ABSTRACT
Background: Cerebral palsy (CP) is a group of persistent (but not necessarily unchanged), movement, posture, muscle tone and motor skills disorders non-progressive, with early onset, due to non-progressive impairments, occurring on an immature brain or a brain under development (prenatal, perinatal, postnatal during the first 3-4 years of life). It is associated to a variable extent with: cognitive disorders, epilepsy, sensory deficits, behaviour disorders.
Aim: The study of the correlations between the clinical forms/subtypes of CP, comorbidities, and severity of functional impairment.
Material and method: It is a retrospective trial aimed only at patients with the diagnosis of cerebral palsy admitted at Paediatric Neurology Clinic of the "Alexandru Obregia" Clinical Hospital in 2010.
Results, discussions and conclusions: Patients with cerebral palsy corresponding with the criteria for inclusion: 379. The spastic CP type has prevailed. Comorbidities like mental retardation, epilepsy, and ophthalmic disorders were found with greater frequency than in the studies in the literature.
The unilateral spastic form was statistically correlated with slight functional impairment (GMFCS I), with the absence of comorbidities or mild mental retardation, or with focal epilepsy when there is epilepsy. The bilateral spastic, tetraparetic and dyskinetic forms were correlated significantly with severe functional impairment (GMFCS IV, V), with profound or severe retardation, microcephaly, swallowing disorders, statural, ponderal hypotrophy, blindness and epilepsy. The bilateral spastic paraparetic form, which in the literature is mentioned as having fewer associated disorders (for example strabismus, slight retardation), when there is severe functional impairment, it may have the same comorbidities as the tetraparetic form (similar to the cases studied in the hospital). Comorbidities are the main admission cause and it correlates with the severity and prognosis.
Keywords: cerebral palsy, clinical type, comorbidities, GMFCS
INTRODUCTION
Cerebral palsy (CP) is a group of persistent (but not necessarily unchanged), movement, posture, muscle tone, and motor skills disorders non-progressive, with early onset, due to non-progressive impairments, occurring on an immature brain or a brain under development (prenatal, perinatal, postnatal during the first 3-4 years of life) (1). It is associated to a variable extent with: cognitive disorders, epilepsy, sensory deficits, behaviour disorders. ❑
AIM
The study of the correlations between the clinical forms/subtypes of CP, comorbidities, and severity of functional impairment. ❑
MATERIAL AND METHOD
It is a retrospective trial aimed only at patients with the diagnosis of cerebral palsy admitted at Paediatric Neurology Clinic of the Clinical Hospital "Alexandru Obregia", in 2010. All patients with the diagnosis of cerebral palsy were reassessed retrospectively based on data recorded in their medical observation sheet based on the previous records, development, clinical examination, laboratory and paraclinical examinations.
Inclusion criteria: patients with the diagnosis of cerebral palsy and who underwent cerebral imaging.
Exclusion criteria: static movement disorders with an onset after the age of 3, more or less progressive movement disorders or psychomotor retardation due to progressive tumoral, metabolic, degenerative, genetic causes; the presence of sensory deficits, ataxia, muscle atrophy, involuntary movements occurred in the development; medullary lesions; movement disorders caused by muscles or peripheral motor neuron disease; hypotonic syndromes, patients with a family history of "cerebral palsy", the presence of unexplainable acute episodes of vomiting, diarrhea, consciousness disorders, abnormal movements, mental or endocrine disorders (growth hormone deficit, adrenal insufficiency, diabetes), patients aged under 1.
From a clinical point of view cerebral palsy was classified according to the classification from 2002 of SCPE (European Cooperation on Surveillance of Cerebral Palsies in Europe) in (2): spastic; ataxic; dyskinetic; (dystonic; choreoathetosic), and refers to a muscle tone, motion and dominant posture disorder.
