ABSTRACT
Introduction: The development of hysteroscopy has provided a minimally invasive approach to common gynecologic problems, such as abnormal uterine bleeding. Diagnostic hysteroscopy is considered now "the gold standard" by the Association of Professors of Gynecology and Obstetrics (2002) in investigation of abnormal uterine bleeding (AUB) in order to rule out organic endouterine causes of AUB. Although the World Health Organization (WHO) recommends hysterosapingography (HSG) alone for management of infertile women many specialists use hysteroscopy as a first-line routine exam for infertility patients regardless of guidelines.
Material and method: This paper is a retrospective study of 1545 diagnostic hysteroscopies performed in the "Prof. Dr. Panait Sirbu" Obstetrics and Gynecology Hospital between January 1, 2008 and June 30, 2011. The following parameters were studied: diagnostic hysteroscopy indications, type of anesthesia used, correlation between pre-and postoperative diagnoses.
Outcomes: Of 1545 diagnostic hysteroscopies, 78% of cases were performed without anesthesia; of the total of 299 cases of primary infertility diagnostic hysteroscopy showed in 34% of cases tubal obstruction and endouterine pathology; of the total 396 cases of secondary infertility under investigation, diagnostic hysteroscopy showed in 40% of cases tubal obstruction and endouterine pathology; the highest accuracy of HSG was noted for uterine malformation and minimal accuracy was observed for intrauterine adhesions.
Conclusions: Our experience supports the opinion that diagnostic hysteroscopy should be a first-line routine exam in infertility.
Because of the high rate of false positive results for HSG in our study and considering the other studies in specialty literature, we always perform a diagnostic hysteroscopy before Assisted Human Reproduction procedures regardless of the HSG aspect.
Keywords: diagnostic hysteroscopy, abnormal uterine bleeding, infertility
INTRODUCTION
Hysteroscopy is performed for evaluation or treatment of different pathologies of the endometrial cavity, tubal ostia, or endocervical canal for diagnostic alone or for diagnostic and treatment in the same operative time (1,2).
A review published on Up To Date in 2011 synthesizes the indications of diagnostic and operative hysteroscopy as follows: abnormal premenopausal or postmenopausal uterine bleeding, endometrial polyps, submucosal, and some intramural, fibroids, intrauterine adhesions, Müllerian anomalies (eg, uterine septum), retained intrauterine devices (retained IUD ) or other foreign bodies, retained products of conception, desire for sterilization, endocervical polips (3).
Contraindications to hysteroscopy are: viable intrauterine pregnancy, active pelvic infection (including genital herpes infection) (4), known cervical or uterine cancer.
The possibility to perform hysteroscopy using no anesthetic or local anesthesia allows use of outpatient settings and speeds recovery. The vaginoscopic, or "no touch," technique is performed without a speculum or tenaculum and without anesthesia (5). Bettocchi introduced the 'no-touch' trans-vaginal approach, where no instruments expose or grasp the cervix (6,7). Most diagnostic and brief or minor operative procedures can be performed without anesthetic or with a local anesthetic. Regional or general anesthesia is reserved for patients who cannot tolerate a procedure under local anesthesia, extensive operative procedures, or patients with comorbidities that necessitate intensive monitoring (7).
One factor in deciding whether to use a para-cervical block versus no anesthetic is the pain of the injection; some women find the injection of the anesthetic agent more painful than the procedure itself (8,9). Some surgeons advocate using no anesthetic (10,11). In our study, about 20% of the diagnostic hysteroscopies were performed using the "no-touch" technique with very good tolerance. This technique is used by a couple of surgeons who were trained by professor Bettocchi at "Prof. Dr. Panait Sirbu" Obstetrics and Gynecology Hospital in 2005. ❑
MATERIAL AND METHOD
This paper is a retrospective study of 1545 diagnostic hysteroscopies performed in the "Prof. Dr. Panait Sirbu" Obstetrics and Gynecology Hospital between January 1, 2008 and June 30, 2011. Total number of hysteroscopies performed in this period was 3220. Those patients who underwent hysteroscopy for pathology suspected via another imagistic method were initially investigated using trans-vaginal ultrasonography or hysterosapingography (HSG).
Before hysteroscopy, the standard investigations were represented by: PAP smear, vaginal bacteriologic tests, hemograms.
