ABSTRACT
Introduction: In this study we evaluate the involvement of Vitamin D Receptor (VDR) FokI (rs10735810) Exon 2 (C/T) and BsmI (rs1544410) Intron 8 (A/G) gene variations in genetic susceptibility to polycystic ovary syndrome (PCOS) in Iranian Azeri Turkish women.
Materials and methods: The RFLP-PCR method was performed on peripheral blood lymphocyte for a total of 46 females with PCOS and 46 controls.
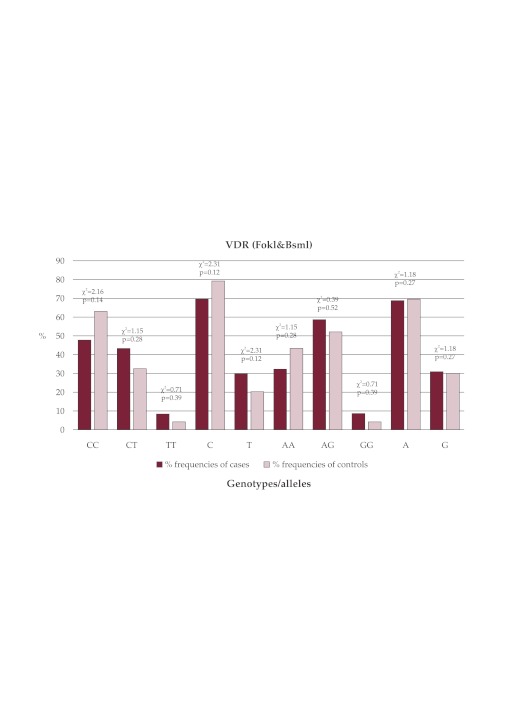
Outcomes: VDR FokI (rs10735810) CC,CT,TT,C and T genotypic/allelic frequencies were 22(47.83), 20(43.48), 4(8.696), 64(69.57) and 28(30.43) in cases and 29(63.04), 15(32.61), 2(4.348), 73(79.35) and 19(20.65) in controls, respectively. The frequencies of VDR FokI C and T alleles were 0.7 and 0.3 in cases, and 0.79 and 0.21 in controls, respectively. VDR BsmI (rs1544410) Intron 8 (A/G) AA,AG,GG,A and G genotypic/allelic frequencies were 15(32.6), 27(58.7), 4(8.7), 57(62), and 35(38) in cases and 20(43.5), 24(52.2), 2(4.35), 64(69.6), and 28(30.4) in controls, respectively. The frequencies of VDR BsmI (rs1544410) Intron 8 A and G alleles were 0.7 and 0.3 in cases, and 0.62 and 0.38 in controls, respectively. Statistical analysis showed that the differences in genotypic/allelic frequencies between the cases and controls were not statistically significant regarding of VDR FokI(rs10735810) Exon 2 (C/T) and VDR BsmI (rs1544410) Intron 8 (A>G) (p >0.05).
Conclusions: It can be concluded that FokI (rs10735810) Exon 2 (C/T) and VDR BsmI (rs1544410) Intron 8 (A>G) were not associated with PCOS susceptibility in studied group. Present investigation is the first study in its own kind in Iranian Azeri Turkish women.
