INTRODUCTION
Cardiovascular complications are extremely common following stroke and represent a major form of morbidity. These complications may be caused by focal cerebral injury or may be a manifestation of preexisting cardiac disease, which is common. According to international guidelines all patients with acute stroke need an ECG performed at the moment of admission to document any heart abnormalities.
Several studies have documented a high prevalence of ECG changes and arrhythmias in patients with acute ischemic stroke (1-3). Common ECG changes include QT prolongation, T-wave abnormalities, prominent U waves, and ST-segment abnormalities (3,4). Atrial fibrillation has been most frequently described, although it is often unclear whether the atrial fibrillation was the cause of a cardioembolic event or secondary to cerebral infarction (5-7). Ventricular ectopy has also been frequently reported and also atrioventricular (AV) block has been reported and has been attributed to excessive vagal stimulation (6).
Subarachnoid hemorrhage can be accompanied by ECG abnormalities, enzyme elevation mimicking myocardial infarction, regional left ventricular wall motion abnormalities and arrhythmias (8-10). ECG abnormalities include alterations in QRS configuration, QT interval prolongation, T-wave abnormalities, and S-T segment elevation or depression. The mechanism is unexplained but is thought to be sustained sympathetic stimulation, perhaps caused by dysfunction of the insular cortex, which results in reversible structural neurogenic damage to the myocardium, such as contraction bands, focal myocardial necrosis and subendocardial ischaemia (7,11-13).
In patients with subarachnoid hemorrhage, repolarization and ischemic-like ECG changes are mainly direct consequences of the cerebral condition and their absence essentially rules out cardiac abnormalities. In patients with ischemic stroke and intracerebral hemorrhage, these ECG abnormalities (and QT prolongation) most often represent preexisting coronary artery disease. The specificity of ECG changes to diagnose acute myocardial infarction is low in the acute phase of stroke.
Although electrocardiographic (ECG) abnormalities are well known in ischemic stroke and subarachnoid hemorrhage, these changes have only rarely been investigated systematically in patients with intracerebral hemorrhage. The objective of this study is to investigate the types of ECG abnormalities in patients with non-traumatic spontaneous intracerebral hemorrhage and their association with radiological characteristics and in-hospital mortality. ❑
MATERIALS AND METHODS
This is a retrospective analysis of the charts of the patients admitted in our clinic between January 1, 2010 and October 1, 2010 with non-traumatic cerebral hemorrhage. From the charts we analyzed the electrocardiogram made at the moment of admission and the CT scan performed during the hospitalization. The 12 lead electrocardiogram was recorded with a commonly used machine for emergency room which has a software that can calculate heart rate, QTc interval, QRS axis, wave amplitudes and use a base 0,05 Hz filter and 35Hz muscle tremor and 50 Hz interference filter. We searched for brady/tachyarrhythmias, atrial fibrilation, QTc abnormalities, S-T segment and T wave abnormalities according to the Minnessota ECG criteria.
The cerebral CT scan was obtained at the moment of admission and we analyzed the location, size, ventricular effraction and other complications of the hemorrhage.
