Abstract
Background
The lateral nucleus of the amygdala (LA) is a crucial part of the neural circuitry underlying the formation and storage of memories established through fear conditioning. To investigate corticotropin-releasing factor (CRF) contributions to fear memory in LA, the present experiments tested the effects of intra-LA infusions on the formation and expression of memory after Pavlovian fear conditioning.
Methods
In experiment 1, CRF was infused bilaterally into LA of rats one hour before fear conditioning training. Two days later, rats were tested for conditioned stimulus (CS)-elicited freezing behavior in a distinct context. In experiment 2 rats were infused with CRF in LA immediately after auditory fear conditioning and then tested two days later. In experiment 3 rats were fear conditioned, and then two days later infused with CRF in LA one hour before fear memory testing to assess effects on the expression of fear memory. Finally, we repeated the pre-training and pre-testing experiments with the central nucleus of the amygdala (CE) infusions.
Results
Rats given either pre- or post-training CRF infusions in LA showed dose-dependent suppression of CS-elicited freezing in the fear memory test session. In contrast, rats given pre-testing CRF showed facilitation of CS-elicited freezing. CRF infusions into the CE had no effect when given before training or testing.
Conclusions
CRF infusions into LA impair the consolidation of memory for fear conditioning, but enhance the expression of pre-established fear memories. These findings may have important implications for understanding mechanisms underlying contributions of CRF to fear-related disorders.
Keywords: Amygdala, corticotrophin-releasing factor, fear conditioning, memory, fear-related disorders
The amygdala is known to be a crucial component of the neural circuitry underlying the learning and storage of emotional events (1,2). Emotional learning and memory are often studied using Pavlovian fear conditioning, a procedure that involves pairing an initially emotionally neutral conditioned stimulus (CS, e.g. a tone) with an aversive unconditioned stimulus (US, e.g. footshock). When the CS is later presented on its own, it elicits a conditioned fear reaction that consists of defensive behavioral responses (e.g. freezing), autonomic and endocrine activation.
The amygdala is a complex region of the temporal lobe consisting of several subareas (3,4). Two areas that are particularly important for fear conditioning are the lateral nucleus (LA) and the central nucleus (CE). LA is an initial site of convergence for CS and US information, and synaptic changes within the LA are thought to be crucial for the storage of the CS-US association (4–8). CE receives projections from LA, and sends outputs to hypothalamic (e.g. paraventricular nucleus and lateral nucleus) and brain stem regions (e.g. periaqueductal grey) that control the defensive, autonomic and endocrine reactions to fear (9,10).
Corticotropin-releasing factor (CRF), a 41-amino acid neuropeptide, is not only a first mediator in hypothalamic-pituitary-adrenal (HPA) axis activation, but also has direct effects in the brain via widely distributed CRF 1 and 2 receptors (11–16). CRF plays an important role in behavioral, endocrine and autonomic responses to stress with actions on the cardiovascular, gastrointestinal and reproductive systems (17,18). In the amygdala, there are many CRF immunoreactive cells and fibers in CE (19). CRF 1 receptors are more densely expressed in LA than in CE, but CRF 2 receptors are not evident in either LA or CE (20,21).
The role of CRF in LA or CE in fear conditioning is not well understood. CRF overexpression in mice attenuates acquisition of auditory fear conditioning (22), and systemic injection of the CRF receptor antagonist, CP-154,526, also impaired contextual fear conditioning (23), but these studies provide little information about the locus and mechanism of the effects. Existing studies of the effects of CRF manipulations in the amygdala on fear conditioning have focused on infusions in CE (24), which lacks CRF receptors, and the basolateral amygdala (BLA) (25), which includes the LA but also other areas (4). We performed the present studies to elucidate the contribution of CRF in LA and CE to the formation and the expression of memories created by auditory fear conditioning.
Methods and Materials
Subjects
Adult male Sprague Dawley rats obtained from Hiltop Laboratories (Scottdale, PA, USA) were housed individually in plastic Nalgene cages with ad libitum food and water and maintained on a 12 h light/dark cycle (lights on at 7:00a.m.). All experiments were performed from 9:00a.m. to 5:00p.m.. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Experimental Animals and were approved by the New York University Animal Care and Use Committee.
Surgery
Rats were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (5 mg/kg), and buprenorphine-HCl (0.02 mg/kg) was given as an analgesic. Cannulae (22 gauge; Plastics One Inc., Roanoke, VA), fitted with 28 gauge internal cannulae that extended 1.5 mm beyond the guides, were surgically implanted bilaterally into the LA [−3.3 mm anterioposterior (AP), 5.4 mm mediolateral (ML), and 7.8 mm dorsoventral (DV) from Bregma] or CE (−2.2 mm AP, 4.0 mm ML, and 8.0 mm DV from Bregma) (26). The guide cannulae were secured to the skull using surgical screws and acrylic dental cement. Twenty-eight gauge dummy cannulae, cut to extend 0.5 mm from the guides, were inserted to prevent clogging.
Apparatus
Context A: The fear conditioning apparatus was constructed of aluminum and Plexiglas walls (Rat Test Cage, Coulbourn Instruments, Allentown, PA) with metal stainless steel rod flooring that was attached to a shock generator (Model H13–15; Coulbourn Instruments). This chamber was enclosed within a sound-isolation cubicle (Model H10-24A; Coulbourn Instruments).
Context B: The testing apparatus was changed by inserting smooth plastic floor covering, and a peppermint scent.
Intra-amygdala infusions
One day before infusion treatments dummy cannulae were removed and injection cannulae inserted to acclimate rats to the injection procedure, and to minimize new tissue damage at the time of infusion. On infusion day, rats received bilateral LA or CE infusions (0.25 μl/side) of either vehicle or CRF (3, 30, or 300 ng/side) at a rate of 0.15 μl/min through infusion cannulae attached to 1.0 μl Hamilton syringes via polyethylene tubing (A-M Systems, Inc.). Cannulae were left in place for an additional 80 sec to allow drug diffusion away from the injection cannula tips, and then the dummy cannulae were replaced. Rats were returned to their home cages and transferred to the colony room.
Fear conditioning (training)
Rats were habituated to the conditioning apparatus for 15 min one day before training. All rats were given a single conditioning trial (CS-US pairing) after a 10 min acclimation period (Context A). The CS was a 30 sec, 5 kHz, 80 dB-SPL sine wave tone that co-terminated with a footshock US (1 sec, 0.7 mA). One min after the fear conditioning trial rats were returned to their home cages and to the colony room.
