Abstract
Following AAV-based gene transfer, the occurrence of adaptive immune responses specific to the vector or the transgene product is a major roadblock to successful clinical translation. These responses include antibodies against the AAV capsid, which can be neutralizing and therefore prevent the ability to repeatedly administer the vector, and CD8+ cytotoxic T lymphocytes, which can eliminate transduced cells. In addition, humans may have both humoral and cellular pre-existing immunity, as a result from natural infection with parent virus or related serotypes. The need for assays to detect and measure these anti-capsid immune responses in humans and in experimental animals is profound. Here, ELISPOT, immunocapture (ELISA), and neutralization assays are explained and provided in detail. Furthermore, such techniques can readily be adapted to monitor and quantify immune responses against therapeutic transgene products encoded by the vector genome.
Keywords: AAV, Viral capsid, CD8+ T cells, ELISPOT, IFN-γ, ELISA, antibody, neutralization
1. Introduction
Among vector systems that allow efficient in vivo gene transfer, recombinant adeno-associated virus vectors (rAAV) hold great potential. However, untoward immunes responses against the viral capsid and/or transgene products have emerged as serious obstacles for successful clinical translation, possibly resulting in prevention or elimination of therapeutic gene expression.
In animal models, rAAV-based gene transfer often resulted in deleterious humoral as well as cellular immune responses towards heterologous and, in some instances, even autologous gene products (1-5). The potential of such responses depends on several parameters including vector construct and dose, viral serotype with its ability to transduce antigen-presenting cells (APCs), the purity of viral preparation, and genetic and non-genetic factors in the recipient. Moreover, the route of vector delivery appears to be a key factor, even able to shift the balance from an immunogenic to a tolerogenic response towards the transgene product as shown following intrahepatic rAAV administration (6-11). Aside anti-transgene responses, animal studies have also shown formation of neutralizing antibodies against vector particles following rAAV delivery that prevents re-administration. In humans, high prevalence of anti-AAV preexisting immunity is an additional challenge for rAAV gene transfer. Preformed neutralizing antibodies are present in most individuals with the highest prevalence for AAV2 serotype (12, 13). Low titers of these preexisting antibodies have been shown to neutralize and to abrogate gene transfer efficiency using several AAV serotypes, even when high-dose vector is administered (14-16). Moreover, antibodies against AAV in humans have been shown to be associated to anti-capsid T cell responses including both CD4+ helper and CD8+ cytotoxic T cell (CTL) responses. Importantly, in recent clinical trials using rAAV of serotypes 1 and 2 (17-19), specific capsid CTLs have been detected, and their activation was reported to be dependent on the vector dose (17). In a clinical trial on treatment of hemophilia, these CTLs have been hypothesized to eliminate transduced hepatocytes in vivo. Supportive evidence was recently obtained using an in vitro killing assay (20). Interestingly and for still unclear reasons, this key issue has not been identified in animal studies even when AAV-specific CTLs have been detected.
Therefore, the evaluation of the immune status of potential recipients of AAV vector gene therapy trials prior and after gene transfer is critical and should include monitoring antibodies as well as T cell responses. Specific and sensitive assays for the detection and the quantification of such responses have been developed. This chapter provides protocols used to detect and measure anti-capsid antibodies in serum of any species using both an ELISA technique as well as an AAV neutralizing transduction assay. The ELISA technique allows rapid detection of most anti-AAV antibodies of even low avidity. The neutralizing assay is longer but allows a more relevant functional analysis of the antibodies; in fact both assays are likely complementary and both may have clinical relevance. In terms of cellular responses against capsid, the chapter also describes an ELISPOT assay for IFN-γ producing cells that utilizes AAV capsid-derived peptides recognized by CD8+ cells from human or mouse origin.
2. Materials
2.1. Isolating White Blood Cells for IFN-γ Secretion. (see Note 1)
PBS: Sterile phosphate-buffered saline.
Hanks Balanced Salt Solution (Invitrogen, Carlsbad, CA).
Culture Media: RPMI 1640 Medium [+] L-Glutamine (Invitrogen, Carlsbad, CA) with 25mM HEPES (Sigma, St. Louis, MO), 10% Heat Inactivated Fetal Bovine Serum (Invitrogen, Carlsbad, CA), and 1% Penicillin – Strepomycin Solution (Mediatech, Manassas, VA).
Red Blood Cell Lysis Buffer (ebiosciences, San Diego, CA).
70μm nylon cell strainer (BD Biosciences, San Jose, CA).
Ficoll Paque Plus (GE Healthcare, Piscataway, NJ).