Keywords: Vitamin D receptor, FokI, BsmI, Polycystic Ovary Syndrome
INTRODUCTION
Polycystic Ovary Syndrome (PCOS) is known as one of the most usual heterogeneous hormonal disease among females in the reproductive age (1). The mechanism and molecular etiology of PCOS is still poorly understood (2). Diagnosis of PCOS was carried out based on abnormal findings in medical checkups regarding hyperandrogenic, polycystic ovarian morphology on ultrasound, ovulatory dysfunction (oligo-anovulation), and amenorrhea as well as exclusion of other related disease such as androgen excess (3,4). It has been shown that not only each female with PCOS suffers from wide range of the symptoms and abnormalities but also those are not the same among affected cases (3,4). PCOS resulting in several disorders such as infertility (5), myocardial infarction (6), dysfunctional uterine bleeding (7), cardiovascular risk (7), endometrial carcinoma (7), coronary artery disease (8,9), insulin resistance (IR) (10-12), diabetes mellitus (11-13), hyperandrogenism (hirsutism, acne, male-pattern hair loss), oligo-anovulation, and polycystic ovaries on ultrasound (14), dyslipidemia (15), amenorrhea (16) and hypertension (17) as well as associated with obesity (18) and high levels of cholesterol (19). PCOS incidence became increased ranging from 5-10% and aging 12 to 45 years old in females (20). The study of PCOS aetiopathogenesis has been suggested that several genes as well as environmental factors have been associated with PCOS in different ethnic groups (21-23). Vitamin D Receptor (VDR) locus variations seem to have important impact on pathogenesis and insulin resistance in PCOS women (24). These findings confirms the influence of VDR genetic variations on intestinal calcium absorption (24). Vitamin D and calcium repletion predict reproductive success following fertilization (25). Calcium is one of the main regulators of process including egg activation, oocyte maturation and follicular development and resulting in embryo development (26). Ranjzad et al (2010) reported that there was significant association between VDR BsmI GG genotype and decreased levels of sex hormone binding globulin (SHBG) in PCOS women (27). The results of several investigations imply that SHBG level became decreased in insulin resistance and actually is known as a very good marker for diagnosis of insulin resistance and PCOS (27-30). Presence of the "F" allele and "FF" genotype of the FokI genetic variation are more important than the "f" allele and "Ff and ff" genotypes (31) and have been associated with increased risk for IR in PCOS women (32). Also, Mahmoudi (2009) reported that "bb" genotype (presence of restriction sites for ApaI and BsmI) has been associated with higher levels of insulin and IR in comparison to ''Ff/ff''and "BB and Bb" genotypes (32). Present investigation is the first to study the role of theVDR locus variations (FokI and BsmI) in genetic susceptibility to PCOS in Iranian Azeri Turkish women. ❑
METHODS AND MATERIALS
The research project was approved by the Ethics Committee of Urmia University of Medical Sciences and all subjects signed informed consents. This investigation was performed in Urmia University of Medical Sciences in the city of Urmia, Iran. Between 2011 and 2012, a total number of 92 females with age ranging from 18 to 40 years (46 patients with PCOS and 46 healthy women as normal controls) entered the study. Cases and controls were genetically unrelated and matched for ethnicity, and geografical region. All participants were clinically examed in ART Reproductive Center and Infertility Clinic by ART and infertility specialists. Familial and medical history, physical evaluations, and clinical tests were carried out by the same physicians for all individuals. All diagnosis was based on the finding of three or more of the criteria proposed by the Rotterdam criteria (33) and on the basis of the NICHD criteria (34). Participants with a history of any known cause of oligomenorrhea, amenorrea, hyperandrogenism including non-classic congenital adrenal hyperplasia, hyperprolactinaemia and other confounding factors as well as individuals taking drugs that affect calcium homeostasis were excluded from the study (27). A 3-4 mL of whole blood was collected with EDTA-containing tubes for extraction of DNA by standard "salting out" method (35). Genotypes of VDR BsmI (rs1544410) Intron 8 (G/A) and FokI (rs10735810) Exon 2 (C/T) were determined using RFLP-PCR method. Optimized primer pairs of the VDR gene (Fok-I and BsmI) were used as reported earlier (27). Type of SNPs, site of SNPs, PCR Conditions (primers and programs), un-cut PCR products, restriction enzymes, incubation temperature, and allele size (bp) are summerized in Table 1. PCR reaction was performed in a 20 µl solution including 100 ng of DNA, 1x reaction buffer 10 pmol of each primer, 200 µmol of each dNTPs, 0.5 unit of Taq DNA polymerase, and 1.5 mmol MgCl2. Following the production of PCR products, PCR fragments were digested with restriction enzymes (Fermentas, Stockholm, Sweden). Digested PCR products were analyzed by electrophoresis on 2.5% agarose gel containing ethidium bromide stain, and presence or absence of fragments were monitored by UV transilluminator.VDR BsmI (rs1544410) Intron 8 (G/A) and FokI (rs10735810) Exon 2 (C/T) genotypic and allelic frequencies were counted directly. The chi-square (χ2) test was performed to compare VDR BsmI (rs1544410) Intron 8 (G/A) and FokI (rs10735810) Exon 2 (C/T) genotypic and allelic distributions between patients and healthy control group. The data were analyzed for deviations from Hardy-Weinberg equilibrium (HWE) at each locus. A minimum sample size of 38 individuals in cases group had a statistical power of about 90% (two-tailed, α = 5%). Calculation of χ2 value, the odds ratio (OR), and 95% confidence interval (CI) as well as analysis of independent T-Test for detection of differences between cases and controls regarding clinical characteristics were performed by SPSS ver. 16.0 software and Microsoft Office Excel 2007. A p-value of less than 0.05 was considered statistically significant difference between tested groups. ❑
Table 1.