Measurement of freezing behavior (testing)
Forty-eight hours after training, rats were replaced in a distinct context (Context B), and given 5 CS alone (no US) after 5 min. The inter-trial interval was 210 sec. A micro-video camera was installed for video recording of freezing behavior. Freezing was defined as the lack of any observable movement of the body and the vibrissae, except for movements related to respiration (27).
Drugs
We used CRF (Corticotropin Releasing Factor Human, Rat; Sigma-Aldrich Co.). CRF was dissolved in artificial cerebrospinal fluid in the morning of the infusion day and kept out of the light when not in use.
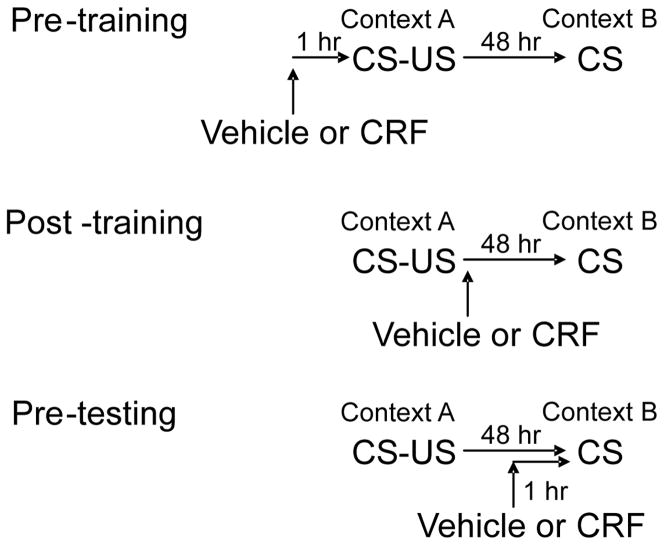
Timing of CRF infusions (Figure 1)
Figure 1.
The timing of CRF infusions. Pre-training The CRF effect infused at pre-training. We infused vehicle or CRF one hour before training (Context A). Two days later, rats were tested for CS-elicited freezing in a distinct context (Context B). Post-training The CRF effect infused at post-training. We infused vehicle or CRF immediately after training (Context A). Two days later, rats were tested for CS-elicited freezing in a distinct context (Context B). Pre-testing The CRF effect infused at pre-testing. Rats received training (Context A). Two days later, we infused vehicle or CRF one hour before testing. Rats were tested for CS-elicited freezing in a distinct context (Context B). CRF: Corticotropin-releasing factor, CS: conditioned stimulus
Pre-training: CRF or vehicle was infused one hour before training.
Post-training: CRF or vehicle was infused immediately (within 5 min) after training.
Pre-testing: CRF or vehicle was infused one hour before testing.
Histology