Trypan blue (Invitrogen, Carlsbad, CA).
2.2. ELISPOT (Enzyme-linked immunospot) Detection of IFN-γ Secreting Cells
Murine or Human IFN-γ Development Module Kit (R&D Systems, Minneapolis, MN) containing IFN-γ capture and detection antibodies.
BCIP/NBT substrate (R&D Systems, Minneapolis, MN): 5-Bromo-4-Chloro3’ Indolylphosphate p-Toluidine Salt (BCIP) and Nitro Blue Tertazolium Chloride (NBT) in organic solvent.
Streptavidin-AP (R&D Systems, Minneapolis, MN)
Specific CD8+ T cell epitope (Anaspec, San Jose, CA).
SEB: Staphlococcal enterotoxin B (Sigma, St Louis, MO).
PBS: phosphate-buffered saline.
Wash buffer: 0.05% Tween 20 in PBS.
Blocking buffer: 1% Bovine Serum Albumin (BSA) and 5% Sucrose in PBS.
Reagent Diluent Concentration (R&D systems, Minneapolis, MN): filter sterilized PBS with 10% BSA.
Tissue culture incubator.
dH2O: Sterile deionized water.
96-well immunospot plate (Millipore, Billerica, MA). (see Note 2)
ELISPOT Reader. (see Note 3)
Optional: multi-channel pipette or automated plate washer.
2.3. Antibody ELISA (Enzyme-linked immunosorbent assay) to rAAV2 Humoral Response
Intact (DNA containing or empty) rAAV particles of the same serotype used for gene transfer.
Experimental host serum (i.e. from rAAV infected mice or humans).
Mouse immunoglobulin standard (IgG2a, Sigma, St. Louis, MO)
Anti-mouse IgG2a-HRP (horseradish peroxidase conjugate; Southern Biotech, Birmingham, Alabama). (see Note 4)
Coating Buffer: 13mM Na2CO, 88mM NaHCO3.
Wash Buffer: 0.05% Tween 20 in PBS.
Dilution Buffer: 5% BSA (bovine serum albumin), 0.05% Tween 20 in PBS.
dH2O: Sterile deionized water
OPD (o-Phenylenediamine dihydrochloride) Tablets (Sigma, St. Louis, MO).
Microtiter Plates (Corning, Lowell, MA); or suitable alternative.
Adhesive sealing film (EXCEL Scientific, Victorville, CA).
Microplate reader (Biorad, Hercules, CA) or suitable alternative.
Optional: Multi-channel pipette or automated plate washer.
Optional: Stop solution for HRP development; 3 M HCl or 3 M H2SO4
2.4. Neutralizing Antibody Assay
Wild type adenovirus type 5 (wt Ad5) particles.
Experimental host serum (i.e. rAAV2 infected mice, non human primates or humans).
HeLa cell line (see Note 6).
Sterile trypsin.
DMEM: Sterile Dulbecco’s Modified Eagle Medium medium.
FCS: Heat inactivated Fetal Calf Serum FCS.
PBS: Sterile phosphate-buffer saline PBS.
X-Gal staining buffer: 50ng/ml 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-Gal) for colorimetric detection of β-galactosidase enzyme activity (Promega), 0.1% MgCl2, 1% potassium ferricyanide, 1% potassium ferrocyanide in PBS (see Note 7).
0.5% Glutaraldehyde in PBS.
dH2O: Sterile deionized water.
Sterile 24 well cell culture plates.
3. Methods
3.1. Isolating White Blood Cells for IFN-γ Secretion
3.1.1 Murine splenocyte isolation
Remove spleen from mouse or rat in a sterile environment and immerse in a container with ice cold Hanks Media and store on ice.
Pulverize spleen on cell strainer, collect cellular content in Hanks Media as it flows through the screen, rinse cell strainer with Hanks Media and bring volume up to 20 – 40 mL in a 50-mL tube with Hanks Media.
Centrifuge isolated splenocytes and red blood cells at 4°C (350g for 10 minutes).
Carefully pour off supernatant, break up the cell pellet, resuspend in red blood cell lysis buffer (2 to 5 mL) and incubate at room temperature for 5 minutes.
Wash with 20 - 40 mL of Hanks Media and centrifuge white blood cell fraction of bulk splenocytes at 4°C (350g for 10 minutes).
Carefully pour off supernatant, break up cell pellet and resuspend in complete RPMI 1640 media (containing 2 μg/mL of the peptide antigen that encodes the specific T cell epitope) at a concentration of 1.0 × 107 cells/mL.
Determine cell count concentration using a 1:10 dilution of trypan blue to exclude dead cells.