In this study we only included patients with supratentorial hemorrhage and excluded patients with cerebellar and brainstem hemorrhage. None of the patients had a previous 12 lead ECG recording performed before stroke. After the recording of the 12 lead ECG and cerebral CT scan we looked for patient outcome during the hospitalization; we didn't have the result of the necropsy of the patients who died attached to the charts and in some cases we couldn't establish the cause of death based on their charts. We used the software MedCalc to analyze the data and used Fischer's exact test for statistical analysis. Stepwise logistic regression was used to analyze these relationships with an entry criterion of p=0.1. P value of 0.005 was considered significant. ❑
RESULTS
We included 120 patients with supratentorial intracranial hemorrhage. The majority of these patients had a basal ganglia hemorrhage (70 patients-58%), followed by thalamic hemorrhage (30 patients, 25%) and lobar hemorrhage (20 patients, 17%). The majority of ECG changes appeared in the first two subgroups; with basal ganglia and thalamic hemorrhage 95%-114 patients and with lobar hemorrhage in 5% of cases-6 patients. In the lobar hemorrhage subgroup we observed mainly brady/tachycardia and only two patients with ST abnormalities; one patient with ST elevation and another with nonspecific ST changes. 84 patients had ECG abnormalities that consisted in QT- c prolongation, nonspecific ST abnormalities; ST and T wave abnormalities and brady/tachycardia (Table 1). 60 patients had QTc prolongation, 50 of which had QT-c prolongation associated with ventricular effraction and 10 patients didn't have ventricular effraction. The most common ECG abnormality was QTc prolongation (50%) followed by brady/tachycardia (33.3%) and by ST abnormalities (21.6%). Almost all patients who had ventricular effraction had QTc prolongation and 25 patients with ventricular effraction had multiple ECG changes. We noticed that 10 patients with basal ganglia and thalamic hemorrhage without ventricular effraction (equally distributed between the two subgroups) had QT-c prolongation. Also they had other abnormalities: ST abnormalities 5 patients and brady/tachycardia (10 patients). We didn't perform an analysis of the ECG changes according to the localization in the right or left hemisphere or according to the size- the majority of patients had a hemorrhage of great dimensions, over 4.5/3 cm in axial size; the volumetry of the hemorrhage wasn't available, so we did not look for any correlations.
Table 1.
Number of ECG abnormalities observed in our patients.
| QTc↑ | ↑/↓ ST | Bradi/TahyC | |
|---|---|---|---|
| Number of patients | 60 | 18 | 40 |
Almost all of the patients with ventricular hemorrhage had QTc prolongation-50 patients and 25 patients had multiple ECG changes (Table 2); it seems that ventricular effraction was the major determinant of these changes (OR=25.735, 95% CI=5.471-166.077). A total of 52 patients from this study died within the first week of admission and the majority had ventricular hemorrhage (Table 3). The majority of the patients with QTc prolongation and ventricular hemorrhage died within first week after admission-41 patients and six patients with QTc prolongation without ventricular effraction died within first week. Ten patients with normal QTc and ventricular hemorrhage died within one week of admission and also 16 patients with ST abnormalities and 10 patients with brady/tachycardia. QTc prolongation was associated with higher mortality in the subgroup of patients with ventricular effraction or hemorrhage (OR=13.364, 95% CI=5.136-35.741) and in the subgroup of patients without ventricular effraction or hemorrhage QTc prolongation was associated with higher mortality (OR=6.682, 95% CI=1.344-35.236). QTc prolongation was a factor significantly associated with mortality regardless of the presence of the blood in the ventricles (OR=6.833, 95% CI=1.317-37.903). ST segment abnormalities were associated with higher mortality (OR=15.314, 95% CI=3.094-102.576) but brady/tachycardia was not associated with higher mortality (OR=0,407, 95% CI=0,163-0.995) (Table 5). To see if the associations we observed had a statistical significance we used logistical regression and found that QTc prolongation and ST segment abnormalities was associated with high mortality (Table 4). ❑
Table 2.
ECG abnormalities distributed between the effraction and non effraction groups.
| QTc↑ | QTcN | ↑/↓ ST | Bradi/TahyC | |
|---|---|---|---|---|
| Efraction | 50 | 2 | 13 | 25 |
| Non efraction | 10 | 16 | 5 | 15 |
Table 3.
Distribution of mortality in our patients.
| Death | Survival | ||
|---|---|---|---|
| QTc↑ | Effraction | 41 | 9 |
| Without effraction | 4 | 6 | |
| QTc N | Effraction | 1 | 1 |
| Without effraction | 10 | 49 | |
| ↑/↓ ST | 16 | 2 | |
| Bradi/TahyC | 10 | 30 |
Table 5.
Statistical analysis of factors associated with mortality.
| OR | 95% CI | p value | ||
|---|---|---|---|---|
| ECG abnormalities plus ventricular effraction | 25.735 | 5.471-166.077 | 0.00378 | |
| QTc prolongation | Efraction | 6.682 | 1.344-35.236 | 0.0078 |
| Non effraction | 6.833 | 1.317-77.903 | 0.00932 | |
| ST abnormalities | 15.314 | 3.094-102.576 | 0.00054 | |
| Bradi/tahycardia | 0.407 | 0.163-0.995 | 2.374 |
Table 4.