To verify cannula placements, rats were anesthetized with an overdose of chloral hydrate (25 %, 1.5 ml) and transcardially perfused with 10 % buffered Formalin solution. Brains were removed and stored in 10 % buffered Formalin with 30 % sucrose. Brains were blocked and sectioned on a microtome at 50 μm. Sections were stained by Nissl (0.5 % cresyl violet or 0.25 % thionin), cover-slipped, and examined on a light microscope for cannula tip locations.
Data analysis
For all experiments a two-way analysis of variance (ANOVA) was used to analyze freezing scores with one between-subjects factor (Dose), and one within-subjects factor (CS Trial). For experiments involving multiple doses, follow-up mean comparisons were performed using Dunnett’s test to compare means for the CRF doses against vehicle.
Results
Cannula tip locations
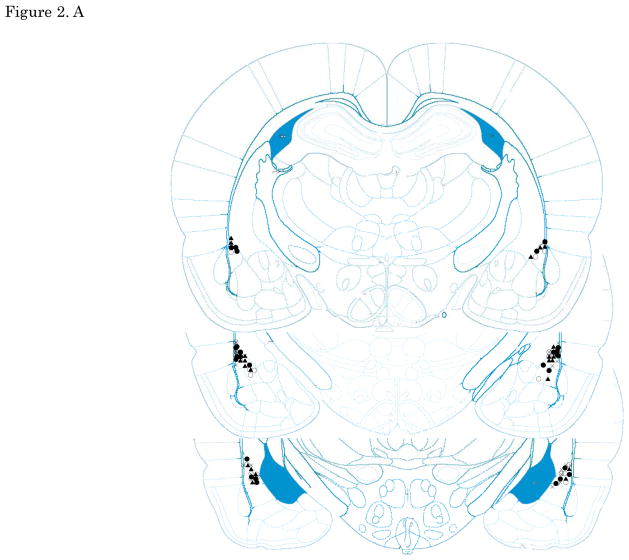
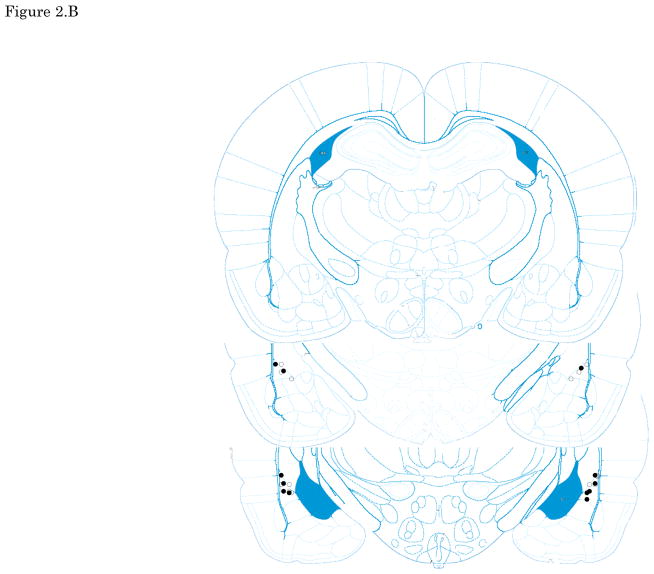
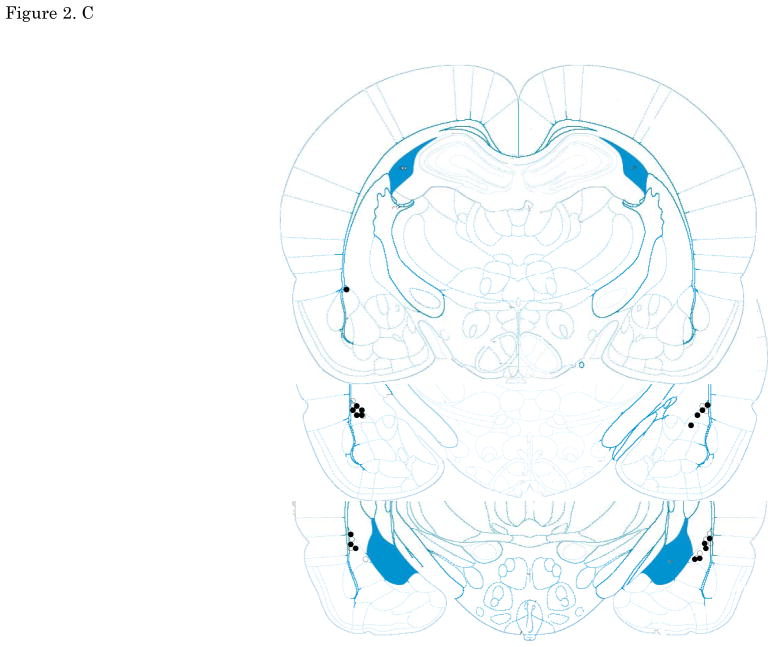
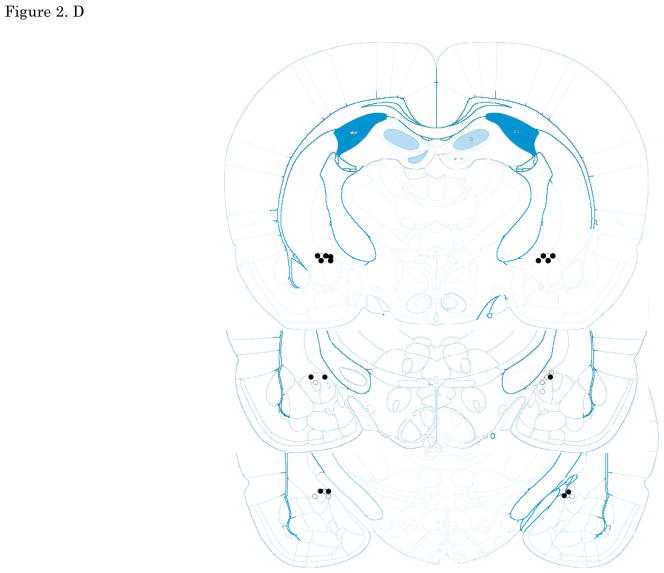
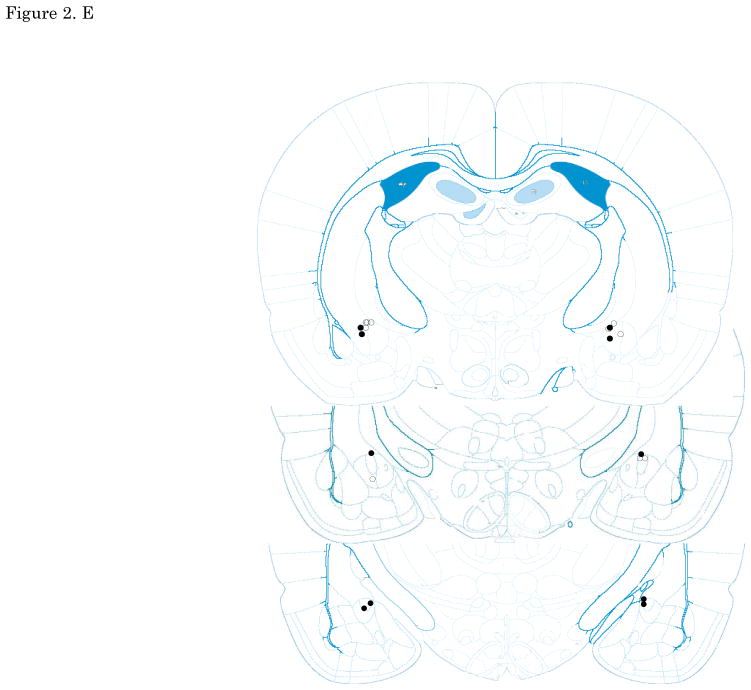
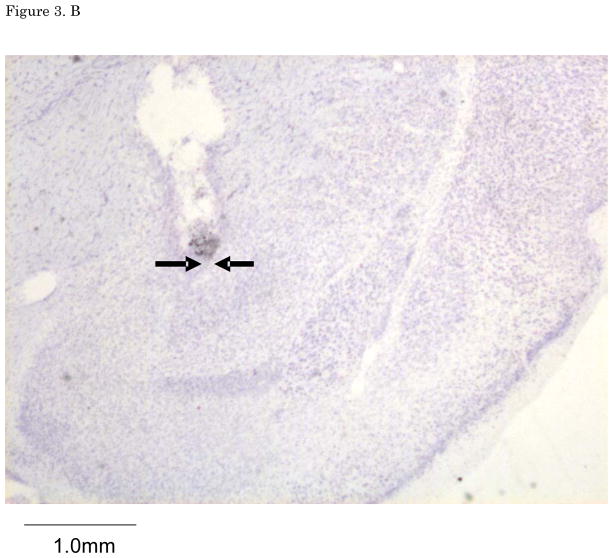
Figures 2. A–E show cannula tip locations. We excluded subjects with cannula placements outside of the LA or CE from further analysis. Figure 3. shows the an example of the injector trace situated within LA (Figure 3A) and CE (Figure 3B).
Figure 2.
The location of injector tips. Vehicle: open circle, CRF 3 ng: cross, CRF 30 ng: closed circle, CRF 300 ng: closed triangle. (A) LA infusion at pre-training. (B) LA infusion at post-training. (C) LA infusion at pre-testing. (D) CE infusion at pre-training. (E) CE infusion at pre-testing. Top to bottom relative to Bregma: (A, B, C)-2.4, -3.0, -3.6, (D, E) -1.8, -2.4, -3.0; adapted from Paxinos and Watson, 2005. CRF: Corticotropin-releasing factor, LA: lateral nucleus of the amygdala, CE: central nucleus of the amygdala
Figure 3.