Type of SNPs, site of SNPs, PCR Conditions (primers and programs), un-cut PCR products, restriction enzymes, incubation temperature, and allele size (bp).
| Gene/SNP(SNP ID) | Location | PCR detailes |
|---|---|---|
| VDR/FokI (rs10735810) | Exon 2 (C/T) | 5'-agctggccctggcactgactctgctct-3' 5'-atggaaacaccttgcttcttctccctc-3' PCR program: x35: 93°C 45 s,66°C30 s, 72°C 45 s un-cut PCR products: 265 bp restriction enzymes,Incubation temperature: FokI, 55°C Alleles: Allele C: 265, Allele T: 169+96 (bp) |
| VDR/BsmI (rs1544410) | Intron 8 (G/A) | 5'-ggcaacctgaagggagacgta-3' 5'-ctctttggacctcatcaccgac-3' PCR program: x35: 93°C 45 s,66°C30 s, 72°C 45 s un-cut PCR products: 461(bp) restriction enzymes,Incubation temperature: BsmI, 37°C Alleles: Allele A: 461, Allele G: 258+203 (bp) |
OUTCOMES
The studied group consisted a total number of 92 females (46 PCOS women (mean±SD age, 26.58±3.33) and 46 healthy women as normal controls (mean±SD age, 28.24±5.25). Statistically significant difference between cases and controls was not found regarding age (p-value >0.05). But in the case of BMI (kg/m2) statistically significant difference between cases and controls was found (p-value <0.05). The prevalence of hirsutism and obesity (BMI >27 kg/m2) in our tested cases were about 100 % and 61.53%, respectively. Our cases (χ2 = 0.03 <3.84, p value with degree of freedom 2 = 0.98 >0.05) and controls (χ2 = 0.001 <3.84, p value with degree of freedom 2 = 0.99 >0.05) were consistent with HWE regarding VDR FokI (rs10735810) Exon 2 (C/T). VDR FokI (rs10735810) CC,CT,TT,C and T genotypic/allelic frequencies were 22(47.83), 20 (43.48), 4(8.696), 64(69.57) and 28(30.43) in cases and 29(63.04), 15(32.61), 2(4.348), 73 (79.35) and 19(20.65) in controls, respectively. The frequencies of VDR FokI C and T alleles were 0.7 and 0.3 in cases, and 0.79 and 0.21 in controls, respectively. In the case of VDR BsmI (rs1544410) Intron 8 (A/G),our cases (χ2 = 2.76 <3.84, p value with degree of freedom 2 = 0.25 >0.05) and controls (χ2 = 2.47 <3.84, p value with degree of freedom 2 = 0.28 >0.05) were consistent with HWE.VDR BsmI (rs1544410) Intron 8 (A/G) AA,AG,GG,A and G genotypic/allelic frequencies were 15(32.6), 27(58.7), 4(8.7), 57(62), and 35(38) in cases and 20(43.5), 24(52.2), 2(4.35), 64 (69.6), and 28(30.4) in controls, respectively. The frequencies of VDR BsmI (rs1544410) Intron 8 A and G alleles were 0.7 and 0.3 in cases, and 0.62 and 0.38 in controls, respectively. Statistical analysis showed that the differences in genotypic/allelic frequencies between the cases and controls were not statistically significant regarding VDR FokI(rs10735810) Exon 2 (C/T) and VDR BsmI (rs1544410) Intron 8 (A>G) (p >0.05) (see Table 2 and Figure 1). ❑
Table 2.