Logistics reggresion analysis of factors associated with increased mortality.
| p | OR | 95% CI | |
|---|---|---|---|
| N QTc Efraction | 0.4193 | 0.85 | 0.57-1.26 |
| N QTc Non efraction | 0.9391 | 0.67 | 0.67-1.41 |
| N ST | 0.8732 | 0.97 | 0.42-1.07 |
| N bradi/tahycardia | 0.3579 | 0.86 | 0.63-1.18 |
| Age | 0.908 | 1.105 | 0.38-1.35 |
| Gender | 0.1667 | 3.23 | 0.61-17.07 |
| Thalamic + QTc | 0.758 | 1.21 | 0.32-12.03 |
| Basal Ganglia + QTc | 0.832 | 0.87 | 0.45-3.34 |
| Cortical + QTc | 0.23 | 0.56 | 0.41-2.21 |
| QTc + efraction | <0.001 | 1.90 | 1.24-2.95 |
| QTc + nonefraction | <0.001 | 2.78 | 1.33-2.70 |
| ST abnormalities | <0.001 | 2.93 | 1.89-3.95 |
| Bradi/tahyc | 0.2288 | 0.78 | 0.52-1.17 |
DISCUSSION
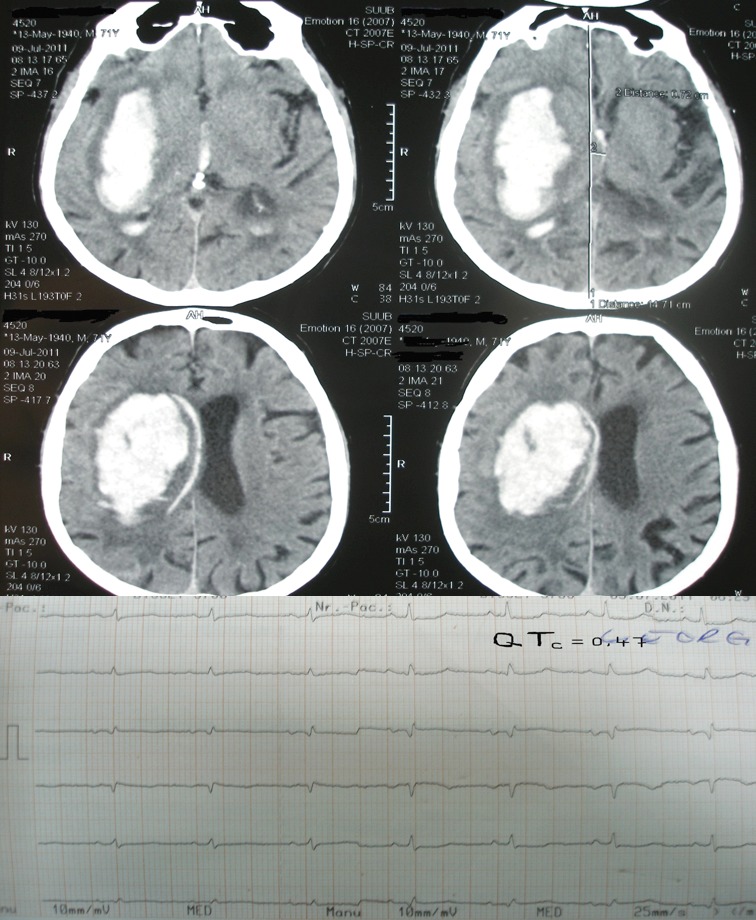
Electrocardiografic changes are common in supratentorial hemorrhagic stroke especially in basal ganglia and thalamic localization. Commonly, ECG changes appear in patients with ventricular effraction; the most frequent ECG changes are: QTc prolongation followed by brady/tachycardia and then ST segment modification. We selected for this paper two pictures of the CT scan and ECG obtained from our patients; first picture shows a lenticular hemorrhage without ventricular effraction and a normal QTc interval and in the second picture a lenticular hemorrhage of greater size with ventricular effraction and a prolonged QTc interval (Figure 1 and 2).