Functional Classification
The motor disorders were evaluated from a functional point of view and classified according to GMFCS (the scale for the classification of the gross motor function) into 5 levels/grades (3,4): Synthetic: Level I – walking without restrictions, Level II – walking without aid devices, but with some limitations when walking outside the house, Level III – assisted walking, Level IV – auto-mobility with limitations, the children are transported or use powered trolleys, Level V – severely limited self-mobility (for children under 2 years, born prematurely, the corrected age at assessment was used).
The associated clinical signs or comorbidities were also recorded for analysis: mental retardation, epilepsy, ocular/ vision disorders, microcephaly, macrocephaly, hearing disorders, swallowing disorders, mental disorders, behaviour and speech disorders, growth deficits, obesity, orthopaedic complications.
Statistical analysis
The data obtained was processed using the statistical software. The Chi square test (Pearson chi- square) was used for the comparison of more than 2 variables. The value p <0.05 is of statistical importance. ❑
RESULTS AND DISCUSSIONS
Patients with cerebral palsy corresponding with the criteria for inclusion: 379 of 495.
Characteristics of the batch:
Distribution by gender: girls 159 (42%), boys 220 (58%), ratio: boys/girls 1.38 /1.Place of origin: urban area 199 children (52.5%), rural area 180 children (47.5%).
The age of the children was between 1 and 17 years: average age 5.94 years (5 years and 11 months) with the following distribution per age groups: 106 children (28%) 1 to 2 years, 106 children (28%) 3 to 5 years, 100 children (26.4%) 6 to 10 years, 67 children (17.7%) >10 years.
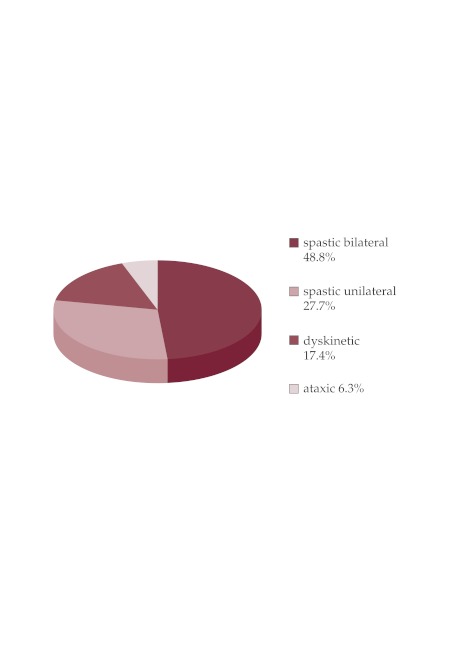
Clinical forms identified: bilateral spastic form 185 children (48.8%), bilateral spastic diparesis 103 (27.2%), bilateral spastic triparetic 14 cases (3.7%), tetraparetic spastic 68 cases (17.9%), unilateral spastic 104 cases (27.4%), dyskinetic 66 cases (17.4%), ataxic 24 cases (6.3%) (Figure 1).
Figure 1. Clinical forms distribution.
The clinical forms identified in my trial had a distribution comparable with those in literature, i.e. the spastic forms have prevailed 76.2% (80-90% in literature) (5-9). The dyskinetic form was more numerous than in literature (17.4% versus 7% in the population-based trials, 6-7% in the hospital-based studies). An explanation might be the fact that these children undergo imaging investigations more frequently, and patients with this forms had severe functional impairment, severe mental retardation and epilepsy and required hospitalization for treatment.
Functional impairment according to the Gross Motor Function Classification System (GMFCS)
From the point of view of severity of the functional impairment, the distribution was relatively uniform in the 5 levels: GMFCS I – 20.3% of the children, GMFCS II – 22.7%, GMFCS III – 17.6%, GMFCS IV – 22.4%, GMFCS V – 16.9 % of the children, different from the population studies where mild impairment predominates (GMFCS I) (6).