Antibiotics are not routinely administered during hysteroscopy for prevention of surgical site infection or endocarditis since post hysteroscopy infection occurs in less than 1 percent of women (12).
The following parameters were studied: diagnostic hysteroscopy indications, type of anesthesia used, correlation between pre-and postoperative diagnoses. ❑
OUTCOMES
During the period 1st January 2008 till 30 June 2011, in the "Prof.dr.Panait Sîrbu" Clinical Hospital of Obstetrics Gynecology, in Bucharest, there was a total number of 3220 hysteroscopies. The distribution of pathologies is expressed in Table 1. Of these 3220 hysteroscopies, a number of 1545 were diagnostic hysteroscopies.
Table 1.
The distribution of pathologies in 3220 hysteroscopies.
| Intervention | Number | % |
|---|---|---|
| Diagnostic hysteroscopies | 1545 | 49 |
| Hysteroscopic adhesiolis of uterine synechiae and cervico-istmic synechiae | 653 | 20 |
| Hysteroscopic polipectomy | 436 | 14 |
| Hysteroscopic myomectomy | 206 | 6 |
| Hysteroscopic metroplasty | 123 | 4 |
| Hysteroscopic endometrial biopsy, | 92 | 3 |
| Others: hysteroscopic endometrial ablation, foreign bodies extraction, hysteroscopic tubar cannulation | 165 | 5 |
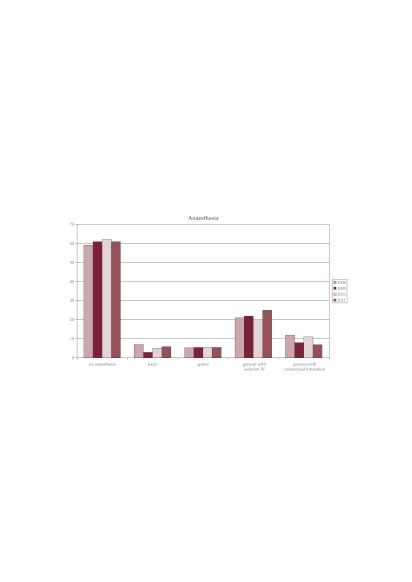
Anesthesia. The type of anesthesia used for the 3220 hysteroscopy shows the prevalence of interventions performed without any type of anesthesia (61%). General anesthesia with orotracheal intubation was used mainly for combined interventions (laparoscopic and hysteroscopic) or hysteroscopic major surgeries like myomectomy or metroplasty with a descending trend between 2008 and 2011 from 12% to 7%. We observe a slight ascending trend for general anesthesia with sedation between 2008 and 2011 from 21% to 25% (Figure 1).
Figure 1. The type of anesthesia used for hysteroscopy.
Diagnostic hysteroscopies were performed without anesthesia in 78% of cases.
Diagnostic hysteroscopies performed during this period had different indications (Table 2).
Table 2.
Indications for diagnostic hysteroscopies.
| Indication | Number | % |
|---|---|---|
| Primary or secondary infertility | 695 | 45 |
| Pathology suspected by HSG or TVS | 309 | 20 |
| Chronic endometritis | 93 | 6 |
| Abnormal uterine bleeding | 139 | 9 |
| Uterine malformations | 62 | 4 |
| Postoperative control | 139 | 9 |
| IVF | 77 | 5 |
| Others | 31 | 2 |
HSG - histerosalpingography; TVS - transvaginal sonography; IVF - in vitro fertilization.
1. Primary and secondary infertility
Primary and secondary infertility cases included a total number of 695 patients, of which 299 (43%) were of primary infertility and 396 (57%) of secondary infertility.
In the "Prof. Dr. Panait Sîrbu" Clinical Hospital of Obstetrics Gynecology in the Department of Assisted Human Reproduction, diagnostic hysteroscopy is used as a first line diagnostic method for the patients with infertility in order to detect endocervical, uterine and proximal tubal factor of infertility.