Figure 1. CT scan of a patient with lenticular hemorrhage and the ECG obtained from this patient.
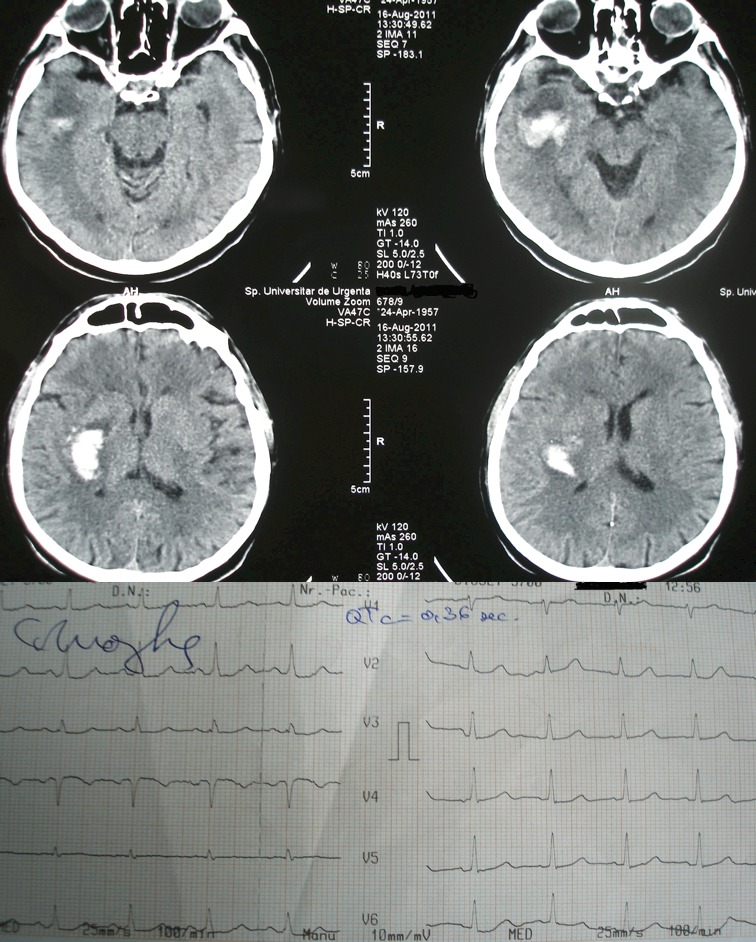
Figure 2. CT scan of a patient with lenticular hemorrhage and ventricular effraction.
We first expected that patients with QTc prolongation and ventricular effraction have a high mortality mainly due to the size of the hemorrhage and complications secondary to the presence of blood in the ventricules. QTc prolongation was also associated with high mortality in patients without ventricular effraction of the hemorrhage. It appears that QTc prolongation associated or not with ventricular effraction and ST segment changes correlate with greater in-hospital mortality.
Previous studies have demonstrated that the most common ECG abnormalities after intracerebral hemorrhage were QT prolongation, ST abnormalities, sinus bradycardia, inverted T wave (11-14). QTc prolongation was associated with hemorrhage involvement of the insular cortex and presence of intraventricular blood and hydrocephalus on admission CT scan. There are also studies that support the hypothesis of a cardiac cortical rhythm control site probably lying within the middle cerebral artery territory or in the anterior cingulate cortex (15,16). Vascular damage to this area could be followed by cardiac arrhythmias related to a disinhibition of the right insular cortex with resulting increased sympathetic tone. Ischemic involvement of the right hemisphere induces a higher risk for cardiac arrhythmia occurrence than that of the left hemisphere. Derangements of autonomic function have been shown to be responsible for these disturbances of rate, rhythm and conduction. The autonomic nervous system receives neural input from various parts of the cerebral cortex, the hypothalamus and the brainstem which is extensively interconnected (17). Tachycardia and pressor responses are more common after stimulation of the right insular cortex and after experimental stimulation of the left vagus which innervates the atrioventricular node and the cardiac conduction system. Bradycardia seems to be more common after stimulation of the left insular cortex or the right vagus nerve which innervates the sinoatrial node or it may be a cause of Cushing effect (17).