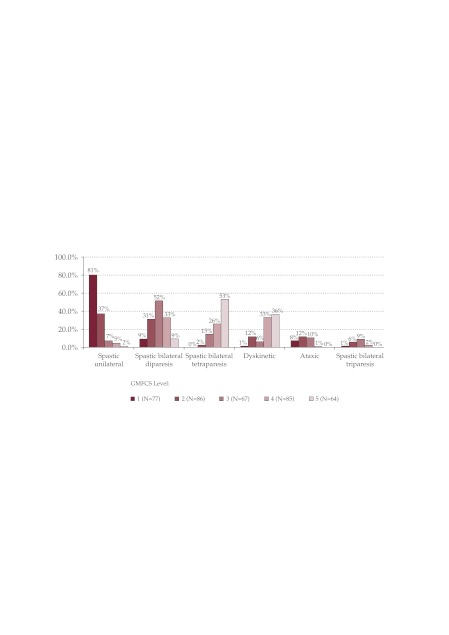
The distribution of clinical forms in GMFCS levels was the following: (Figure 2).
Figure 2. The distribution of clinical forms in GMFCS levels (% of pacients with GMFCS level).
GMFCS I – the majority unilateral spastic forms 80.5%. No tetraparesis was classified at this level. GMFCS II – unilateral spastic forms 37.2%, bilateral spastic (39.5%) – predominantly diparesis, GMFCS III – bilateral spastic forms (76%) predominantly diparesis, GMFCS IV – bilateral spastic forms (61.2% – 28 spastic diparesis, 2 spastic triparesis, 22 tetraparesis), GMFCSV – 62.5% bilateral spastic forms, most of which are cases of spastic tetraparesis, and dyskinetic forms 35.9%.
Statistically significant correlations (p = 0.000) in the study were: unilateral spastic forms with mild functional impairment GMFCS I, II, bilateral spastic diparetic forms with moderate and severe functional impairment GMFCS II, III, IV, bilateral spastic tetraparetic forms with severe functional impairment GMFCS III, IV, V, dyskinetic forms with severe functional impairment IV, V, and ataxic forms with moderate functional impairment GMFCS level II. This data is consistent with the data in the literature (Beckung and Hagberg, 2000) (10).
What is different is the classification with a greater frequency of the spastic diparesis in the study in levels III IV, which could be explained by the extent of the lesions affecting the motor skills, in these CPs, the most severe forms being superposable to the spastic tetraparetic form.
Disorders associated signs or identified comorbidities: mental retardation was present in 80.2% of the cases, epilepsy was present in 49.9 % of the cases, and absent in 50.1% of the cases (the second in terms of frequency between the associated disorders). Most cases were cases of focal epilepsy (73.5%), primary generalized (23.3%) and undetermined (3.2%). Most epilepsies were symptomatic and cryptogenic (when MRI, CT were normal and motor deficit were not concordant with seizure type, in the absence of family history of seizure). Ophthalmological disorders were present in 49.6% of the cases (the third in terms of frequency among associated disorders), microcephaly 32.7% of the cases, weight hypotrophy 18.2% of the cases, stature hypotrophy 15.6% of the cases, swallowing disorders 10% of the cases, speech disorders were 7% of the cases.
Mental retardation, epilepsy, and ocular and vision disorders were found with greater frequency than in the studies in the literature (29-52%) (9-14), and the speech disorders less frequent (9,10,15). A possible explanation would be that, some of the children with CP arrive for admission in the hospital because of comorbidities, and some comorbidities may be underdiagnosed due to the young age of the children (speech and hearing disorders) or due to significant mental retardation.
The distribution of comorbidities and the correlations between the clinical form and comorbidities
Mental retardation: The intellect was normal at 28.2% (compared to 69% in the literature) of the spastic diparesis cases, and 38.5% (compared to 50-82% in the literature) of the spastic hemiparesis cases studied. Normal intellect was correlated with the bilateral spastic diparetic or hemiparetic form (p = 0.000) (Table 1), with the absence of epilepsy (p = 0.000) and the absence of comorbidities. The values are smaller than in the literature, but the correlations are consistent with those in the literature (9,16).