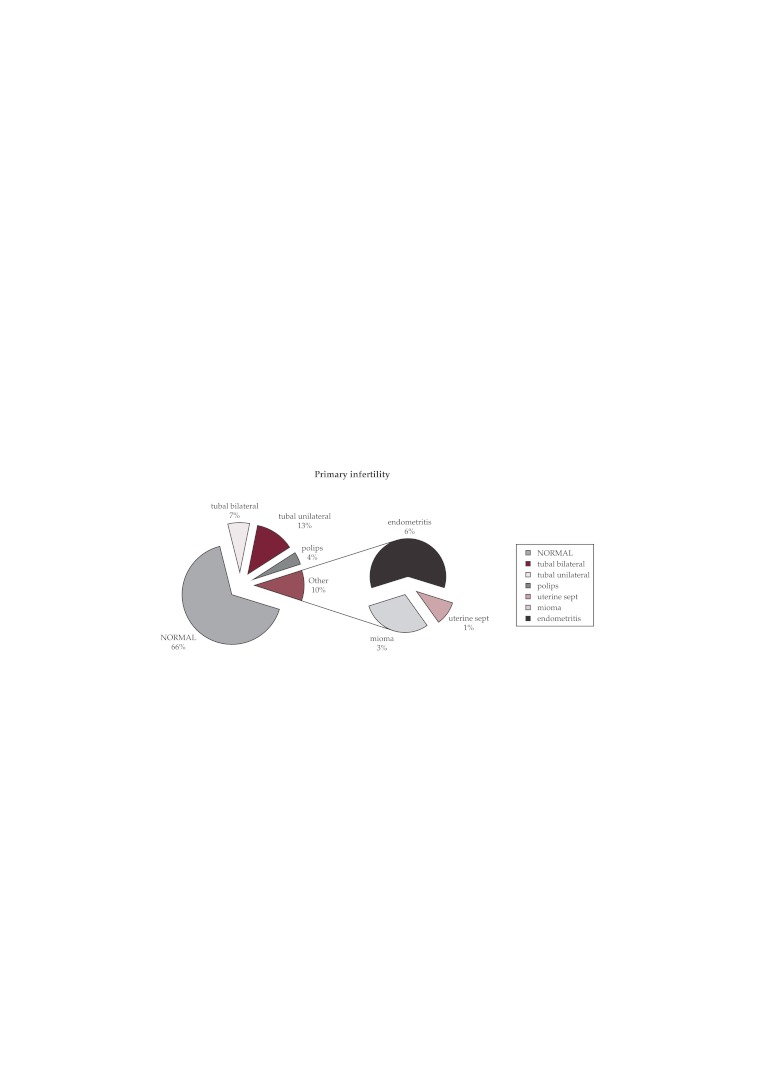
Of the total 299 cases of primary infertility, diagnostic hysteroscopy showed 197 cases (66%) with a normal hysteroscopy aspect; 60 cases (20%) indicated a proximal tubal obstruction uni- or bilateral (cornual adhesions obstructing the tubal ostia, small polyps, endometrial hyperplasia) and in other 42 cases (14%) there were findings of endouterine pathology: polyps, miomas, chronic endometritis (Figure 2).
Figure 2. Primary infertility -hysteroscopic results.
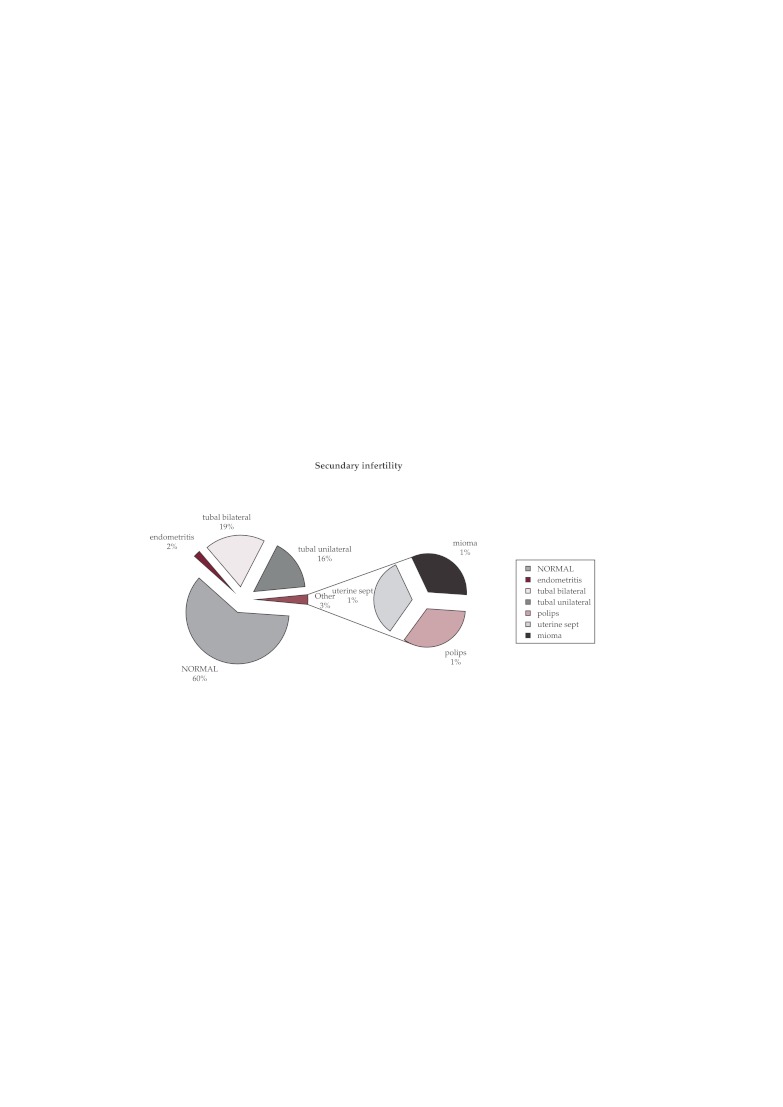
Of the total 396 cases of secondary infertility under investigation, diagnostic hysteroscopy showed a normal hysteroscopy aspect in 238 cases (60%); in 138 cases (35%) there was proximal tubal uni or bilateral obstruction and in other 20 cases (5%) there was endouterine pathology: polyps, miomas, chronic endometritis (Figure 3).
Figure 3. Secondary infertility -hysteroscopic results.
2. There were 309 cases which required diagnostic hysteroscopy in order to confirm the pathology suspected as a result of hysterosapingography or trans-vaginal sonography. The type of pathology under investigation is illustrated in Table 3.
Table 3.
Pathology suspected via another imagistic method.
| Pathology | Number | % |
|---|---|---|
| Submucous mioma | 127 | 41 |
| Endometrial polyp | 32 | 10 |
| Proximal tubal disease | 80 | 26 |
| Uterine sinechiae and cervico-istmic sinechiae | 70 | 23 |
The imagistic methods used before hysteroscopy were HSG in 55% of cases and trans-vaginal sonography in 45% of cases.