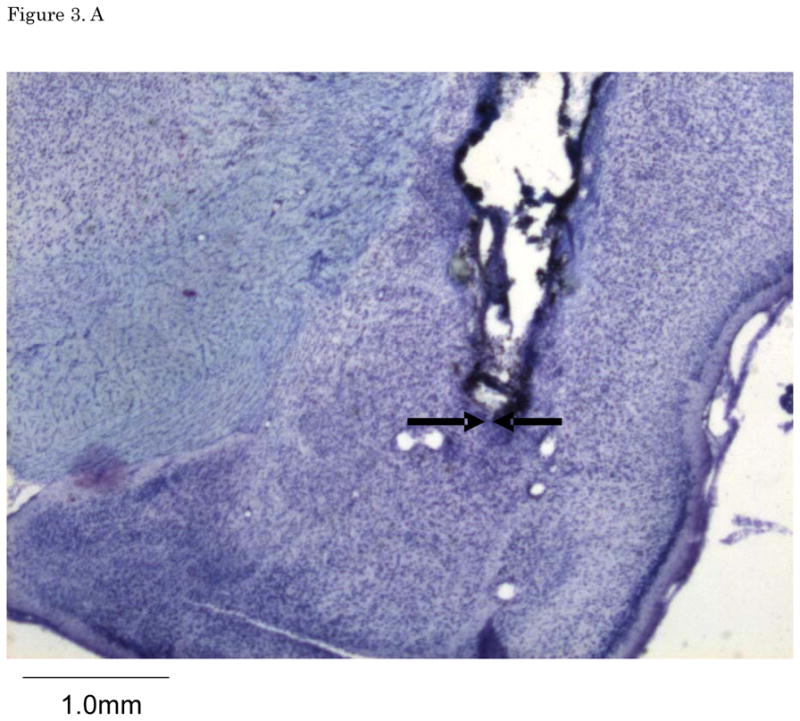
(A) The example of LA infusion injector trace. (B) The example of CE injector trace. LA: lateral nucleus of the amygdala, CE: central nucleus of the amygdala
Effects of Pre-training Infusions in LA
Results from the pre-training LA CRF experiment showed that CRF caused a significant and dose-dependent reduction in CS-elicited freezing during the drug-free long-term memory (LTM) test. The LTM test included 5 trials, and we analyzed only the first 2 trials for all experiments to focus on fear retrieval, apart from within-session extinction across multiple trials.
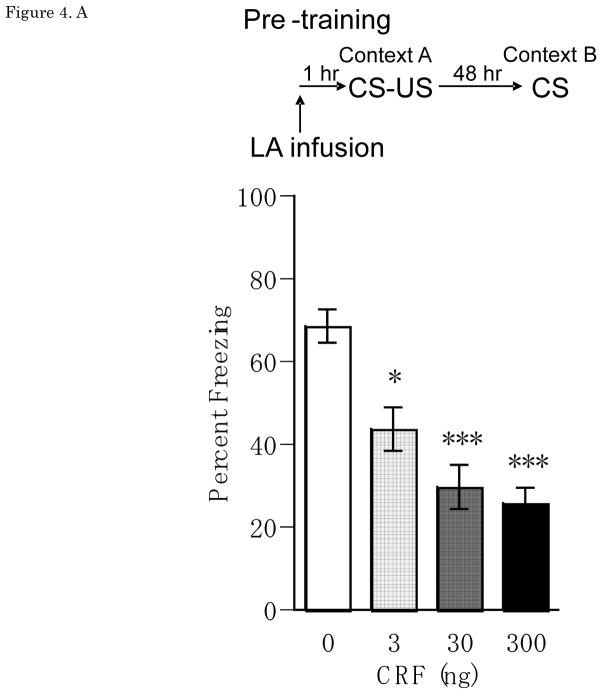
Figure 4A shows mean ± SEM for percentage freezing averaged across the two test trials for groups receiving vehicle or CRF infusion into LA at pre-training. Treatment with CRF (3, 30, or 300 ng/0.25 μl/side) one hour before auditory fear conditioning significantly and dose-dependently reduced CS-elicited freezing when tested 48 hrs later in a distinct context, with a significant main effect of Dose [F(3,34) = 8.03, p<0.001], and significant differences between vehicle and the 3ng (p<0.05), 30ng (p<0.001) and 300 ng (p<0.001) doses. There was no main effect of Trial [F(1,34)=0.19, n.s.], and no Dose × Trial interaction [F(3,34)=0.36, n.s.].
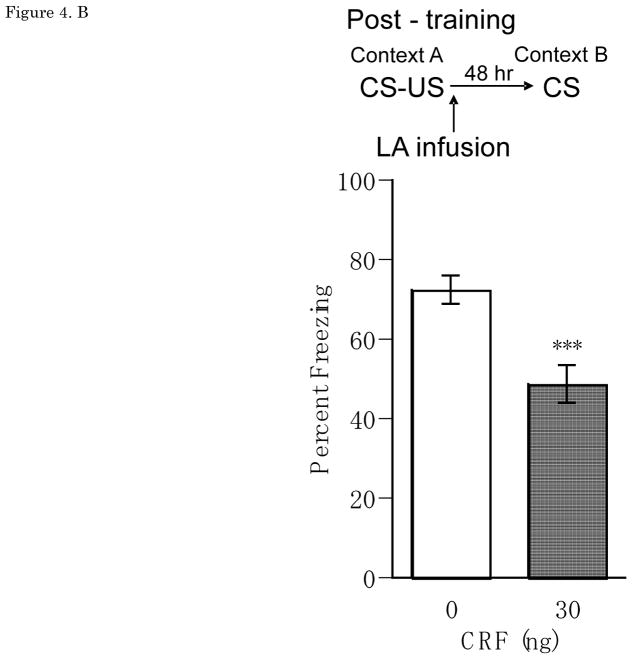
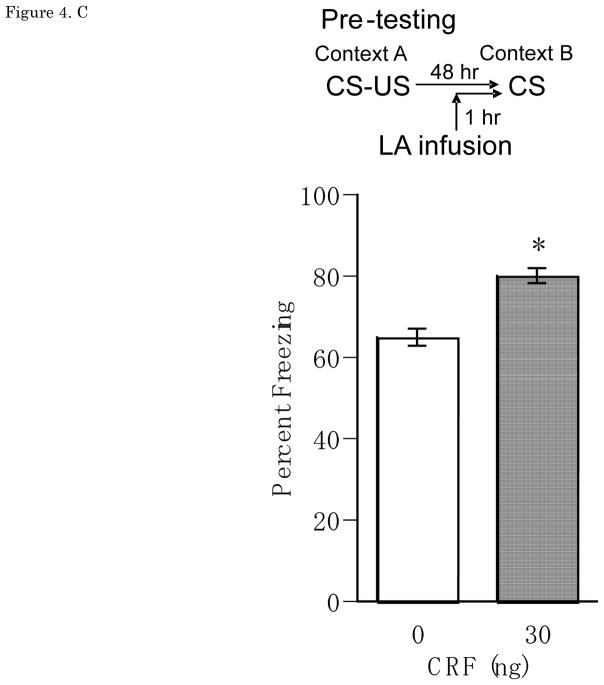
Figure 4.