Table 1.
Correlations between mental retardation and CP type.
| Mental | CP clinical type | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| retardation | Bilateral spastic diparesis | Bilateral spastic triparesis | Bilateral spastic triparesis | Spastic unilateral | Dyskinetic | Ataxic | Total | |||||||
| Count | Column N% | Count | Column N% | Count | Column N% | Count | Column N% | Count | Column N% | Count | Column N% | Count | Column N% | |
| absent | 29 | 28.2% | 0 | .0% | 1 | 1.5% | 40 | 38.5% | 3 | 4.5% | 2 | 8.3% | 75 | 19.8% |
| mild | 24 | 23.3% | 5 | 35.7% | 5 | 7.4% | 31 | 29.8% | 6 | 9.1% | 6 | 25.0% | 77 | 20.3% |
| moderate | 19 | 18.4% | 4 | 28.6% | 5 | 7.4% | 20 | 19.2% | 15 | 22.7% | 6 | 25.0% | 69 | 18.2% |
| severe | 25 | 24.3% | 5 | 35.7% | 35 | 51.5% | 11 | 10.6% | 28 | 42.4% | 8 | 33.3% | 112 | 29.6% |
| profound | 6 | 5.8% | 0 | .0% | 22 | 32.4% | 2 | 1.9% | 14 | 21.2% | 2 | 8.3% | 46 | 12.1% |
| Total | 103 | 100.0% | 14 | 100.0% | 68 | 100.0% | 104 | 100.0% | 66 | 100.0% | 24 | 100.0% | 379 | 100.0% |
Person Chi-Square Tests.
| Clinical type of CP | ||
|---|---|---|
| Mental retardation | Chi-square | 139.298 |
| df | 20 | |
| Sig. | .000*,a |
Mental retardation was predominant in spastic tetraparetic forms (98.5%), dyskinetic forms (95.5%), ataxic forms (91.3%). It was however present also in 71.8% of the spastic diparesis cases, and in 61.5% of the spastic hemiparesis cases (but here almost half were with mild retardation). Except for the ataxic and tetraparetic form, the presence of mental retardation was higher than in the literature in correlation with the clinical forms, which is due to more severe functional impairment in the diparetic, dyskinetic and hemiparetic forms (which is obvious in the GMFCS classification of spastic diparesis). Mild retardation was correlated with the unilateral spastic form (p = 0.000) and the presence of visual field anomalies (p = 0.016).
Severe and profound retardation were correlated with the tetraparetic and dyskinetic forms (p = 0.000), with the presence of epilepsy (p = 0.000), seeing disorders (blindness) (p = 0.016), microcephaly, swallowing disorders, stature-weight hypotrophy. The type of epilepsy was not correlated with retardation (p = 0.190). The data is consistent with the data in the literature, with the exception of the fact that in dyskinetic forms the intellect is usually normal in 64-86% of the cases. Severe retardation was described in children with dyskinetic CP with severe impairment of basal ganglia, thalamus and hippocampal atrophy, associated with seeing disorders (Krageloh-Mann 2002 (17). Our patients with dyskinetic forms were severely impaired functionally and had thalamus lesions.
Epilepsy: 41.7% of the spastic diparesis cases (compared to 16-27% in the literature), 61.8% of the spastic tetraparesis cases (as compared to 50% in the literature), 53.8% of spastic hemiparesis (27-44% in the literature), 41% of the dyskinetic forms (as compared to 25% in the literature), 45.8% of the ataxic forms (as compared to 20-30% in the literature), had epilepsy (8,10,11). The higher frequency of epilepsy in the patients in my study as compared to the data in the literature is due to the fact that epilepsy is a frequent reason for hospitalization in the case of children with CP. The bilateral spastic tetraparetic form was correlated significantly (p = 0.03) with the presence of epilepsy, the unilateral spastic form was correlated with the presence of focal epilepsy and the bilateral spastic diaretic form with undetermined epilepsy (p = 0.001).