In Table 4 there is the concordance between the preoperative and postoperative diagnostic and the rate of false positive results
Table 4.
Concordance between the preoperative and postoperative diagnostic.
| Diagnostic | Preoperative | Postoperative | False positive results |
|---|---|---|---|
| Submucous mioma | 127 | 90 (70.86%) | 29.10% |
| Endometrial polyp | 32 | 20 (62.50%) | 37.50% |
| Proximal tubal disease | 80 | 48 (60.00%) | 40.00% |
| Uterine sinechiae and cervico-istmic sinechiae | 70 | 15 (21.42%) | 79.50% |
Looking at Table 4, we can conclude that the best diagnostic accuracy was for trans-vaginal sonography in cases of sub mucosal miomas (70.86%) and the lowest rate of detection was for HSG in cases with uterine and cervico-istmic sinechiae (21.42%).
3. The uterine malformations for which a diagnostic hysteroscopy was performed showed the following distribution: uterine septum 35% of cases, 10% of cases unicorn uterus, arcuate uterus 45% of cases and other uterine malformations in 10% of cases (Table 5).
Table 5.
Distribution of uterine malformation in the study.
| Uterine malformations | Number | % |
|---|---|---|
| Uterine septum | 21 | 35 |
| Unicorn uterus | 6 | 10 |
| arcuate uterus | 28 | 45 |
| Other malformations | 6 | 10 |
Concordance between HSG and diagnostic hysteroscopy was 100% for septate uterus and the unicorn, decreased to 66.6% for arcuate uterus and 50% for other uterine malformations.
In conclusion, the highest accuracy of HSG was noted for uterine malformation and minimal accuracy was observed for intrauterine adhesions. Trans-vaginal ultrasonography had a better accuracy in sub mucosal miomas than in polyps.