Genotype and allele frequencies of FokI (rs10735810) Exon 2 (C/T) and VDR BsmI (rs1544410) Intron 8 in tested groups.
| VDR | Genotype/Allele | Cases F(F%) | Controls F(F%) | OR(95% CI) | χ2 | P-Value |
|---|---|---|---|---|---|---|
| FokI (rs10735810) Exon 2 (C/T) | CC TC TT C T |
22(47.83) 20(43.48) 4(8.696) 64(69.57) 28(30.43) |
29(63.04) 15(32.61) 2(4.348) 73(79.35) 19(20.65) |
0.54(0.23-1.24) 1.59(0.68-3.71) 2.1(0.36-12) 0.59(0.3-1.17) 1.68(0.86-3.29) |
2.16 1.15 0.71 2.31 2.31 |
0.142 0.283 0.398 0.128 0.128 |
| BsmI (rs1544410) Intron 8 (G/A) | AA AG GG A G |
15(32.6) 27(58.7) 4(8.7) 57(62) 35(38) |
20(43.5) 24(52.2) 2(4.35) 64(69.6) 28(30.4) |
0.629(0.269-1.469) 1.303(0.571-2.97) 2.095(0.364-12.05) 0.713(0.386-1.314) 1.404(0.761-2.588) |
1.153 0.396 0.713 1.183 1.183 |
0.283 0.529 0.398 0.277 0.277 |
Figure 1. FokI (rs10735810): CC,CT,TT,C, and T; BsmI (rs1544410): AA, AG, GG, A, and G.
DISCUSSION
Results of several investigations formed our understanding of VDR genotypes and intestinal calcium absorption in postmenopausal women as well as women's reproductive health (24,25). The aim of present study was to assess the role of VDR FokI (rs10735810) (C/T) and BsmI (rs1544410) (A/G) genetic variation in PCOS for the first time in Iranian Azeri women. PCOS is defined as a syndrome that greatly influences ovarian functions. Research studies indicated that the reason of the ovarian overproduction of testosterone in PCOS women is due by inability of women to mediate insulin effectively (IR or Hyperinsulinemia) (32,36). Hyperandrogenemia and IR are important indicators of PCOS (29). Higher insulin levels and IR is more frequent in PCOS women with F allele and FF genotype of the VDR FokI considering biochemical markers related to PCOS (32). In this condition, level of insulin hormon whithin the blood is too high, therefore the ovaries produce higher level of testosterone (36). SHBG is a carrier protein which regulates the level of unbound steroids in peripheral blood (28,29). It has been demonstrated that VDR genetic variations have been associated with LH and SHBG levels in PCOS women (27). It has been demonstrated that SHBG expression is reduced in the stromal compartment of endometria of women with polycystic ovary syndrome (30). Increasing of androgens bioavailability result in hyperandrogenemia by hyperinsulinemia in PCOS women with VDR BsmI GG genotypes via lower serum level of SHBG (27). In our study, statistical analysis showed that the differences in genotypic/allelic frequencies between the cases and controls were not statistically significant regarding VDR FokI(rs10735810) Exon 2 (C/T) and VDR BsmI (rs1544410) Intron 8 (A>G) (p >0.05). The exact etiopathogenesis of PCOS are not known regarding vitamin D and IR. Several molecular mechanisms have been suggested to describe the relationship between the VDR locus variations and PCOS in different ethic groups. We had some limitations regarding low sample size, registry data of participants because of poor quality of registration systems. Studies with a large sample size and more information such as other candidate gene variants, haplotypes and genetic linkage assessment are needed for further analysis (37-39). ❑
CONCLUSION
It can be concluded that FokI (rs10735810) Exon 2 (C/T) and VDR BsmI (rs1544410) Intron 8 (A>G) were not associated with PCOS susceptibility in studied group. Present investigation is the first study in its own kind in Iranian Azeri Turkish women.