Other comorbidities (Table 2): Swallowing disorders, microcephaly, stature-weight hypotrophy were statistically significantly associated with the bilateral spastic tetraparetic and dyskinetic forms. Blindness (mostly cortical blindness) was significantly more frequent in the spastic tetraparetic form (p = 0.000).
Table 2.
Correlations between comorbidities and CP type.
| Clinical type of CP | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spastic bilateral diparesis | Spastic bilateral triparesis | Spastic bilateral tetraparesis | Spastic unilateral | Dyskinetic | Ataxic | Total | ||||||||
| Comorbidities | Count | Column N% | Count | Column N% | Count | Column N% | Count | Column N% | Count | Column N% | Count | Column N% | Count | Column N% |
| Ophtalmic disordrs | 60 | 58.3% | 7 | 50.0% | 39 | 57.4% | 43 | 41.3% | 28 | 42.4% | 11 | 45.8% | 188 | 49.6% |
| Speech disorders | 8 | 7.8% | 1 | 7.1% | 4 | 5.9% | 10 | 9.6% | 6 | 9.1% | 4 | 16.7% | 33 | 8.7% |
| Hearing disorders | 3 | 2.9% | 0 | .0% | 3 | 4.4% | 2 | 1.9% | 2 | 3.0% | 0 | .0% | 10 | 2.6% |
| Swallowing disorders | 2 | 1.9% | 0 | .0% | 13 | 19.1% | 1 | 1.0% | 21 | 31.8% | 1 | 4.2% | 38 | 10.0% |
| Microcephaly | 30 | 29.1% | 4 | 28.6% | 32 | 47.1% | 17 | 16.3% | 37 | 56.1% | 4 | 16.7% | 124 | 32.7% |
| Macrocephaly | 0 | .0% | 0 | .0% | 1 | 1.5% | 3 | 2.9% | 1 | 1.5% | 1 | 4.2% | 6 | 1.6% |
| Orthopaedic disorders | 7 | 6.8% | 1 | 7.1% | 6 | 8.8% | 5 | 4.8% | 2 | 3.0% | 1 | 4.2% | 22 | 5.8% |
| Weight defficit | 19 | 18.4% | 1 | 7.1% | 22 | 32.4% | 2 | 1.9% | 20 | 30.3% | 5 | 20.8% | 69 | 18.2% |
| Statural-hypotrophy | 18 | 17.5% | 1 | 7.1% | 18 | 26.5% | 2 | 1.9% | 18 | 27.3% | 2 | 8.3% | 59 | 15.6% |
| Mental disorders | 9 | 8.7% | 0 | .0% | 3 | 4.4% | 14 | 13.5% | 1 | 1.5% | 6 | 25.0% | 33 | 8.7% |
| Dyslexia, et al | 1 | 1.0% | 1 | 7.1% | 0 | .0% | 2 | 1.9% | 0 | .0% | 1 | 4.2% | 5 | 1.3% |
| Obesity | 2 | 1.9% | 0 | .0% | 1 | 1.5% | 7 | 6.7% | 0 | .0% | 1 | 4.2% | 11 | 2.9% |
| Hypperkinesia | 7 | 6.8% | 0 | .0% | 1 | 1.5% | 7 | 6.7% | 0 | .0% | 4 | 16.7% | 19 | 5.0% |
| Absent | 21 | 20.4% | 3 | 21.4% | 6 | 8.8% | 33 | 31.7% | 13 | 19.7% | 5 | 20.8% | 81 | 21.4% |
| Total | 103 | 100.0% | 14 | 100.0% | 68 | 100.0% | 104 | 100.0% | 66 | 100.0% | 24 | 100.0% | 379 | 100.0% |
Person Chi-Square Tests.
| Clinical type of | ||
|---|---|---|
| Comorbidities | Chi-square | 246.781 |
| Df | 70 | |
| Sig. | .000*,a,b |
Stature-weight hypotrophy and strabismus were associated significantly from a statistical point of view with spastic diparesis compared with unilateral spastic forms (p = 0.000).
