI-06-0829. [DOI] [PubMed] [Google Scholar]
- 14.Hartge P, Chatterjee N, Wacholder S, Brody LC, Tucker MA, Struewing JP. Breast cancer risk in Ashkenazi BRCA1/2 mutation carriers: effects of reproductive history. Epidemiology. 2002;13:255–61. doi: 10.1097/00001648-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Andrieu N, Goldgar DE, Easton DF, Rookus M, Brohet R, Antoniou AC, et al. Pregnancies, breast-feeding, and breast cancer risk in the International BRCA1/2 Carrier Cohort Study (IBCCS) J Natl Cancer Inst. 2006;98:535–44. doi: 10.1093/jnci/djj132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisen A, Lubinski J, Klijn J, Moller P, Lynch HT, Offit K, et al. Breast cancer risk following bilateral oophorectomy in BRCA1 and BRCA2 mutation carriers: an international case-control study. J Clin Oncol. 2005;23:7491–6. doi: 10.1200/JCO.2004.00.7138. [DOI] [PubMed] [Google Scholar]
- 17.Strauss JF, Barbieri RL. Yen and Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. 5th ed. Elsevier Saunders; Philadelphia: 2004. [Google Scholar]
- 18.Liao DJ, Dickson RB. Roles of androgens in the development, growth, and carcinogenesis of the mammary gland. J Steroid Biochem Mol Biol. 2002;80:175–89. doi: 10.1016/s0960-0760(01)00185-6. [DOI] [PubMed] [Google Scholar]
- 19.Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet. 1997;350:1047–59. [PubMed] [Google Scholar]
- 20.Brinton LA, Schairer C, Hoover RN, Fraumeni JF., Jr Menstrual factors and risk of breast cancer. Cancer Invest. 1988;6:245–54. doi: 10.3109/07357908809080645. [DOI] [PubMed] [Google Scholar]
- 21.Titus-Ernstoff L, Longnecker MP, Newcomb PA, Dain B, Greenberg ER, Mittendorf R, et al. Menstrual factors in relation to breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1998;7:783–9. [PubMed] [Google Scholar]
- 22.Burger HG. The endocrinology of the menopause. Maturitas. 1996;23:129–36. doi: 10.1016/0378-5122(95)00969-8. [DOI] [PubMed] [Google Scholar]
- 23.Eliassen AH, Hankinson SE. Endogenous hormone levels and risk of breast, endometrial and ovarian cancer: prospective studies. In: Berstein RJ, editor. Innovative Endocrinology of Cancer. Landes Bioscience; New York: 2008. pp. 148–65. [PubMed] [Google Scholar]
- 24.Eisen A, Lubinski J, Gronwald J, Moller P, Lynch HT, Klijn J, et al. Hormone therapy and the risk of breast cancer in BRCA1 mutation carriers. J Natl Cancer Inst. 2008;100:1361–7. doi: 10.1093/jnci/djn313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rebbeck TR, Friebel T, Wagner T, Lynch HT, Garber JE, Daly MB, et al. Effect of short-term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. 2005;23:7804–10. doi: 10.1200/JCO.2004.00.8151. [DOI] [PubMed] [Google Scholar]
- 26.Chand AL, Simpson ER, Clyne CD. Aromatase expression is increased in BRCA1 mutation carriers. BMC Cancer. 2009;9:148. doi: 10.1186/1471-2407-9-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finch A, Metcalfe KA, Chiang JK, Elit L, McLaughlin J, Springate C, et al. The impact of prophylactic salpingo-oophorectomy on menopausal symptoms and sexual function in women who carry a BRCA mutation. Gynecol Oncol. 2011 doi: 10.1016/j.ygyno.2010.12.326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.