(A) Pre-training Percent freezing of rats in CS test for vehicle (n=11), 3 ng (n=12), 30 ng (n=9), 300 ng (n=6) of CRF/0.25 μl/side infusions into LA at pre-training. Administrations of CRF (3 ng, 30 ng, 300 ng of CRF/0.25 μl/side) one hour before auditory fear conditioning significantly and dose-dependently reduced CS-elicited freezing in a distinct context. (B) Post-training Percent freezing of rats in CS test for vehicle (n=7) and CRF 30 ng (n=6)/0.25 μl/side infusions into LAat post-training.Administrations of CRF 30 ng/0.25 μl/side immediately after auditory fear conditioning reduced CS-elicited freezing in a distinct context. (C) Pre-testing Percent freezing of rats in CS test for vehicle (n=7) and 30 ng (n=9) of CRF/0.25 μl/side infusions into LA at pre-testing. Administrations of CRF 30 ng/0.25 μl/side one hour before testing increased CS-elicited freezing in a distinct context. *P<0.05, **P<0.01, ***P<0.001. CRF: Corticotropin-releasing factor, CS: conditioned stimulus, LA: lateral nucleus of the amygdala
Effects of Post-training Infusion in LA
Results from the post-training LA CRF experiment showed that CRF caused a significant reduction in CS-elicited freezing during the drug-free LTM test. Figure 4B shows mean ± SEM for percentage freezing averaged across two test CS presentations for groups receiving vehicle or CRF (30ng) infusions into the LA immediately after training. Treatment with CRF 30 ng/0.25μl/side significantly reduced CS-elicited freezing when tested 48 hrs later in a distinct context, with a significant main effect of Dose [F(1,11)=10.61, p<0.01]. There was no main effect of Trial [F(1,11)=0.04, n.s.], and no Dose × Trial interaction [F(1,11)=1.0, n.s.].
Effects of Pre-testing Infusions in LA
Results from the pre-testing LA CRF experiment showed that CRF caused a significant increase in CS-elicited freezing during the test. Figure 4C shows mean ± SEM for percentage freezing averaged across two test CS presentations for groups receiving pre-testing vehicle or CRF (30ng) LA infusions. CRF 30 ng/0.25μl/side, given 1 hr before the LTM test in a distinct context 48hrs after training, significantly increased CS-elicited freezing, with a significant main effect of Dose [F(1,13) = 18.83, p < 0.001]. There was no main effect of Trial [F(1,13)=1.97, n.s.], and no Dose × Trial interaction [F(1,13)=0.15, n.s.].
Effects of Pre-training and Pre-testing Infusions in CE
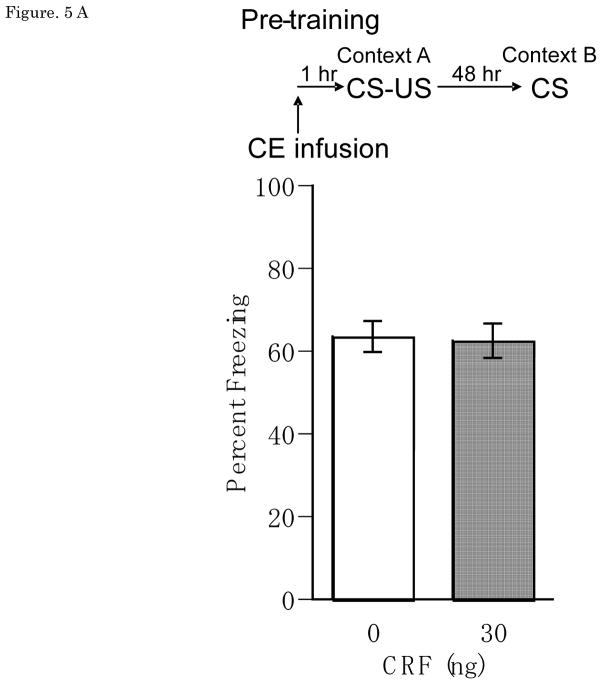
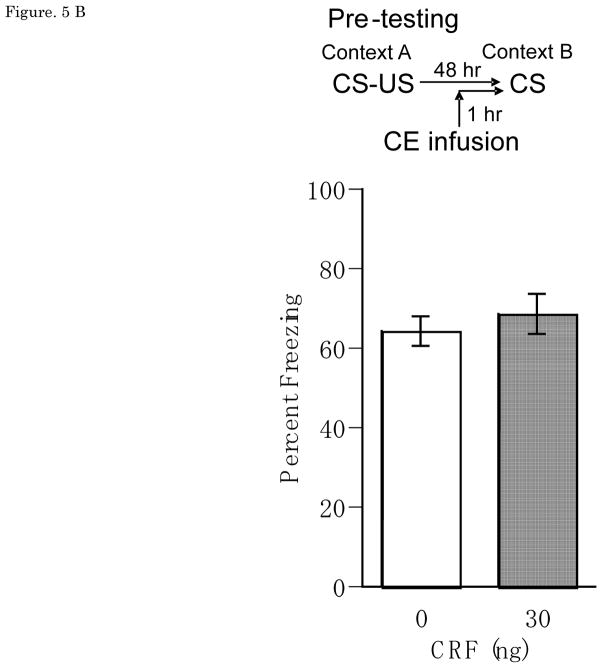
Results from the CE infusion experiments showed that CRF had no effect on CS-elicited freezing from either pre-training or pre-testing manipulations. Figure 5A and 5B show mean ± SEM for percentage freezing averaged across two test tone presentations for groups receiving vehicle or CRF (30ng) pre-training or pre-testing CE infusions, with no pre-training main effect of Dose [F(1,14) = 0.01, n.s.], Trial [F(1,14)=0.66, n.s.] or Dose × Trial interaction [F(1,14)=0.81, n.s.], and no pre-testing main effect of Dose [F(1,9) = 0.23, n.s.], Trial [F(1,9)=0.072, n.s.], or Dose × Trial interaction [F(1,9)=0.014, n.s.]. Note that the effect of post-training CE CRF was not tested because there was no pre-training CE CRF effect, obviating the need to distinguish between effects on acquisition versus consolidation.
Figure 5.
