ABSTRACT
Some patients with ischemic stroke are subject to hemorrhagic transformation, a complication leading to increased patient morbidity and mortality. The discovery of biomarkers that can be used to identify ischemic strokes prone to this complication are very important for the clinical practice because therapy could be altered to mitigate the risk. We discuss here the results of a trial that evaluated for the first time tight junction proteins as biomarkers of blood-brain barrier disruption and hemorrhagic transformation in ischemic stroke.
Keywords: stroke, hemorrhagic transformation
Water molecules' motion or diffusion was found to be much faster along the white matter fibers than perpendicular to them. The difference between these two motions (parallel and perpendicular to the fibers, also termed diffusion anisotropy) is the basis of DTI (1-4).
Diffusion tensor imaging (DTI) has become one of the most popular MRI techniques in brain research, as well as in clinical practice. It is used to study the white matter architecture and integrity of the normal and diseased brains (multiple sclerosis, stroke, aging, tumors, dementia, schizophrenia, etc.) (5-8). ❑
PHYSICAL PRINCIPLES
Brownian motion is traditionally regarded as discovered by the botanist Robert Brown in 1827 while studying, under a microscope, pollen grains suspended in water.
The molecules of any substance in a continuous media have a random movement determined by their thermal energy. The motion takes place over a distance in space which is determined by the diffusion coefficient (D), in accordance with Stokes-Einstein's law:
D=kT/f,
where k represents Boltzmann constant, and
f depends on particle dimensions and fluid viscosity.
Diffusion Tensor Imaging (DTI) is a MRI-based technique, used for describing biologic tissues microstructures, that exploit quantification of water diffusion in tissues.
The diffusion can be either:
-
-
isotropic, when there are no hinders to diffusion, being statistically the same in all directions in space, or (ex in CSF)
-
-
anisotropic: when has barriers against diffusion for some directions (ex. white matter).
Diffusion parameters are fractional anizotropy (FA) and apparent diffusion coefficient (ADC).
- FA (fractional anisotropy) – that represents the degree of directionality of diffusion, the preference for a single direction of diffusion.
FA=0 (isotropic diffusion, equal in all space directions).
FA=1 (diffusion occurs only along one axis).
When axons or myelin are destructed FA decrease (diffusion will not be restricted only to one direction).
- ADC (apparent diffusion coefficient), estimates total diffusion for each voxel analyzed. We also determine the magnitude of axial (11) and radial diffusivity (13, 12).
ADC increase if biological tissues are affected (no diffusion hinders) – due to increase in radial diffusion.
In nervous tissues (white matter), water diffuses preferentially along axons and nerve fiber bundles (γ1- axial difusivity).
On perpendicular direction diffusion process is hindered by axonal membranes and is modulated by myelin. (l2 & 3 - radial difusivity) (9-11).
A diffusion tensor matrix can be generated for each voxel from aquired DWI diffusion weighted images.
RGB (red–green–blue) color-coded scheme attributes a color for each orientation of the fibers:
fibers crossing from left to right are visualized in red,
fibers crossing anteriorly–posteriorly are visualized in green, and
fibers crossing inferiorly–superiorly are visualized in blue.
Figure 1. Isotropic and anisotropic diffusion.
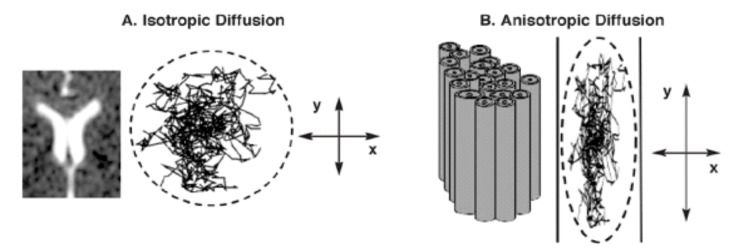
Figure 2. Normal subject – reconstruction of corpus calosus.