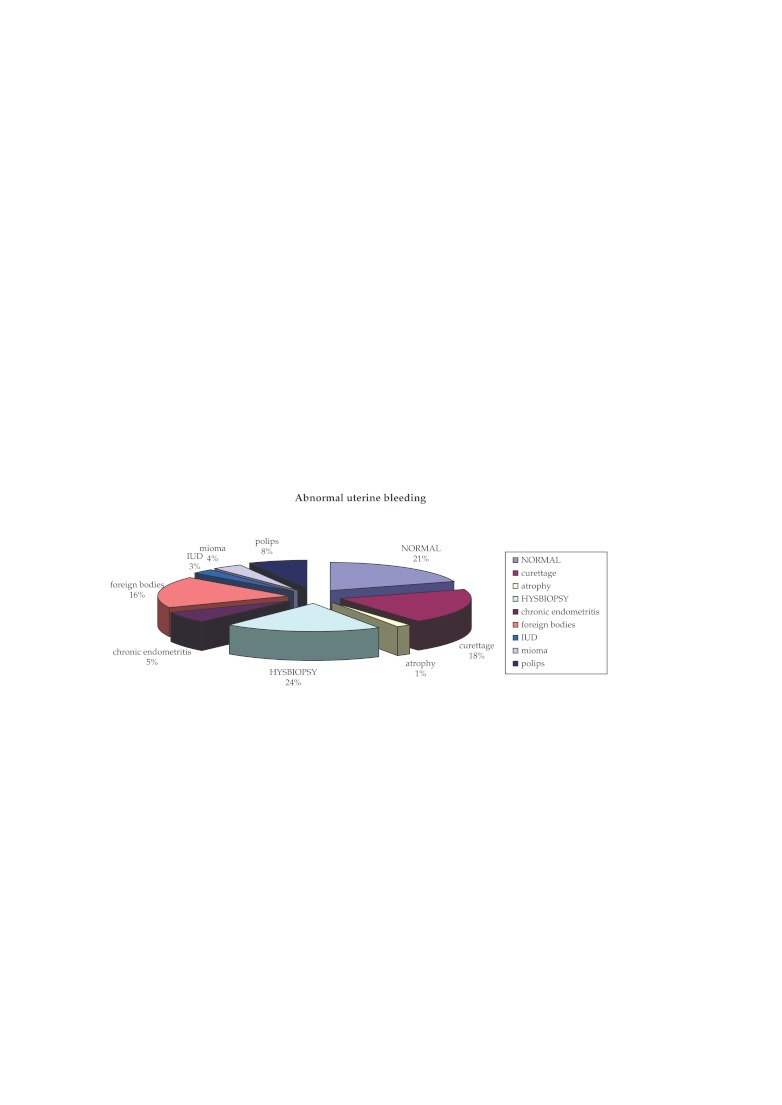
4. In our study, in 9% from the 1.545 hysteroscopies were performed for abnormal uterine bleeding. Distribution of different types of pathology diagnosed by hysteroscopy is illustrated in Figure 4.
Figure 4. Distribution of different types of pathology for abnormal uterine bleeding.
HYSBIOPSY: hysteroscopic biopsy; IUD : intrauterine devices.
Figure 4 shows following aspects: no endouterine pathology observed in 21% of cases; in 18% of cases hysteroscopy findings interested entire endometrial cavity requiring curettage biopsy for endometrial hyperplasia, in 24% of cases focal pathology was detected (focal hyperplasia of the endometrium, small polyps) which was followed by hysteroscopy biopsy (HYSBIOPSY); a percentage of 19% was represented by the intracavitary foreign bodies (70% suture material remaining post caesarian) and imprisoned IUD 8% of endometrial polyps over 1 cm resected with resectoscope; 4% sub mucosal miomas; 5% chronic endometritis which underwent hysteroscopy biopsy. ❑
DISCUSSION
Since 1999, the specialists in infertility of the University of Jerusalem from the Department of Obstetrics and Gynecology started a debate about the opportunity of including hysteroscopy in the basic/common investigations of infertility (13). The specialists' conclusions, based in the studies performed throughout the years, lead to hysteroscopy being currently considered as absolutely necessary in the infertility investigations (14).