(A) Pre-training Percent freezing of rats in CS test for vehicle (n=8) and 30 ng (n=9) of CRF/0.25 μl/side infusions into CE at pre-training. (B) Pre-testing Percent freezing of rats in CS test for vehicle (n=5) and 30 ng (n=5) of CRF/0.25 μl/side infusions into CE at pre-testing. There was no significant effect on CS-elicited freezing in CRF infusions into CE at either pre-training or pre-testing. CRF: Corticotropin-releasing factor, CS: conditioned stimulus, CE: central nucleus of the amygdala
Discussion
In the present study we found that pre or post-training infusions of CRF into LA impaired long-tem memory for fear conditioning. Since post-training infusions produce the same suppressive effect as pre-training infusions, the findings suggest that memory consolidation rather than acquisition was affected. In contrast, the expression of pre-established fear memories was enhanced by pre-test infusions. These findings provide evidence that CRF exogenously applied in the LA can modulate fear memory formation and expression. Given the well established role of CRF in stress, this suggests that CRF may be a candidate for modulation of fear memory processes by stress.
Impaired fear memory formation by CRF infusions targeted to LA
The finding that pre- and post-training CRF impaired fear memory formation is surprising, considering that previous results from experiments with CRF receptor antagonists resulted in fear memory impairments. Hikichi et al. (23) reported that systemic injection of the CRF receptor antagonist, CP-154,526, 30 min before contextual fear conditioning impaired context-elicit fear memory. Bilateral microinjections of DMP 696, the selective CRF receptor 1 antagonist, into BLA at 5 min or 3 hrs, but not 9 hrs, after exposure to contextual fear conditioning reduced contextual freezing in the conditioned fear test (25). However, in contrast with these results, Tovote et al. (22) reported that CRF overexpressing mice showed low acquisition of memory for auditory fear conditioning. Thus, interference (23,25) and enhancement (22, current study) of CRF function both produce impairments of fear memory consolidation.
One explanation for the similar effects of amygdala CRF receptor blockade and stimulation could be that receptor stimulation (22, current study) overshadow normal patterns of endogenous CRF release that are triggered naturally by the fear conditioning experience. US exposure may trigger endogenous CRF release in the LA as part of a crucial post-conditioning signal that is needed to support Pavlovian associative learning and memory formation. CRF1 receptor stimulation is itself able to trigger unconditioned fearful behaviors (28). Footshock US exposure during fear conditioning leads to CRF release in the amygdala, as measured by immunocytochemical imaging of CRF (29). And immediate post-training infusions into the basal and lateral amygdala of the CRF–binding protein ligand inhibitor CRF 6–33 (0.1μg, but not 1.0μg or 0.01μg), which increases the concentration of free endogenous CRF, induced enhancement of 48 hr retention latencies test for inhibitory avoidance training, which involves both Pavlovian and instrumental conditioning (29). When CRF receptors are blocked as with an antagonist (23,25) the fear memory may not be coded with sufficient intensity.
Conversely, when CRF receptors are stimulated with exogenous CRF unrelated to fear memory consolidation (current study) the signal may not be sufficiently coupled to the learning experience to support appropriate learning. Exogenous CRF treatments could disrupt this endogenous mechanism by interfering with the coupling between the aversive learning experience and subsequent CRF release. In essence, exogenous CRF could be overshadowing the endogenous signal that would normally support fear memory formation.
In stress experiments, Blank et al. (30) reported that one hour of immobilization stress immediately before context-dependent fear conditioning impaired contextual fear memory, and that systemic injection of CRF receptor 1 antagonist CP-154,526 pretreatment 15 min before immobilization stress reversed the stress-induced fear memory impairment in mice. Thus, pre-training stress exposure was found to suppress fear learning via a CRF-dependent mechanism, similar to the effects of CRF observed in the current study.
In summary, the literature suggests that endogenous CRF activation necessary for fear memory consolidation appears to have facilitating effects on emotional learning. In contrast, exogenous LA CRF (current study) or increased endogenous CRF that is not related to fear memory consolidation (30,22) may overshadow discrete emotional learning experiences, with a resulting suppressive effect on memory formation.
Clinical research from Schelling et al. (31) has shown a similar post-training effect. Patients admitted to the hospital for emergency treatment for trauma (e.g. car accidents) and given systemic treatment with glucocorticoids showed lower incidence of post-traumatic stress, suggestive of weaker aversive memory for the traumatic event. It is not known if the current findings reflect a similar mechanism to the Schelling et al study, but interestingly, CRF in the amygdala is increased, even though CRF in the paraventricular nucleus is decreased, by corticosterone treatments (32). If patients receive systemic injection of CRF soon after a traumatic event, it is possible that aversive memory might be weakened.
Facilitated fear memory expression by CRF infusions into LA prior to testing
CRF infusions into LA one hour before auditory fear memory testing, which occurred 48 hours after training, increased the expression of conditioned fear. In other studies, systemic injection of CRF receptor antagonist, CP-154,526, 30 min before testing impaired the expression of contextual fear memory (23). Similarly, the intracerebroventricular (icv) administration of alpha-helical CRF 20 μg/rat 20 min before testing attenuated ultrasonic vocalizations in rats triggered by a fear CS (33). Kumar and Karanth (34) reported that icv CRF (0.05 μg, 0.1 μg and 0.2 μg), given 20 min before testing in previously fear conditioned rats, produced a dose-dependent increase in latencies to enter the previously shocked goal arm in a T-maze, and alpha-helical CRF reversed this effect. Our results on fear memory expression are consistent with these findings, and suggest that CRF may augment aversive emotional responses to pre-potent conditioned fear stimuli. In contrast to other studies though we use infusions targeted to the LA. Because the role of LA in fear conditioning is well understood, this paves the way for more mechanistic studies of CRF contribution to fear and anxiety. Some of the output from the LA will pass through the remainder of the BLA prior to reaching the CE. If the CRF injected into LA spread to other BLA areas, there is a possibility that the expression of fear memory could be enhanced (35). Diffusion to CE is unlikely to be a factor, as described next.
No effect on formation and expression by CRF infusions into CE
CRF infusions into CE one hr before auditory fear training or testing had no effect on aversive emotional memory. Wilensky et al. (36) reported that CE is not only involved in fear expression but, like LA, is also involved in the learning and consolidation of Pavlovian fear conditioning. There are many CRF immunoreactive cells and fibers in CE (19). But CRF receptor 1 in situ hybridization studies indicate that density of these receptors is low in the CE (12,14,20,21,37,38). Combining these findings with those of the current study suggests that the LA and other BLA areas are more important than the CE for CRF modulation of the formation and expression of fear conditioning.
Where dose the endogenous CRF in LA come from?