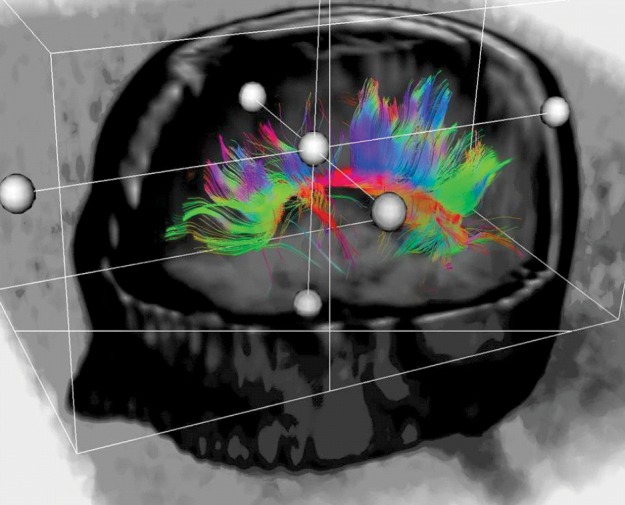
Figure 3. Normal subject – reconstruction of cortico-spinal tract.
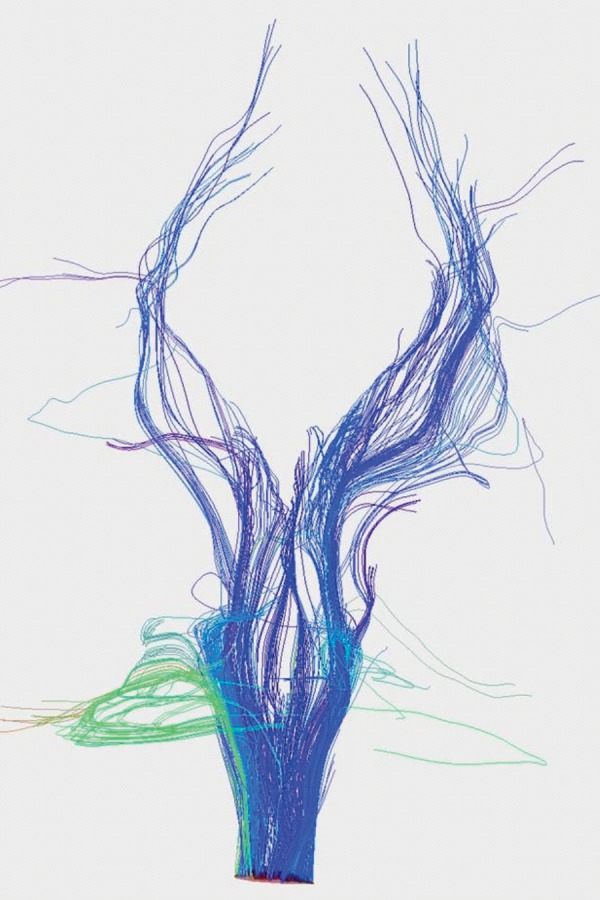
Figure 4. DTI recontruction of white matter tracts in normal subject.