In a review of ECG abnormalities after stroke Khechinashvili G and Asplund K demonstrated that ECG changes were present in more than 90% of unselected patients with ischemic stroke and intracerebral hemorrhage, but the prevalence was much lower after exclusion of patients with preexisting heart disease. Compared with other abnormal cardiac findings (cardiac wall motion abnormality detected by echocardiography, elevated levels of biochemical markers of myocardial injury, autopsy findings, thallium scintigraphy), these ECG changes were characterized by a high sensitivity but a very low specificity. In patients with subarachnoid hemorrhage, repolarization and ischemic-like ECG changes are direct consequences of the cerebral condition. In patients with ischemic stroke and intracerebral hemorrhage QT prolongation most often represents preexisting coronary artery disease.
There are some conflicting results weather QT prolongation represent a risk factor for in-hospital mortality after intracerebral hemorrhage. A study by Gölbaşi Z et al demonstrated that QT, QT peak, and QT-QT peak dispersion values were significantly greater in patients with intracerebral hemorrhage than in the control subjects, but QT, QT peak, and QT-QT peak dispersions were not independent risk factors for in-hospital mortality in patients with intracerebral hemorrhage (18). Other studies have clearly demonstrated that QT abnormalities represent an independent risk factor for in-hospital mortality (19). Interestingly QT prolongation was found to be an independent risk factor for stroke besides traditional risk factors (20).
We demonstrated that ECG changes are frequent in hemorrhagic stroke especially in those patients with ventricular effraction and these modifications correlate with greater in-hospital mortality. After logistical regression we found that QTc prolongation with or without ventricular effraction and ST segment abnormalities were the only two factors associated with poor outcome. QTc prolongation and ST abnormalities are the 12 lead ECG abnormalies that correlate with high in-hospital mortality. ECG changes appeared mainly in patients who had ventricular effraction; these changes might be due to sympathetic and parasympathetic imbalance that occurs after the compression of hypothalamus and secondary to compression of the periaqueductal grey matter or it may be secondary to a preexisting coronary artery disease.
It is very important to have an ECG performed at the moment of admission and it might be important to do follow up recordings to see what happens with these ECG changes. Also it is very important to look for QTc prolongation and to select drugs that do not prolong QT interval (certain Antibiotics and Antipsychotics).
This study has limitations due to the retrospective form of data retrieval; we didn't investigate ECG changes according to the site of hemorrhage in the right or left hemisphere. There are also some limitations due to the fact that we didn't have any ECG made prior to the hospitalization of these patients and these changes could exist before the occurrence of the hemorrhage. ❑
CONCLUSION
ECG changes in supratentorial intracerebral hemorrhage are very common and consist in QT-c prolongation, ST segment abnormalities and brady/tachycardia. QT-c prolongation appears with predominance in patients with intraventricular hemorrhage and together with ST segment abnormalities represents a risk factor for in-hospital mortality.