There are many CRF immunoreactive cells and fibers in CE (19) and CRF release in CE is increased by stress like single (39,40) or repeated episodes of restraint stress (41). However, there is no evidence that axon terminals of neurons located in CE might reach LA (3). Bittencourt and Sawchenko (21) reported that icv CRF spread into the brain and induced Fos expression in several brain regions. Swiergiel et al. (42) reported that pre-testing infusion of a CRF antagonist into CE reduced contextual conditioned freezing, and Pitts et al. (24) showed that fear memory formation was impaired in rats that received CRF antisense into CE after fear conditioning. Interestingly, Roosendaal et al. (43) showed evidence indicating that the CRF in the CE may diffuse into LA after footshock administration. Therefore, the endogenous CRF in LA might come from close proximity to CE by non-synaptic diffusion (i.e. volume neurotransmission).
Clinical Implications
Post-traumatic stress disorder (PTSD) and major depressive disorder (MDD) patients show impaired acquisition of new aversive emotional memories, but strong retrieval of pre-existing aversive emotional memories.
In PTSD patients, CRF in cerebrospinal fluid (CSF) is higher than in control subjects (44), and amygdala hyperactivity was also reported (45). PTSD patients were also found to have deficits in the formation of new memories for fear conditioning. However, when PTSD patients do experience successful fear conditioning, they then show delayed extinction for the new memories (46).
MDD patients frequently and excessively retrieve aversive memories with anxiety and fear implications. MDD patients showed visible HPA axis abnormality (47), CRF in CSF was higher than in control subjects (48), and amygdala hyperactivity was reported (49). Weniger et al. (50) reported that MDD patients were significantly impaired in learning fearful emotional facial expression and larger left amygdala volumes were significantly related to worse memory performance and to higher anxiety score of MDD patients. Thus, similar to our observed effects of LA CRF on the formation and expression of fear conditioning, PTSD and MDD patients show impaired formation, and enhanced expression of aversive memories.
Given the presence of higher CRF levels in the CSF of these patients, there is a possibility that CRF could contribute to these behavioral symptoms of the disorder. In view of our findings of persistent individual differences in fear acquisition and extinction in rats (51), it would be interesting to examine the extent to which endogenous differences in CRF function could contribute to extreme fear phenotypes, towards advancing understanding of the potential role of CRF in the etiology of PTSD, MDD and other disorders involving extreme fear symptoms.
Conclusion
CRF infusion into LA impaired the formation and facilitated the expression of aversive emotional memories. It is interesting that the primary role of CRF is similar to human psychopathological phenomena. CRF modulation of aversive memories in LA could be involved in etiology of emotional symptoms in MDD and PTSD. The detailed understanding of LA and its role in fear conditioning paves the way for mechanistic studies of the contribution of CRF to fear and anxiety.
Acknowledgments
The present study was supported by the National Institute of Mental Health Grant Nos. R37 MH038774, P50 MH058911, R01 MH046516 and K05 MH067048 to Joseph E. LeDoux. We thank Drs. Hiroki Hamanaka and Christopher Cain for their helpful discussion.
Footnotes
All authors reported no biomedical financial interests or potential conflicts of interest.
References
- 1.LeDoux JE. Emotional networks and motor control: a fearful view. Prog Brain Res. 1996;107:437–446. doi: 10.1016/s0079-6123(08)61880-4. [DOI] [PubMed] [Google Scholar]
- 2.Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- 3.Pitkänen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- 4.LeDoux JE. The amygdala. Curr Biol. 2007;17:R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Maren S. Synaptic mechanisms of associative memory in the amygdala. Neuron. 2005;47:783–786. doi: 10.1016/j.neuron.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Sah P, Westbrook RF. Behavioural neuroscience: The circuit of fear. Nature. 2008;454:589–90. doi: 10.1038/454589a. [DOI] [PubMed] [Google Scholar]
- 7.Johansen JP, Hamanaka H, Monfils MH, Behnia R, Deisseroth K, Blair HT, et al. Optical activation of lateral amygdala pyramidal cells instructs associative fear learning. Proc Natl Acad Sci U S A. 2010;107:12692–12697. doi: 10.1073/pnas.1002418107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LeDoux JE. In: Emotion and the Amygdala. The Amygdala. Aggleton J, editor. New York: Academic Press; 1992. pp. 339–351. [Google Scholar]
- 10.Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 11.Koob GF, Heinrichs SC. A role for corticotrophin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848:141–152. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- 12.Sánchez MM, Young LJ, Plotsky PM, Insel TR. Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain. J Comp Neurol. 1999;408:365–377. [PubMed] [Google Scholar]
- 13.Isogawa K, Akiyoshi J, Hikichi T, Yamamoto Y, Tsutsumi T, Nagayama H. Effect of corticotropin releasing factor receptor 1 antagonist on extracellular norepinephrine, dopamine and serotonin in hippocampus and prefrontal cortex of rats in vivo. Neuropeptides. 2000;34:234–239. doi: 10.1054/npep.2000.0806. [DOI] [PubMed] [Google Scholar]
- 14.Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K, et al. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci U S A. 1994;91:8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isogawa K, Akiyoshi J, Tsutsumi T, Kodama K, Horinouti Y, Nagayama H. Anxiogenic-like effect of corticotropin-releasing factor receptor 2 antisense oligonucleotides infused into rat brain. J Psychopharmacol. 2003;17:409–413. doi: 10.1177/0269881103174004. [DOI] [PubMed] [Google Scholar]
- 17.Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- 18.Linthorst AC, Flachskamm C, Hopkins SJ, Hoadley ME, Labeur MS, Holsboer F, et al. Long-term intracerebroventricular infusion of corticotropin-releasing hormone alters neuroendocrine, neurochemical, autonomic, behavioral, and cytokine responses to a systemic inflammatory challenge. J Neurosci. 1997;17:4448–4460. doi: 10.1523/JNEUROSCI.17-11-04448.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- 20.Chan RK, Vale WW, Sawchenko PE. Paradoxical activational effects of a corticotropin-releasing factor-binding protein “ligand inhibitor” in rat brain. Neuroscience. 2000;101:115–129. doi: 10.1016/s0306-4522(00)00322-5. [DOI] [PubMed] [Google Scholar]