References
- 1.Carmina E, Lobo RA. Polycystic ovary syndrome (PCOS): arguably the most common endocrinopathy is associated with significant morbidity in women. J Clin Endocrinol Metab. 1999;84:1897–9. doi: 10.1210/jcem.84.6.5803. [DOI] [PubMed] [Google Scholar]
- 2.Barry JA, Kay AR, Navaratnarajah R, et al. Umbilical vein testosterone in female infants born to mothers with polycystic ovary syndrome is elevated to male levels. J Obstet Gynaecol. 2010;30:444–6. doi: 10.3109/01443615.2010.485254. [DOI] [PubMed] [Google Scholar]
- 3.Pasquali R, Gambineri A, Cavazza C, et al. Heterogeneity in the responsiveness to long-term lifestyle intervention and predictability in obese women with polycystic ovary syndrome. Eur J Endocrinol. 2011;164:53–60. doi: 10.1530/EJE-10-0692. [DOI] [PubMed] [Google Scholar]
- 4.Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456–88. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 5.Simoni M, Tempfer CB, Destenaves B, et al. Functional genetic polymorphisms and female reproductive disorders. Part I: polycystic ovary syndrome and ovarian response. Hum Reprod Update. 2008;14:459–484. doi: 10.1093/humupd/dmn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahlgren E, Janson PO, Johansson S, et al. Polycystic ovary syndrome and risk for myocardial infarction. Evaluated from a risk factor model based on a prospective population study of women. Acta Obstet Gynecol Scand. 1992;71:599–604. doi: 10.3109/00016349209006227. [DOI] [PubMed] [Google Scholar]
- 7.Costello MF. Polycystic ovary syndrome – a management update. Aust Fam Physician. 2005;34:127–33. [PubMed] [Google Scholar]
- 8.Glueck CJ, Morrison JA, Goldenberg N, et al. Coronary heart disease risk factors in adult premenopausal white women with polycystic ovary syndrome compared with a healthy female population. Metabolism. 2009;58:714–21. doi: 10.1016/j.metabol.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 9.de Groot PC, Dekkers OM, Romijn JA, et al. PCOS, coronary heart disease, stroke and the influence of obesity: a systematic review and meta-analysis. Hum Reprod Update. 2011;17:495–500. doi: 10.1093/humupd/dmr001. [DOI] [PubMed] [Google Scholar]
- 10.Jones H, Sprung VS, Pugh CJ, et al. Polycystic Ovary Syndrome with Hyperandrogenism Is Characterized by an Increased Risk of Hepatic Steatosis, Compared to Nonhyperandrogenic PCOS Phenotypes and Healthy Controls, Independent of Obesity and Insulin Resistance. J Clin Endocrinol Metab. 2012 Jul 26; doi: 10.1210/jc.2012-1382. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Ovalle F, Azziz R. Insulin resistance, polycystic ovary syndrome, and type 2 diabetes mellitus. Fertil Steril. 2002;77:1095–105. doi: 10.1016/s0015-0282(02)03111-4. [DOI] [PubMed] [Google Scholar]
- 12.Sir-Petermann T, Angel B, Maliqueo M, et al. Prevalence of Type II diabetes mellitus and insulin resistance in parents of women with polycystic ovary syndrome. Diabetologia. 2002;45:959–64. doi: 10.1007/s00125-002-0836-3. [DOI] [PubMed] [Google Scholar]
- 13.Pelusi B, Gambineri A, Pasquali R. Type 2 diabetes and the polycystic ovary syndrome. Minerva Ginecol. 2004;56:41–51. [PubMed] [Google Scholar]
- 14.Setji TL, Brown AJ. Comprehensive clinical management of polycystic ovary syndrome. Minerva Med. 2007;98:175–89. [PubMed] [Google Scholar]
- 15.Wild RA. Dyslipidemia in PCOS. Steroids. 2012;77:295–9. doi: 10.1016/j.steroids.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Rachmiel M, Kives S, Atenafu E, et al. Primary amenorrhea as a manifestation of polycystic ovarian syndrome in adolescents: a unique subgroup? Arch Pediatr Adolesc Med. 2008;162:521–5. doi: 10.1001/archpedi.162.6.521. [DOI] [PubMed] [Google Scholar]
- 17.Ben Salem Hachmi L, Ben Salem Hachmi S, Bouzid C, et al. [Hypertension in polycystic ovary syndrome]. Arch Mal Coeur Vaiss. 2006;99:687–90. [PubMed] [Google Scholar]
- 18.Gambineri A, Pelusi C, Vicennati V, et al. Obesity and the polycystic ovary syndrome. Int J Obes Relat Metab Disord. 2002;26:883–96. doi: 10.1038/sj.ijo.0801994. [DOI] [PubMed] [Google Scholar]