3.1.2 Human PBMC isolation
Pipette 25 mL of human blood collected with anti-coagulant onto 12.5 mL of Ficoll-Paque Plus solution.
With centrifuge set to “0” acceleration and “0” brake, centrifuge at 500g for 30 minutes at room temperature.
Isolate the white blood cell fraction (see Note 8), transfer to 50-mL tube and bring volume to 50 mL with PBS and centrifuge at room temperature (350g for 10 minutes).
Carefully pour off supernatant, break up cell pellet, resuspend in 5 to 10 mL of red blood cell lysis buffer, and incubate for 5 minutes at room temperature.
Add 25 mL of Hanks buffer and centrifuge PBMCs at room temperature (350g for 10 minutes).
Carefully pour off supernatant and resuspend in complete RPMI 1640 media (containing 2 μg/mL of the peptide antigen that encodes the specific T cell epitope) at a concentration of 1.0 × 107 cells/mL.
Determine cell count concentration using a 1:10 dilution of trypan blue to exclude dead cells.
3.2. ELISPOT (Enzyme-linked immunospot) Detection of IFN-γ Secreting Cells
3.2.1. Prepare ELISPOT plate to culture IFN-γ secreting white blood cells. (see Note 9)
Pre-wet and coat a 96 well ELISPOT plate 24 hours prior to isolating white blood cells from murine splenocytes or human PBMCs.
Pre-wet plate with 15 μL/well of 35% ethanol for 15 seconds and flick out ethanol (DO NOT VACUUM) by inverting plating. (see Note 10)
To coat plate, prepare 1:60 dilution (see Note 11) of IFN-γ capture antibody in PBS, add 100 μL/well of diluted antibody to 96-well plate and incubate overnight 4°C.
Remove capture antibody and wash wells 3 times with wash buffer using multi-channel pipette or automatic plate washer and remove excess wash buffer by inverting and blotting the plate on a paper towel.
Block membrane with 200 μL/well of blocking solution for 2 hours at room temperature.
Repeat step 3 to remove blocking buffer and wash the plate.
After washing, rinse the plate with 200 μL/well of complete RMPI 1640 media and remove the rinse media when IFN-γ secreting cells are ready to be cultured.
3.2.2. Stimulate activated CD8+ rAAV specific IFN-γ secreting cells. (see Note 12)
Remove rinse media from ELISPOT plate by flicking the plate while inverted and blot remaining rinse media on a paper towel
Add 1.0 × 106 cells/well, which is 100 μL of the isolated white blood cells re-suspended at a concentration of 1.0 × 107 cells/mL in the appropriate stimulation media and incubate for 24 to 36 hours (see Note 13) at 37°C in a 5% CO2 cell culture incubator.
Experimental stimulation media consists of the complete RPMI 1640 media with 2 μg/ml of appropriate CD8+ epitope (see Table 1 for examples) to determine the frequency of capsid-specific IFN-γ secreting cells (18, 20-23).
Negative control stimulation media consists of complete RMPI 1640 media without epitope to determine background level of IFN-γ secreting cells.
A non-specific, positive control stimulation media consists of the complete RMPI 1640 culture media with 5 μg/ml of a highly immunogenic superantigen, Staphylococcal enterotoxin B, to ensure that IFN-γ secreting cells are present and to determine the maximal frequency of IFN-γ secreting T cells.
An internal positive control can be used by the addition of recombinant IFN-γ protein at 2 ug/mL to the culture media alone, without cells, to verify that there are no procedural errors and to ensure the quality of the reagents. This entire well will appear completely bluish- purple after color development.
Table 1.
Examples of dominant CD8+ rAAV epitopes
| Host | AAV Serotype | Peptide Sequence | MHC I restriction |
|---|---|---|---|
| Mouse; BALB/c (H-2d) | AAV2 | VPQYGYLTL | Ld |
| AAV8 | IPQYGYLTL | Ld | |
| Mouse; C57BL/6 (H-2b) | AAV2 | SNYNKSVNV | Kb |
| AAV8 | NSLANPGIA | Db | |
| Human (HLA-A) | AAV2 | SADNNNSEY | HLA-A*0101 |
| Human (HLA-B) | AAV2 | VPQYGYLTL | HLA-B*0702 |
3.2.3. Detection of IFN-γ Protein Secreted from Stimulated CD8+ rAAV Specific T-Cells
Remove stimulation media and wash 3 times with wash buffer as described above.
Dilute 10X Reagent Diluent Concentrate in sterile dH2O and use to prepare a 1:60 dilution of IFN-γ detection antibody, add 100 μL/well of diluted antibody to 96-well plate and incubate overnight at 4°C.