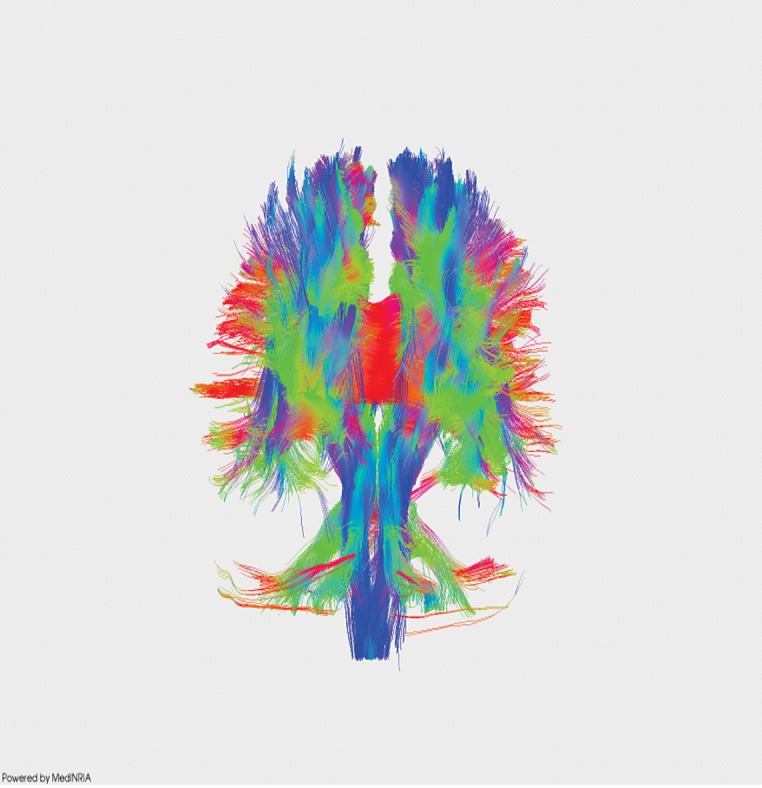
Figure 5. DTI recontruction of white matter tracts in MS patient.
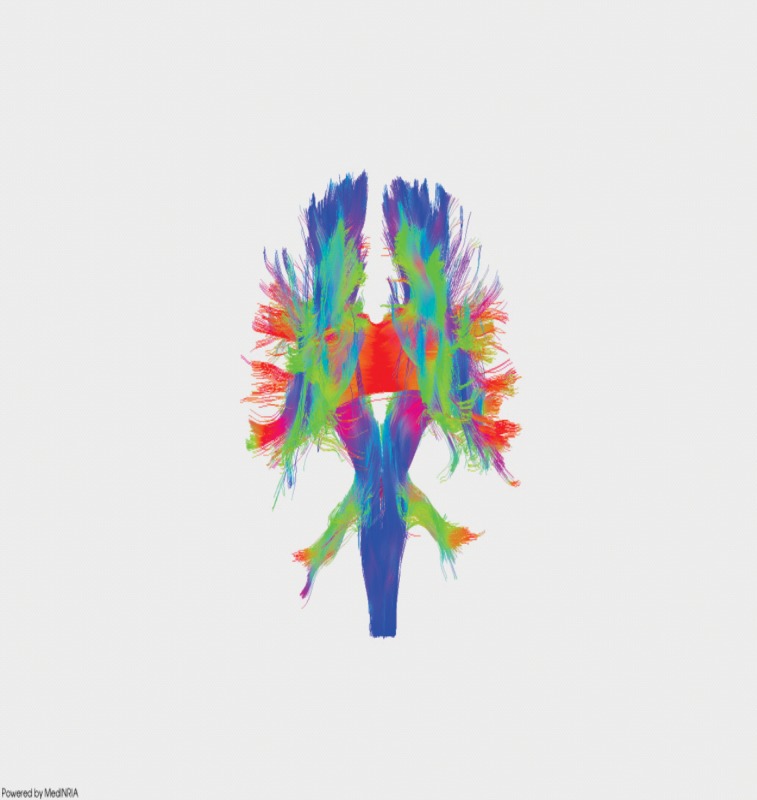
At the Department of Neurology of Emergency University Hospital Bucharest and "Theodor Burghele" Hospital Bucharest, we take advantage of this neuroimaging technique to study how neurological pathologies affect nervous fibers in multiple sclerosis patients (12-13).
Equipment used is GE Signa Excite 1.5 T MRI equipment, with 6 or 25 gradients, with NEX 1-3, 128x128 matrix, b = 1000 s/mm2.
As protocol, we start with a classic MRI exam which has a better spatial resolution, and then we apply the pulsed gradients of DTI, with exposure times that vary from 10 to 15 minutes.