References
- 1.Dimant J, Grob D. Electrocardiographic changes and myocardial damage in patients with acute cerebrovascular accidents. Stroke. 1977;8:448–455. doi: 10.1161/01.str.8.4.448. [DOI] [PubMed] [Google Scholar]
- 2.Kenneth W, Mahaffey D. In Topol J. Textbook of Cardiovascular Medicine. Third eddition. Lippincott Williams & Wilkins; 2007. Cardiac Manifestations of Selected Neurologic Disorders. pp. 627–628. [Google Scholar]
- 3.Khechinashvili G, Asplund K. Electrocardiographic changes in patients with acute stroke: a systematic review. Cerebrovasc Dis. 2002;14:67–67. doi: 10.1159/000064733. [DOI] [PubMed] [Google Scholar]
- 4.Ramani A, Shetty U, Kundaje GN. Electrocardiographic abnormalities in cerebrovascular accidents. Angiology. 1990;41:681–681. doi: 10.1177/000331979004100902. [DOI] [PubMed] [Google Scholar]
- 5.Chua HC, Sen S, et al. Neurogenic ST depression in stroke. Clin Neurol Neurosurg. 1999;101:44–44. doi: 10.1016/s0303-8467(99)00007-4. [DOI] [PubMed] [Google Scholar]
- 6.Oppenheimer SM, Kedem G, Martin WM. Left-insular cortex lesions perturb cardiac autonomic tone in humans. Clin Auton Res. 1996;6:131–131. doi: 10.1007/BF02281899. [DOI] [PubMed] [Google Scholar]
- 7.Laowattana S, Zeger SL, Lima JA, et al. Left insular stroke is associated with adverse cardiac outcome. Neurology. 2006;66:477–477. doi: 10.1212/01.wnl.0000202684.29640.60. [DOI] [PubMed] [Google Scholar]
- 8.Yamour BJ, Sridharan MR, Rice JF, et al. Electrocardiographic changes in cerebrovascular hemorrhage. Am Heart J. 1980;99:294–300. doi: 10.1016/0002-8703(80)90343-9. [DOI] [PubMed] [Google Scholar]
- 9.Caplan L. In Caplan's Stroke A Clinical Approach Fourth Edition by Saunders, an imprint of Elsevier Inc. 2009. Subarachnoid Hemorrhage, Aneurysms, and Vascular Malformations; pp. 459–460. [Google Scholar]
- 10.Hravnak M, Frangiskakis JM, Crago EA, et al. Elevated Cardiac Troponin I and Relationship to Persistence of Electrocardiographic and Echocardiographic Abnormalities After Aneurysmal Subarachnoid Hemorrhage. Stroke. 2009;40:3478–3484. doi: 10.1161/STROKEAHA.109.556753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Bree MD, Roos YB, van der Bilt IA, et al. Prevalence and characterization of ECG abnormalities after intracerebral hemorrhage. Neurocrit Care. 2010;12:50–5. doi: 10.1007/s12028-009-9283-z. [DOI] [PubMed] [Google Scholar]
- 12.Huang CH, Chen WJ, Chang WT, et al. QTc dispersion as a prognostic factor in intracerebral hemorrhage. Am J Emerg Med. 2004;22:141–141. doi: 10.1016/j.ajem.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 13.Yamour BJ, Sridharan MR, Rice JF, et al. Electrocardiographic changes in cerebrovascular hemorrhage. Am Heart J. 1980;99:294–294. doi: 10.1016/0002-8703(80)90343-9. [DOI] [PubMed] [Google Scholar]
- 14.Liu Q, Ding Y, Yan P, et al. Electrocardiographic Abnormalities in Patients with Intracerebral Hemorrhage. Acta Neurochir Suppl. 2011;111:353–615. doi: 10.1007/978-3-7091-0693-8_59. [DOI] [PubMed] [Google Scholar]
- 15.Caravaglios G, Fierro B, Natalè E. Stroke and cardiac arrhythmias. Stroke Cerebrovasc Dis. 2002;11:28–33. doi: 10.1053/jscd.2002.123972. [DOI] [PubMed] [Google Scholar]
- 16.Critchley HD, Mathias CJ, Josephs O, et al. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- 17.Keller C, Williams A. Cardiac dysrhythmias associated with central nervous system dysfunction. J Neurosci Nurs. 1993;25:349–55. doi: 10.1097/01376517-199312000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Gölbasi Z, Selçoki Y, Eraslan T, et al. QT dispersion. Is it an independent risk factor for in-hospital mortality in patients with intracerebral hemorrhage? Jpn Heart J. 1999;40:405–11. doi: 10.1536/jhj.40.405. [DOI] [PubMed] [Google Scholar]
- 19.Pickham D, Helfenbein E, Shinn JA, et al. High prevalence of corrected QT interval prolongation in acutely ill patients is associated with mortality: results of the QT in Practice (QTIP) Study. Crit Care Med. 2012;40:394–9. doi: 10.1097/CCM.0b013e318232db4a. [DOI] [PubMed] [Google Scholar]
- 20.Soliman EZ, Howard G, Cushman M, et al. Prolongation of QTc and risk of stroke: The REGARDS (REasons for Geographic and Racial Differences in Stroke) study. J Am Coll Cardiol. 2012;59:1460–7. doi: 10.1016/j.jacc.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]