- 19.Nidhi R, Padmalatha V, Nagarathna R, et al. Effect of a yoga program on glucose metabolism and blood lipid levels in adolescent girls with polycystic ovary syndrome. Int J Gynaecol Obstet. 2012;118:37–41. doi: 10.1016/j.ijgo.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 20.Azziz R, Woods KS, Reyna R, et al. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–9. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 21.Prapas N, Karkanaki A, Prapas I, et al. Genetics of polycystic ovary syndrome. Hippokratia. 2009;13:216–23. [PMC free article] [PubMed] [Google Scholar]
- 22.Fratantonio E, Vicari E, Pafumi C, et al. Genetics of polycystic ovarian syndrome. Reprod Biomed Online. 2005;10:713–20. doi: 10.1016/s1472-6483(10)61114-5. [DOI] [PubMed] [Google Scholar]
- 23.Diamanti Kandarakis E, Kandarakis H, Legro R. The role of genes and environment in the etiology of PCOS. Endocrine. 2006;30:19–26. doi: 10.1385/ENDO:30:1:19. [DOI] [PubMed] [Google Scholar]
- 24.Gennari L, Becherini L, Masi L, et al. Vitamin D receptor genotypes and intestinal calcium absorption in postmenopausal women. J Clin Endocrinol Metab. 1997;82:1772–5. [Google Scholar]
- 25.Grundmann M, von Versen-Höynck F. Vitamin D - roles in women's reproductive health? Reprod Biol Endocrinol. 2011;9:146–146. doi: 10.1186/1477-7827-9-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang SL, Zhao QJ, Li XC, et al. Dynamic analysis of Ca2+ level during bovine oocytes maturation and early embryonic development. J Vet Sci. 2011;12:133–42. doi: 10.4142/jvs.2011.12.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ranjzad F, Mahban A, Shemirani AI, et al. Influence of gene variants related to calcium homeostasis on biochemical parameters of women with polycystic ovary syndrome. J Assist Reprod Genet. 2011;28:225–32. doi: 10.1007/s10815-010-9506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golbahar J, Al-Ayadhi M, Das NM, et al. Sensitive and specific markers for insulin resistance, hyperandrogenemia, and inappropriate gonadotrophin secretion in women with polycystic ovary syndrome: a case-control study from Bahrain. Int J Womens Health. 2012;4:201–6. doi: 10.2147/IJWH.S30661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajkhowa M, Bicknell J, Jones M, et al. Insulin sensitivity in women with polycystic ovary syndrome: relationship to hyperandrogenemia. Fertil Steril. 1994;61:605–12. doi: 10.1016/s0015-0282(16)56633-3. [DOI] [PubMed] [Google Scholar]
- 30.Maliqueo M, Bacallao K, Quezada S, et al. Sex hormone-binding globulin expression in the endometria of women with polycystic ovary syndrome. Fertil Steril. 2007;87:321–8. doi: 10.1016/j.fertnstert.2006.06.038. [DOI] [PubMed] [Google Scholar]
- 31.Whitfield GK, Remus LS, Jurutka PW, et al. Functionally relevant polymorphisms in the human nuclear vitamin D receptor gene. Mol Cell Endocrinol. 2001;177:145–59. doi: 10.1016/s0303-7207(01)00406-3. [DOI] [PubMed] [Google Scholar]
- 32.Mahmoudi T. Genetic variation in the vitamin D receptor and polycystic ovary syndrome risk. Fertil Steril. 2009;92:1381–3. doi: 10.1016/j.fertnstert.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41–7. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 34.Zawadski JK, Dunaif A. In: Dunaif A, Givens JR,Haseltine FP, Merriam GE, Hershman SM, editors. Polycystic ovary syndrome. Current issues in endocrinology and metabolism. Blackwell; Boston: 1992. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. pp. 377–84. [Google Scholar]
- 35.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wehr E, Trummer O, Giuliani A, et al. Vitamin D-associated polymorphisms are related to insulin resistance and vitamin D deficiency in polycystic ovary syndrome. Eur J Endocrinol. 2011;164:741–9. doi: 10.1530/EJE-11-0134. [DOI] [PubMed] [Google Scholar]
- 37.Tabor HK, Risch NJ, Myers RM. Candidate-gene approaches for studying complex genetic traits: practical considerations. Nat Rev Genet. 2002;3:391–7–391–7. doi: 10.1038/nrg796. [DOI] [PubMed] [Google Scholar]
- 38.Cardon LR, Palmer LJ. Population stratification and spurious allelic association. Lancet. 2003;361:598–604. doi: 10.1016/S0140-6736(03)12520-2. [DOI] [PubMed] [Google Scholar]
- 39.Colhoun HM, McKeigue PM, Davey Smith G. Problems of reporting genetic associations with complex outcomes. Lancet. 2003;361:865–72. doi: 10.1016/s0140-6736(03)12715-8. [DOI] [PubMed] [Google Scholar]