Mental and hyperkinetic disorders and nystagmus were associated (p = 0.000) with the ataxic form and the number of cases with these comorbidities was too small.
Comorbidities were correlated with the severity of CP as follows:
Unexpectedly, epilepsy was present in my study with the same frequency of approximately 50% regardless of the severity of the functional impairment GMFCS. This was due to the fact that patients with diparetic and dyskinetic forms arrived more frequently (almost double) than in the literature and this is one frequent reason for hospitalization. Focal epilepsy was significantly more frequent in children with PC classified as GMFCS levels I and II (p = 0.007). (Patients with spastic hemiparesis that are hospitalized usually for epilepsy were classified more frequently).
I have found statistically significant correlations between mental retardation and the severity of the GMFCS functional impairment (p = 0.000), the level of retardation being correlated with the GMFCS functional impairment level: the absence of mental retardation or mild retardation were associated statistically significant with mild functional impairment, GMFCS I, severe or profound mental retardation was associated with statistically significant with severe functional impairment, GMFCS V, mild and moderate retardation were associated significantly from a statistical point of view with moderate functional impairment, GMFCS II, III.
Correlations between other comorbidities and the GMFCS level: the absence of comorbidities was associated significantly from a statistical point of view with GMFCS I (p = 0.000), mental and hyperkinetic disorders with GMFCS II, microcephaly and hypotrophy weight with GMFCS III, microcephaly, swallowing disorders, stature and weight hypotrophy with GMFCS IV, V. Correlations between ocular/visual disorders and GMFCS (p = 0.000): visual field anomalies were correlated with GMFCS I (spastic hemiparesis is included here), strabismus with GMFCS IV (explainable by the fact that spastic diparesis are classified here), blindness with GMFCS V (spastic tetraparesis is included here). ❑
CONCLUSIONS
The unilateral spastic form was statistically correlated with mild functional impairment (GMFCS I), with the absence of comorbidities or mild mental retardation, or with focal epilepsy when there is epilepsy.
The bilateral spastic, tetraparetic and dyskinetic forms were correlated significantly with severe functional impairment (GMFCS IV, V), with profound or severe retardation, microcephaly, swallowing disorders, stature, weight hypotrophy, blindness and epilepsy. The bilateral spastic diparetic form, which in the literature is mentioned as having fewer associated disorders (for example strabismus, mild retardation), when there is severe functional impairment, it may have the same comorbidities as the tetraparetic form (similar to the cases studied in the hospital).
Certain comorbidities are suggestive of certain clinical forms, therefore they need to be investigated, and there is a tight connection between certain comorbidities. The severity of the cerebral palsy was dependent on the clinical form and on the presence of associated comorbidities.
Comorbidities are the main admission cause and it correlates with the severity in CP and also with prognosis.
We can estimate, to a certain extent, a clinical picture with associated comorbidities and we can assess the evolution and formulate a functional prognosis.
Etiology and the time of the occurrence of the lesion are also important in cerebral palsy. An important role in the etiological diagnosis and in establishing the occurrence of the lesion was played by imaging investigations. Finding the etiology is the first step in finding treatment and prophylaxis.