Remove detection antibody and wash 3X as described above.
Dilute 10X Reagent Diluent Concentrate in sterile dH2O and use to prepare a 1:60 dilution of Streptavidin-AP, add 100 μL/well of diluted Streptavidin-AP and incubate for 2 hours at room temperature.
Remove Streptavidin-AP and wash plate three times as described above.
Add 100 μL/well of BCIP/NBT solution. Cover and develop the plate in the dark for 10 to 30 minutes at room temperature. (see Note 14)
After development remove BCIP/NBT, rinse THOROUGHLY with dH2O (see Note 15) and allow plate to air dry at room temperature.
The visible spots that have formed can be counted either manually with a dissecting microscope or with an ELISPOT reader (see Fig. 1). Results are typically reported as the average number of spot forming units (SFU) per 106 cells plated.
Fig. 1.
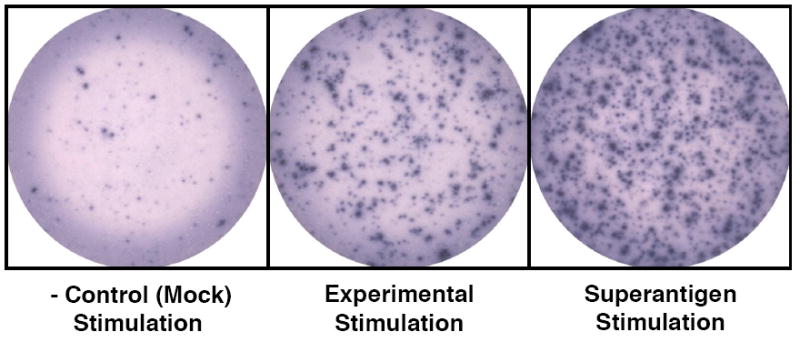
Examples of ELISPOT wells with SFUs (Spot Forming Units) after color development.
3.3. Antibody ELISA (Enzyme-linked immunosorbent assay) to rAAV2 Humoral Response
3.3.1. Preparing Standard curve for measuring antibody titers
Prepare the first point of standard curve using purified mouse IgG2a (Sigma) at a concentration of 2000 ng/ml in coating buffer and then make 1:2 serial dilutions using coating buffer as diluent to get a total of 8 points with the final point being at a concentration of 15.625 ng/ml (see Note 4 and Fig. 2).
Add 50 μL/well of each standard concentration in duplicate to microtiter plate.
Fig. 2.
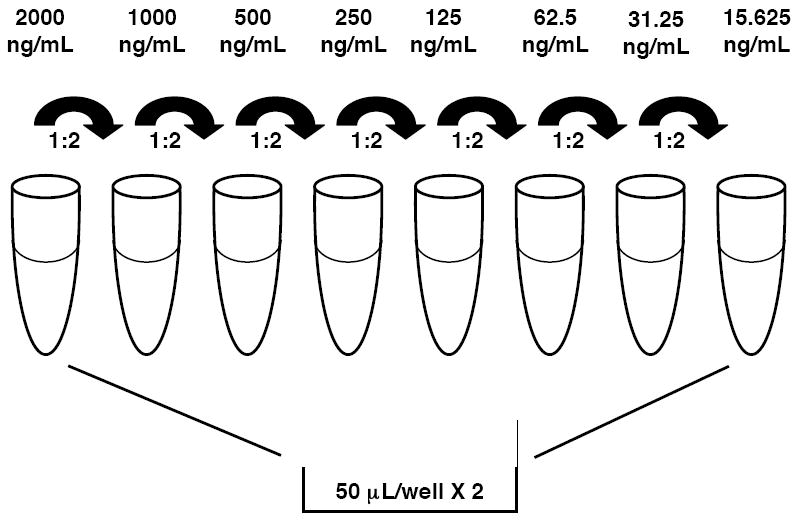
Schematic to preparing immunoglobulin standard used for ELISA by serial dilution.
3.3.2. Measuring rAAV2 antibody titers
After the standard curve wells have been established, coat the remaining wells with 8.0 × 107 intact rAAV viral particles of the respective serotype in 50 μL aliquots using coating buffer. Cover the plate with adhesive sealing film and incubate microtiter plate overnight at 4°C.
Wash plate 3X with 200 μL/well of wash buffer using multi-channel pipette or automated plate washer. Blot inverted microtiter plate on a paper towel after washing to remove residual wash buffer.
Block microtiter plate using 200 μL/well of dilution buffer at room temperature for 2 hours and then wash plate as previously described.