However, the World Health Organization (WHO) recommends hysterosapingography (HSG) alone for management of infertile women (1). The explanation for this discrepancy is that HSG provides information on tuba patency or blockage. Office hysteroscopy is only recommended by the WHO when clinical or complementary exams (ultrasound, HSG) suggest intrauterine abnormality (15) or after in vitro fertilization (IVF) failure (16). Nevertheless, many specialists feel that hysteroscopy is a more accurate tool because of the high false-positive and false-negative rates of intra uterine abnormality with HSG (17-19). This explains why many specialists use hysteroscopy as a first-line routine exam for infertility patients regardless of guidelines (2).
Our experience in the Department of Assisted Human Reproduction at the "Prof.dr.Panait Sîrbu" Clinical Hospital of Obstetrics Gynecology with exploratory hysteroscopy used as a first line diagnostic method for the patients with infertility (in order to detect endocervical, endouterine or proximal tubal factor of infertility) supports the opinion that it should be a first-line routine exam for infertility.
The importance of diagnostic hysteroscopy in elucidating pathological aspects suspected by other diagnostic methods is obvious if we look at the high rate of false positive results given by trans-vaginal ultrasound and HSG. Note that high false positive rates are dependent on the performance of equipment used on the one hand and the experience of medical staff on the other hand. The group of 309 patients who have undergone diagnostic hysteroscopy to elucidate the diagnosis is extremely heterogeneous in terms of equipment used and the practitioner who performed the initial investigation. This could explain the differences between false positive rates existing in our study compared with other published studies in the literature: in the 1996 study run by Wang et al, which compared the diagnostic value of hysteroscopy and HSG, it was demonstrated that out of 135 patients with abnormal HSG, the hysteroscopy aspect was normal in 21 cases, which means a false positive rate of 15.6%. In the same study, the sensibility of HSG to diagnose the intrauterine abnormalities was 80.3% and the specificity was 70.1% (20). There are many independent studies (17,21) in specialty literature with similar results which show that in about one third of cases interpreted with HSG as normal, there may be a false positive result. These false positive results may lead to a wrong diagnostic and therapeutic decision in these patients (22).
Diagnostic hysteroscopy for patients with abnormal uterine bleeding in our study used to investigate organic endouterine causes of abnormal bleeding showed only in 21% of cases no endouterine pathology. The group of 83 cases (60%) benefited from a diagnosis of certainty with this investigation and the possibility of treating in the same operative time the pathology detected by diagnostic hysteroscopy. ❑
CONCLUSIONS
Our experience supports the opinion that diagnostic hysteroscopy should be a first –line routine exam in infertility.
Because of the high rate of false positive results for HSG in our study and considering the other studies in specialty literature, we always perform a diagnostic hysteroscopy before Assisted Human Reproduction procedures regardless of the HSG aspect.
References
- 1.Rowe PC, Hargreave T, Mellows H. The Press Syndicate of the University of Cambridge; Cambridge, UK: 1993. WHO Manual for the Standardized Investigation and Diagnosis of the Infertile Couple. [Google Scholar]
- 2.Koskas M, Mergui JL, Yazbeck C, et al. Office hysteroscopy for infertility: a series of 557 consecutive cases. Obstet Gynecol Int. 2010:168096–168096. doi: 10.1155/2010/168096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley LD. Overview of hysteroscopy. UpToDate Last literature review version. 2010;18:3–3. [Google Scholar]
- 4.Price TM, Harris JB. Fulminant hepatic failure due to herpes simplex after hysteroscopy. Obstet Gynecol. 2001;98:954–954. doi: 10.1016/s0029-7844(01)01511-3. [DOI] [PubMed] [Google Scholar]