References
- 1.Cans C, SCP A collaboration of cerebral palsy surveys and registers. Dev Med Child Neurol. 2000;42:816–824. doi: 10.1017/s0012162200001511. [DOI] [PubMed] [Google Scholar]
- 2.Surveillance of Cerebral palsy in Europe, prevalence and characteristics of children with cerebral palsy in Europe. Dev Med Child Neurol. 2002;44:633–664. [PubMed] [Google Scholar]
- 3.Palisano R, Walter S, Russell D, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 4.Palisano R, Rosenbaum P, et al. Content validity of the expanded and revised Gross Motor Function Classification System. Dev Med Child Neurol. 2008;50:744–750. doi: 10.1111/j.1469-8749.2008.03089.x. [DOI] [PubMed] [Google Scholar]
- 5.Krägeloh-Mann I, Horber V. The role of magnetic resonance imaging in elucidating the pathogenesis of cerebral palsy: a systematic review. Dev Med Child Neurol. 2007;49:144–151. doi: 10.1111/j.1469-8749.2007.00144.x. [DOI] [PubMed] [Google Scholar]
- 6.Towsley K, Shevell M, Dagenais L. Population-based study of neuroimaging findings in children With cerebral Palsy. Eur J Paediatr Neurol. 2011;15:29–35. doi: 10.1016/j.ejpn.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Korzeniewsky SJ, Birbeck G, et al. A systematic review of Nuroimaging for Cerebral Palsy. J Child Neurol. 2008;23:216–227. doi: 10.1177/0883073807307983. [DOI] [PubMed] [Google Scholar]
- 8.Shervell M, Majnemer A, Morin I. Etiologic Yield of Cerebral Palsy: A Contemporary Case series. Pediatr Neurol. 2003;28:352–359. doi: 10.1016/s0887-8994(03)00006-7. [DOI] [PubMed] [Google Scholar]
- 9.Krageloh Mann I, Bax M. In Aicardi J, Diseases of the nervous System in Childhood, 3rd edit. Vol. 7. Mac Keith Press; London: 2009. Cerebral Palsy. pp. 210–242. [Google Scholar]
- 10.Beckung E, Hagberg G. Correlation between ICIDH handicape code and Gross Motor Function Classification System in children with cerebral palsy. Dev Med Child Neurol. 2000;42:669–673. doi: 10.1017/s0012162200001237. [DOI] [PubMed] [Google Scholar]
- 11.Schenk-Rootlieb AJ, van Nieuwenhuizen O, et al. The prevalence of cerebral visual disturbance in children with cerebral palsy. Dev Med Child Neurol. 1992;34:473–80. doi: 10.1111/j.1469-8749.1992.tb11467.x. [DOI] [PubMed] [Google Scholar]
- 12.Kwong KL, Wong SK, So KT. Epilepsy in children with cerebral palsy. Pediatr Neurol. 1998;19:31–36. doi: 10.1016/s0887-8994(98)00011-3. [DOI] [PubMed] [Google Scholar]
- 13.Carlsson M, Hagberg G, Olsson I. Clinical and aetiological aspects of epilepsy in children with cerebral palsy. Dev Med Child Neurol. 2003;45:371–376. doi: 10.1017/s0012162203000719. [DOI] [PubMed] [Google Scholar]
- 14.Ashwal S, Russman BS, Blasco PA. Practice Parameter: Diagnostic assessment of the child with cerebral palsy: Report of the Quality Standards Subcomitee of the Child Neurology Society. Neurology. 2004;62:851–851. doi: 10.1212/01.wnl.0000117981.35364.1b. [DOI] [PubMed] [Google Scholar]
- 15.Thorogood C. Medscape; 2005. Apr, Rehabilitation and Cerebral palsy. [Google Scholar]
- 16.Fedrizzi E, Inverno M, Bruzzone MG. MRI features of cerebral lesions and cognitive functions in preterm spastic diplegic children. Pediatr Neurol. 1996;15:207–212. doi: 10.1016/s0887-8994(96)00174-9. [DOI] [PubMed] [Google Scholar]
- 17.Krägeloh-Mann I, Helber A., et al. Bilateral lesions of thalamus and basal ganglia: origin and outcome. Dev Med Child Neurol. 2002;44:477–84. doi: 10.1017/s0012162201002389. [DOI] [PubMed] [Google Scholar]