Make a 1:20 dilution (see Note 16) of experimental host serum using dilution buffer. Add 50 μL of diluted experimental host serum in duplicate. Incubate at 37°C for 2 hours and then wash plate as previously described. For standard curve wells just add 50 μL of dilution buffer alone. Additionally, blank wells and positive control wells should be used. Dilution buffer alone should be added to blank wells, and known anti-rAAV positive sera should be used as a positive control to ensure that there have been no procedural errors and to verify that efficient reagents are being used.
Make a 1:2000 dilution (see Note 17) of the rabbit anti-mouse IgG2a-HRP secondary antibody in dilution buffer. Add 100 μL/well of the diluted HRP-labeled secondary antibody to the microtiter plate and incubate at 37°C for 2 hours than wash plate as previously described.
For HRP-catalyzed color reaction, dissolve OPD tablets in 20 mL of dH2O and add 200 μL/well to plate.
Allow plate to develop for 5 to 15 minutes (see Note 18) followed by measurement of absorbance at 450 nm wavelength using a microtiter plate reader. Alternatively, 100 μL/well of stop solution can be added to the plate and the absorbance can be measured later.
3.4. Neutralizing Antibody Assay
On day 1, prepare 24 well cell culture plate(s) with 2×105 HeLa cells (see Note 19) in 500μl DMEM 10% FCS per well.
On day 2, inactivate experimental, and possibly control sera (see Note 20) by heating them at 56°C for 30 minutes.
Prior to the assay, remove HeLa adherent cells from 3 wells using trypsin, count and calculate the average number of cells per well.
Aspirate the medium from the wells.
Add 500μl of DMEM 2% FCS containing wt Ad5 preparation at a Multiplicity of Infection (MOI) of 8 infectious particles/cell
Incubate at 37°C for 2 hours.
-
During the incubation of the cells with the wt Ad5
-
7.1
Prepare dilutions of the experimental serum (i.e 1:5, 1:10, 1:20, 1:50, 1:100, 1:200) in DMEM 2% FCS medium (see Note 21).
-
7.2
Add to serum dilutions rAAV2-CMV-LacZ viral particles. The number of AAV particles should achieve in the well a MOI of 4000 particles. The final volume per well of each diluted serum/rAAV should be 500μl.
-
7.3
Incubate the serum with rAAV at room temperature for 30 minutes to allow AAV contact with neutralizing antibodies.
-
7.1
Aspirate the wells and add 500μl of diluted serum/rAAV per well.
Incubate the plate at 37°C for 24 hours.
Aspirate the wells and fix the cells by adding 500μl of glutaraldehyde 0.5% in PBS per well.
Incubate at room temperature for 5 minutes.
Wash the wells 1X with deionized water (dH2O).
Add 500μl of X-Gal solution per well.
Incubate at 37°C for 6 hours (see Note 22).
Wash the wells 1X with deionized water (dH2O) and read the X-Gal coloration (blue cells) by light microscopy (see Fig. 3 and Notes 23, 24).
Fig. 3.
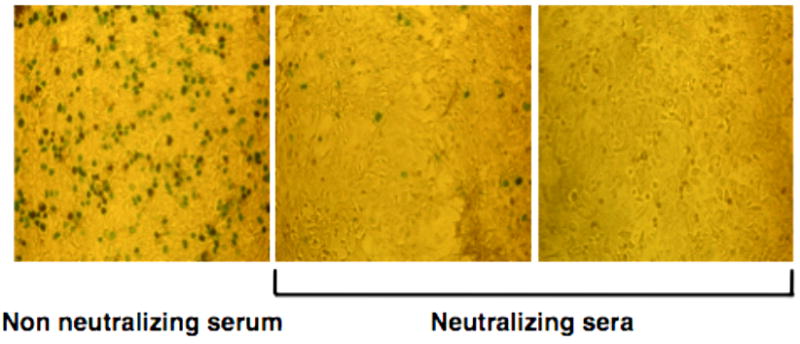
Examples of X-Gal coloration detected by microscopy. Left panel: absence of neutralization.Blue spots correspond to transduced cells.Left and middle panels: neutralizing sera with different amounts of neutralizing antibodies. Far right panel, the serum abrogates completely AAV-mediated transduction.
Footnotes
Described white blood cell source is from either murine splenocytes or human peripheral blood mononuclear cells (PMBCs).
There is a range of immunospot plates with different binding membranes; consult technical support to suit individual needs.
In the absence of an ELISPOT reader, a dissecting microscope can be used to count spots.