- 21.Bittencourt JC, Sawchenko PE. Do centrally administered neuropeptides access cognate receptors?: an analysis in the central corticotropin-releasing factor system. J Neurosci. 2000;20:1142–1156. doi: 10.1523/JNEUROSCI.20-03-01142.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tovote P, Meyer M, Ronnenberg A, Ogren SO, Spiess J, Stiedl O. Heart rate dynamics and behavioral responses during acute emotional challenge in corticotropin-releasing factor receptor 1-deficient and corticotropin-releasing factor-overexpressing mice. Neuroscience. 2005;134:1113–1122. doi: 10.1016/j.neuroscience.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 23.Hikichi T, Akiyoshi J, Yamamoto Y, Tsutsumi T, Isogawa K, Nagayama H. Suppression of conditioned fear by administration of CRF receptor antagonist CP-154,526. Pharmacopsychiatry. 2000;33:189–193. doi: 10.1055/s-2000-7587. [DOI] [PubMed] [Google Scholar]
- 24.Pitts MW, Todorovic C, Blank T, Takahashi LK. The central nucleus of the amygdala and corticotropin-releasing factor: insights into contextual fear memory. J Neurosci. 2009;29:7379–7388. doi: 10.1523/JNEUROSCI.0740-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hubbard DT, Nakashima BR, Lee I, Takahashi LK. Activation of basolateral amygdala corticotropin-releasing factor 1 receptors modulates the consolidation of contextual fear. Neuroscience. 2007;150:818–828. doi: 10.1016/j.neuroscience.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5. San Diego: Academic; 2005. [DOI] [PubMed] [Google Scholar]
- 27.Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- 28.Blank T, Nijholt I, Eckart K, Spiess J. Priming of long-term potentiation in mouse hippocampus by corticotropin-releasing factor and acute stress: implications for hippocampus-dependent learning. J Neurosci. 2002;22:3788–3794. doi: 10.1523/JNEUROSCI.22-09-03788.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roozendaal B, Schelling G, McGaugh JL. Corticotropin-releasing factor in the basolateral amygdala enhances memory consolidation via an interaction with the beta-adrenoceptor-cAMP pathway: dependence on glucocorticoid receptor activation. J Neurosci. 2008;28:6642–6651. doi: 10.1523/JNEUROSCI.1336-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blank T, Nijholt I, Vollstaedt S, Spiess J. The corticotropin-releasing factor receptor 1 antagonist CP-154,526 reverses stress-induced learning deficits in mice. Behav Brain Res. 2003;138:207–213. doi: 10.1016/s0166-4328(02)00244-9. [DOI] [PubMed] [Google Scholar]
- 31.Schelling G, Roozendaal B, De Quervain JF. Can posttraumatic stress disorder be prevented with Glucocorticoids? Ann N Y Acad Sci. 2004;1032:158–66. doi: 10.1196/annals.1314.013. [DOI] [PubMed] [Google Scholar]
- 32.Makino S, Gold PW, Schulkin J. Corticosterone effects on corticotropin-releasing hormone mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Res. 1994;640:105–12. doi: 10.1016/0006-8993(94)91862-7. [DOI] [PubMed] [Google Scholar]
- 33.Kikusui T, Takeuchi Y, Mori Y. Involvement of corticotropin-releasing factor in the retrieval process of fear-conditioned ultrasonic vocalization in rats. Physiol Behav. 2000;71:323–328. doi: 10.1016/s0031-9384(00)00352-8. [DOI] [PubMed] [Google Scholar]
- 34.Kumar KB, Karanth KS. Alpha-helical CRF blocks differential influence of corticotropin releasing factor (CRF) on appetitive and aversive memory retrieval in rats. J Neural Transm. 1996;103:1117–1126. doi: 10.1007/BF01291796. [DOI] [PubMed] [Google Scholar]
- 35.Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci. 2004;24:3471–9. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makino S, Schulkin J, Smith MA, Pacák K, Palkovits M, Gold PW. Regulation of corticotropin-releasing hormone receptor messenger ribonucleic acid in the rat brain and pituitary by glucocorticoids and stress. Endocrinology. 1995;136:4517–4525. doi: 10.1210/endo.136.10.7664672. [DOI] [PubMed] [Google Scholar]
- 38.Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 39.Kalin NH, Takahashi LK, Chen FL. Restraint stress increases corticotropin-releasing hormone mRNA content in the amygdala and paraventricular nucleus. Brain Res. 1994;656:182–186. doi: 10.1016/0006-8993(94)91382-x. [DOI] [PubMed] [Google Scholar]
- 40.Merali Z, McIntosh J, Kent P, Michaud D, Anisman H. Aversive and appetitive events evoke the release of corticotropin-releasing hormone and bombesin-like peptides at the central nucleus of the amygdala. J Neurosci. 1998;18:4758–4766. doi: 10.1523/JNEUROSCI.18-12-04758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hatalski CG, Guirguis C, Baram TZ. Corticotropin releasing factor mRNA expression in the hypothalamic paraventricular nucleus and the central nucleus of the amygdala is modulated by repeated acute stress in the immature rat. J Neuroendocrinol. 1998;10:663–9. doi: 10.1046/j.1365-2826.1998.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swiergiel AH, Takahashi LK, Kalin NH. Attenuation of stress-induced behavior by antagonism of corticotropin-releasing factor receptors in the central amygdala in the rat. Brain Res. 1993;623:229–234. doi: 10.1016/0006-8993(93)91432-r. [DOI] [PubMed] [Google Scholar]
- 43.Roozendaal B, Brunson KL, Holloway BL, McGaugh JL, Baram TZ. Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. Proc Natl Acad Sci U S A. 2002;99:13908–13913. doi: 10.1073/pnas.212504599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, et al. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997;154:624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chung YA, Kim SH, Chung SK, Chae JH, Yang DW, Sohn HS, et al. Alterations in cerebral perfusion in posttraumatic stress disorder patients without re-exposure to accident-related stimuli. Clin Neurophysiol. 2006;117:637–642. doi: 10.1016/j.clinph.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 46.Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behav Res Ther. 2007;45:2019–2033. doi: 10.1016/j.brat.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 47.Isogawa K, Nagayama H, Tsutsumi T, Kiyota A, Akiyoshi J, Hieda K. Simultaneous use of thyrotropin-releasing hormone test and combined dexamethasone/corticotropine-releasing hormone test for severity evaluation and outcome prediction in patients with major depressive disorder. J Psychiatr Res. 2005;39:467–473. doi: 10.1016/j.jpsychires.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 48.Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 49.Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Ann N Y Acad Sci. 2003;985:420–444. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- 50.Weniger G, Lange C, Irle E. Abnormal size of the amygdala predicts impaired emotional memory in major depressive disorder. J Affect Disord. 2006;94:219–229. doi: 10.1016/j.jad.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 51.Bush DE, Sotres-Bayon F, LeDoux JE. Individual differences in fear: isolating fear reactivity and fear recovery phenotypes. J Trauma Stress. 2007;20:413–422. doi: 10.1002/jts.20261. [DOI] [PubMed] [Google Scholar]