After the acquisitions of DTI data we further process them using sophisticated software in terms of generating fibers and analyzing the parameters of the diffusion (12).
Multiple sclerosis is a demyelinating autoimmune inflammatory disease of central nervous system (CNS), affecting young patients.
Multiple sclerosis in its most typical form is characterized by relapses and remissions of CNS dysfunction. Disease and disability progression is variable.
In addition to simple MRI, other new neuroradiologic techniques and measurements offer a better estimation of disease status and amplitude of injures. MR Diffusion Tensor Imaging and Fiber Tracking Technique are the means for better understanding the cerebral white matter configuration and the different pathologies that could influence it.
References
- 1.Basser PJ, Jones DK. Diffusion-tensor MRI: theory, experimental design and data analysis – a technical review. NMR Biomed. 2002;15:456–467. doi: 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- 2.Stieltjes B, Kaufmann WE, van Zijl PCM, et al. Diffusion tensor imaging and axonal tracking in the human brainstem. Neuroimage. 2001;14:723–735. doi: 10.1006/nimg.2001.0861. [DOI] [PubMed] [Google Scholar]
- 3.Pierpaoli C, Jezzard P, Basser PJ, et al. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–48. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- 4.Hasan KM, Narayana PA. Computation of the fractional anisotropy and mean diffusivity maps without tensor decoding and diagonalization: Theoretical analysis and validation. Magn Reson Med. 2003;50:589–598. doi: 10.1002/mrm.10552. [DOI] [PubMed] [Google Scholar]
- 5.Werring DJ, Toosy AT, Clark CA, et al. Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. J Neurol Neurosurg Psychiatry. 2000;69:269–72. doi: 10.1136/jnnp.69.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mori S, Frederiksen K, van Zijl PC, et al. Brain white matter anatomy of tumor patients evaluated with diffusion tensor imaging. Ann Neurol. 2002;51:377–80. doi: 10.1002/ana.10137. [DOI] [PubMed] [Google Scholar]
- 7.Tievsky AL, Ptak T, Farkas J. Investigation of apparent diffusion coefficient and diffusion tensor anisotropy in acute and chronic multiple sclerosis lesions. Am J Neuroradiol. 1999;20:1491–9. [PMC free article] [PubMed] [Google Scholar]
- 8.Antochi FA, Onu M, Roceanu AM. DTI parameters changes in axonal loss due to multiple sclerosis. European Journal of Neurology; 14th Congress of the European Federation of Neurological Societies; September 25-28, 2010; Geneva, Switzerland. pp. 72–350. p. 279 (poster 1535) [Google Scholar]
- 9.Chenevert TL, Brunberg JA, Pipe JG. Anisotropic diffusion in human white matter: demonstration with MR techniques in vivo. Radiology. 1990;177:401–405. doi: 10.1148/radiology.177.2.2217776. [DOI] [PubMed] [Google Scholar]
- 10.Lazar M, Weinstein DM, Tsuruda JS, et al. White Matter Tractography Using Diffusion Tensor Deflection. Human Brain Mapping. 2003;18:306–321. doi: 10.1002/hbm.10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westin C, Maier S, Mamata H, et al. Processing and visualization for diffusion tensor MRI. Medical Image Analysis. 2002;6:93–108. doi: 10.1016/s1361-8415(02)00053-1. [DOI] [PubMed] [Google Scholar]
- 12.Ferastraoaru V, Roceanu A, Bajenaru O. Tractography and parameters analysis methods for cerebral fibers using Diffusion Tensor Imaging. The 7th Congress of Romanian Society of Neurology, Romanian Journal of Neurology. 2009;VII(Suppl. 1):27–27. [Google Scholar]
- 13.M Onu, A Roceanu, U Soboto-Frankenstein, et al. Diffusion abnormality maps in demyelinating disease: Correlations with clinical scores. European Journal of Radiology. 2012;81:e386–e391. doi: 10.1016/j.ejrad.2011.12.014. [DOI] [PubMed] [Google Scholar]