Mice typically produce immunoglobulin of IgG2a subclass against rAAV particles. However, other subclasses such as IgG3 have also been described and can be tested for and quantified using analogous reagents for the alternate subclass (murine immunoglobulin standards and detecting antibody) (24). In humans, the dominant response is IgG1 (i.e. the human equivalent to IgG2a, reflecting a Th1-driven response), while IgG2, 3, and 4 may also be found (25). Respective analogous reagents to measure such titers in humans are available. The identical assay can be used to determine antibody titers against the transgene product by coating the microtiter plate with the respective protein instead of with vector particles (8, 26, 27).
Other transgenes (i.e luciferase (13), Green Fluorescent Protein, GFP) can be used for this type of assay and specific detection methods can also be performed (i.e flow cytometry for GFP+ cells).
The assay can be adapted to other rAAV serotypes. Optimal MOI may need to be determined for each serotype. Using Hela or Huh7 hepatoma cell line, a recent study showed that maximal transduction was reached at a MOI of 5000 vector genomes [Vg]/cell for AAV2, 5 and 6, at MOI of 10,000 Vg/cell for AAV1 and 8, and at a MOI of 100 000 Vg/cell for AAV9 (13).
Aside MOI, optimal cell line must also be tested for specific serotypes. For instance, even if the assay can be performed with HeLA cell line, it is not optimal for rAAV8 serotype. Boutin et al. showed that HeLA cell were best transduced with recombinant AAV1, 2, 5, 6 and 9, whereas Huh7 cell line was more suitable for serotype 8 (13). In another study, Huh7 cells were used for serotypes 1, 2, 7 and 8 (12).
This solution is stable 1 month in dark at 4°C.
White blood cell fraction is the white ring below the upper plasma layer.
Culture isolated white blood cells the day of isolation for ELISPOT analysis.
Pre-wetting plate helps minimize background after detection.
A 1:60 dilution is recommended, but optimization of both capture and detection antibodies maybe required.
CD8+ T-cell responses specific for the transgene product can be assessed with the same assay using the appropriate CD8+ T cell epitope. Similarly, the assay can be used to measure CD4+ T cell responses using peptides encoding the respective epitopes. In this case, frequencies of IFN-γ secreting cells are determined to measure Th1 responses, while for Th2 responses, IL-4 or IL-5 producing cells are typically measured.
Begin with a 32 hr incubation time but optimization maybe necessary. Optimizing incubation time can be done by using naïve isolated white blood cells and the positive control stimulation media with 5 ug/mL Staphylococcal enterotoxin B. Too short of a time period may result in minimal spot formation and differences in the experimental groups may not be accurately assessed while excessive incubation time may cause saturation of spot formation.
Watch development carefully to prevent over-development, which will lead to high background and make it difficult to accurately count the spots that have formed.
If the plate is not thoroughly rinsed, the residual BCIP/NBT can cause over-development of plate while drying. Additionally, the thin plastic sheet on the bottom of the plate that protects the membrane can now be removed manually with pliers so the bottom of the membrane can be rinsed as well.
This dilution may need to be optimized depending on antibody titers in sera (i.e very high levels may need to be diluted more and very low levels may need to be diluted less). Absorbance values should be within the linear range of the standard curve.
This dilution may need to be optimized to suit individual needs.
Color development should be such to achieve a linear range of immunoglobulin concentration as a function of the absorbance (on a semi-log plot). Therefore, the time of color development has to be optimized. Alternatively, the plate can be measured several times over 5 to 15 minute range in order to identify an optimal readout
Huh7 and 2V6.11 embryonic kidney cell lines have also been described for neutralizing assays for AAV2 serotype (12, 25).
Several controls are recommended to validate the assay: C1) cells alone; C2) cells + wt Ad5; C3) cells + rAAV2; C4) cells + wt Ad5 + rAAV2 (X-Gal positive control); and if possible C5) cells + wt Ad5 + rAAV2 + control neutralizing negative serum (also X-Gal positive control) and C6) cells + wt Ad5 + rAAV2 + control neutralizing positive serum (X-Gal negative coloration control). In animal experimental studies, internal neutralizing negative serum can be obtained before rAAV immunization.
This is recommended serum dilution but it may need to be adapted to suit individual needs and depending on levels of rAAV2 antibodies (i.e very high levels may need to be diluted more, up to 1:5000).
Other variants of incubation with X-Gal are 2 hours at 42°C or overnight at 30°C.
The plate can be stored at 4°C for further reading.