- 5.Garbin O, Kutnahorsky R, Göllner JL, et al. Vaginoscopic versus conventional approaches to outpatient diagnostic hysteroscopy: a two-centre randomized prospective study. Hum Reprod. 2006;21:2996–3000. doi: 10.1093/humrep/del276. [DOI] [PubMed] [Google Scholar]
- 6.Bettocchi S, Selvaggi L. A vaginoscopic approach to reduce the pain of office hysteroscopy. J Am Assoc Gynecol Laparosc. 1997;4:255–258. doi: 10.1016/s1074-3804(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 7.Bettocchi S, Ceci O, Di Venere R, et al. Advanced operative office hysteroscopy without anesthesia: analysis of 501 cases treated with a 5 Fr bipolar electrode. Hum Reprod. 2002;17:2435–2438. doi: 10.1093/humrep/17.9.2435. [DOI] [PubMed] [Google Scholar]
- 8.Giorda G, Scarabelli C, Franceschi S, et al. Feasibility and pain control in outpatient hysteroscopy in postmenopausal women: a randomized trial. Acta Obstet Gynecol Scand. 2000;79:593–593. [PubMed] [Google Scholar]
- 9.Broadbent JA, Hill NC, Molnár BG, et al. Randomized placebo controlled trial to assess the role of intracervical lignocaine in outpatient hysteroscopy. Br J Obstet Gynaecol. 1992;99:777–777. doi: 10.1111/j.1471-0528.1992.tb13886.x. [DOI] [PubMed] [Google Scholar]
- 10.De Iaco P, Marabini A, Stefanetti M, et al. Acceptability and pain of outpatient hysteroscopy. J Am Assoc Gynecol Laparosc. 2000;7:71–71. doi: 10.1016/s1074-3804(00)80012-2. [DOI] [PubMed] [Google Scholar]
- 11.Kremer C, Barik S, Duffy S. Flexible outpatient hysteroscopy without anaesthesia: a safe, successful and well tolerated procedure. Br J Obstet Gynaecol. 1998;105:672–672. doi: 10.1111/j.1471-0528.1998.tb10185.x. [DOI] [PubMed] [Google Scholar]
- 12.ACOG Committee on Practice Bulletins. ACOG Practice Bulletin No. 74. Antibiotic prophylaxis for gynecologic procedures. Obstet Gynecol. 2006;108:225–225. doi: 10.1097/00006250-200607000-00057. [DOI] [PubMed] [Google Scholar]
- 13.Shushan A, Rojansky N. Should hysteroscopy be a part of the basic infertility workup? Hum Reprod. 1999;14:1923–1924. doi: 10.1093/humrep/14.8.1923. [DOI] [PubMed] [Google Scholar]
- 14.Campo R, Van Belle Y, Rombauts L, et al. Office mini-hysteroscopy. Hum Reprod Update. 1999;5:73–81. doi: 10.1093/humupd/5.1.73. [DOI] [PubMed] [Google Scholar]
- 15.De Sa Rosa e de Silva AC, Rosa e Silva JC, Candido dos Reis FJ, et al. Routine office hysteroscopy in the investigation of infertile couples before assisted reproduction. J Reprod Med. 2005;50:501–506. [PubMed] [Google Scholar]
- 16.Balmaceda JP, Ciuffardi I. Hysteroscopy and assisted reproductive technology. Obstet Gynecol Clin North Am. 1995;22:507–518. [PubMed] [Google Scholar]
- 17.Golan A, Eilat E, Ron-El R, et al. Hysteroscopy is superior to hysterosalpingography in infertility investigation. Acta Obstet Gynecol Scand. 1996;75:654–656. doi: 10.3109/00016349609054692. [DOI] [PubMed] [Google Scholar]
- 18.Valle RF. Hysteroscopy in the evaluation of female infertility. Am J Obstet Gynecol. 1980;137:425–431. doi: 10.1016/0002-9378(80)91122-9. [DOI] [PubMed] [Google Scholar]
- 19.Prevedourakis C, Loutradis D, Kalianidis C, et al. Hysterosalpingography and hysteroscopy in female infertility. Hum Reprod. 1994;9:2353–2355. doi: 10.1093/oxfordjournals.humrep.a138451. [DOI] [PubMed] [Google Scholar]
- 20.Wang CW, Lee CL, Lay YM, et al. Comparison of hysterosalpingography and hysteroscopy in female infertility. J Am Assoc Gynecol Laparosc . 1996;3:581–4. doi: 10.1016/s1074-3804(05)80170-7. [DOI] [PubMed] [Google Scholar]
- 21.Prevedourakis C, Loutradis D, Kalianidis C, et al. Hysterosalpingography and hysteroscopy in female infertility. Hum Reprod. 1994;9:2353–5. doi: 10.1093/oxfordjournals.humrep.a138451. [DOI] [PubMed] [Google Scholar]
- 22.Kumar S, Awasthi RT, Gokhale N. Assessment of Uterine Factor in Infertile Women: Hysterosalpingography vs Hysteroscopy. MJAFI. 2003;60:39–41. doi: 10.1016/S0377-1237(04)80156-4. [DOI] [PMC free article] [PubMed] [Google Scholar]