This assay is semi-quantitative (blue cell count using miscroscope) and does not allow precise determination of neutralizing antibody titers. For more precise quantification, the colorimetric detection of beta-galactosidase may be assessed by bioluminiscence after cell lysis and measurement in a microplate luminometer as described elsewhere (12, 25). In assays using luciferase transgene, similar luminescent quantification is described (13).
References
- 1.Ross CJ, Twisk J, Bakker AC, Miao F, Verbart D, Rip J, Godbey T, Dijkhuizen P, Hermens WT, Kastelein JJ, Kuivenhoven JA, Meulenberg JM, Hayden MR. Correction of feline lipoprotein lipase deficiency with adeno-associated virus serotype 1-mediated gene transfer of the lipoprotein lipase S447X beneficial mutation. Hum Gene Ther. 2006;17:487–499. doi: 10.1089/hum.2006.17.487. [DOI] [PubMed] [Google Scholar]
- 2.Herzog RW, Mount JD, Arruda VR, High KA, Lothrop CD., Jr Muscle-directed gene transfer and transient immune suppression result in sustained partial correction of canine hemophilia B caused by a null mutation. Mol Ther. 2001;4:192–200. doi: 10.1006/mthe.2001.0442. [DOI] [PubMed] [Google Scholar]
- 3.Gao G, Lebherz C, Weiner DJ, Grant R, Calcedo R, McCullough B, Bagg A, Zhang Y, Wilson JM. Erythropoietin gene therapy leads to autoimmune anemia in macaques. Blood. 2004;103:3300–3302. doi: 10.1182/blood-2003-11-3852. [DOI] [PubMed] [Google Scholar]
- 4.Favre D, Blouin V, Provost N, Spisek R, Porrot F, Bohl D, Marme F, Cherel Y, Salvetti A, Hurtrel B, Heard JM, Riviere Y, Moullier P. Lack of an immune response against the tetracycline-dependent transactivator correlates with long-term doxycycline-regulated transgene expression in nonhuman primates after intramuscular injection of recombinant adeno-associated virus. J Virol. 2002;76:11605–11611. doi: 10.1128/JVI.76.22.11605-11611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chenuaud P, Larcher T, Rabinowitz JE, Provost N, Cherel Y, Casadevall N, Samulski RJ, Moullier P. Autoimmune anemia in macaques following erythropoietin gene therapy. Blood. 2004;103:3303–3304. doi: 10.1182/blood-2003-11-3845. [DOI] [PubMed] [Google Scholar]
- 6.Niemeyer GP, Herzog RW, Mount J, Arruda VR, Tillson DM, Hathcock J, van Ginkel FW, High KA, Lothrop CD., Jr Long-term correction of inhibitor-prone hemophilia B dogs treated with liver-directed AAV2-mediated factor IX gene therapy. Blood. 2009;113:797–806. doi: 10.1182/blood-2008-10-181479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mount JD, Herzog RW, Tillson DM, Goodman SA, Robinson N, McCleland ML, Bellinger D, Nichols TC, Arruda VR, Lothrop CD, Jr, High KA. Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood. 2002;99:2670–2676. doi: 10.1182/blood.v99.8.2670. [DOI] [PubMed] [Google Scholar]
- 8.Mingozzi F, Liu YL, Dobrzynski E, Kaufhold A, Liu JH, Wang Y, Arruda VR, High KA, Herzog RW. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest. 2003;111:1347–1356. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mingozzi F, Hasbrouck NC, Basner-Tschakarjan E, Edmonson SA, Hui DJ, Sabatino DE, Zhou S, Wright JF, Jiang H, Pierce GF, Arruda VR, High KA. Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood. 2007;110:2334–2341. doi: 10.1182/blood-2007-03-080093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dobrzynski E, Mingozzi F, Liu YL, Bendo E, Cao O, Wang L, Herzog RW. Induction of antigen-specific CD4+ T-cell anergy and deletion by in vivo viral gene transfer. Blood. 2004;104:969–977. doi: 10.1182/blood-2004-03-0847. [DOI] [PubMed] [Google Scholar]
- 11.Dobrzynski E, Fitzgerald JC, Cao O, Mingozzi F, Wang L, Herzog RW. Prevention of cytotoxic T lymphocyte responses to factor IX-expressing hepatocytes by gene transfer-induced regulatory T cells. Proc Natl Acad Sci U S A. 2006;103:4592–4597. doi: 10.1073/pnas.0508685103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calcedo R, Vandenberghe LH, Gao G, Lin J, Wilson JM. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boutin S, Monteilhet V, Veron P, Leborgne C, Benveniste O, Montus MF, Masurier C. Prevalence of serum IgG and neutralizing factors against adeno-associated virus types 1, 2, 5, 6, 8 and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- 14.Scallan CD, Jiang H, Liu T, Patarroyo-White S, Sommer JM, Zhou S, Couto LB, Pierce GF. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood. 2006;107:1810–1817. doi: 10.1182/blood-2005-08-3229. [DOI] [PubMed] [Google Scholar]
- 15.Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, Ozelo MC, Hoots K, Blatt P, Konkle B, Dake M, Kaye R, Razavi M, Zajko A, Zehnder J, Rustagi PK, Nakai H, Chew A, Leonard D, Wright JF, Lessard RR, Sommer JM, Tigges M, Sabatino D, Luk A, Jiang H, Mingozzi F, Couto L, Ertl HC, High KA, Kay MA. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 16.Jiang H, Couto LB, Patarroyo-White S, Liu T, Nagy D, Vargas JA, Zhou S, Scallan CD, Sommer J, Vijay S, Mingozzi F, High KA, Pierce GF. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. 2006;108:3321–3328. doi: 10.1182/blood-2006-04-017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mingozzi F, Meulenberg JJ, Hui DJ, Basner-Tschakarjan E, Hasbrouck NC, Edmonson SA, Hutnick NA, Betts MR, Kastelein JJ, Stroes ES, High KA. AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood. 2009;114:2077–2086. doi: 10.1182/blood-2008-07-167510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, Rasko JE, Ragni MV, Manno CS, Sommer J, Jiang H, Pierce GF, Ertl HC, High KA. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med. 2007;13:419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- 19.Brantly ML, Chulay JD, Wang L, Mueller C, Humphries M, Spencer LT, Rouhani F, Conlon TJ, Calcedo R, Betts MR, Spencer C, Byrne BJ, Wilson JM, Flotte TR. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc Natl Acad Sci U S A. 2009;106:16363–16368. doi: 10.1073/pnas.0904514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pien GC, Basner-Tschakarjan E, Hui DJ, Mentlik AN, Finn JD, Hasbrouck NC, Zhou S, Murphy SL, Maus MV, Mingozzi F, Orange JS, High KA. Capsid antigen presentation flags human hepatocytes for destruction after transduction by adeno-associated viral vectors. J Clin Invest. 2009;119:1688–1695. doi: 10.1172/JCI36891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Figueredo J, Calcedo R, Lin J, Wilson JM. Cross-presentation of adeno-associated virus serotype 2 capsids activates cytotoxic T cells but does not render hepatocytes effective cytolytic targets. Hum Gene Ther. 2007;18:185–194. doi: 10.1089/hum.2007.001. [DOI] [PubMed] [Google Scholar]
- 22.Sabatino DE, Mingozzi F, Hui DJ, Chen H, Colosi P, Ertl HC, High KA. Identification of mouse AAV capsid-specific CD8+ T cell epitopes. Mol Ther. 2005;12:1023–1033. doi: 10.1016/j.ymthe.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Wu Q, Yang P, Hsu HC, Mountz JD. Determination of specific CD4 and CD8 T cell epitopes after AAV2- and AAV8-hF.IX gene therapy. Mol Ther. 2006;13:260–269. doi: 10.1016/j.ymthe.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Xiao W, Chirmule N, Schnell MA, Tazelaar J, Hughes JV, Wilson JM. Route of administration determines induction of T-cell-independent humoral responses to adeno-associated virus vectors. Mol Ther. 2000;1:323–329. doi: 10.1006/mthe.2000.0045. [DOI] [PubMed] [Google Scholar]
- 25.Murphy SL, Li H, Mingozzi F, Sabatino DE, Hui DJ, Edmonson SA, High KA. Diverse IgG subclass responses to adeno-associated virus infection and vector administration. J Med Virol. 2009;81:65–74. doi: 10.1002/jmv.21360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herzog RW, Fields PA, Arruda VR, Brubaker JO, Armstrong E, McClintock D, Bellinger DA, Couto LB, Nichols TC, High KA. Influence of vector dose on factor IX-specific T and B cell responses in muscle-directed gene therapy. Hum Gene Ther. 2002;13:1281–1291. doi: 10.1089/104303402760128513. [DOI] [PubMed] [Google Scholar]
- 27.Fields PA, Kowalczyk DW, Arruda VR, Armstrong E, McCleland ML, Hagstrom JN, Pasi KJ, Ertl HC, Herzog RW, High KA. Role of vector in activation of T cell subsets in immune responses against the secreted transgene product factor IX. Mol Ther. 2000;1:225–235. doi: 10.1006/mthe.2000.0032. [DOI] [PubMed] [Google Scholar]


