Abstract
Objective:
To describe the rationale, design and first data from PATRO Children, a postmarketing surveillance of the long-term efficacy and safety of somatropin (Omnitrope®) for the treatment of children requiring growth hormone treatment.
Methods:
PATRO Children is a multicentre, open, longitudinal, noninterventional study being conducted in children’s hospitals and specialised endocrinology clinics. The primary objective is to assess the long-term safety of Omnitrope® in routine clinical practice. Eligible patients are infants, children and adolescents (male or female) who are receiving treatment with Omnitrope® and who have provided informed consent. Patients who have been treated with another recombinant human growth hormone (rhGH) product before starting Omnitrope® are eligible for inclusion. All adverse events (AEs) are monitored and recorded, with particular emphasis on: long-term safety; the recording of malignancies; the occurrence and clinical impact of anti-hGH antibodies; the development of diabetes during Omnitrope® treatment in children short for gestational age (SGA); safety issues in patients with Prader–Willi syndrome (PWS). Efficacy assessments include auxological parameters, plus insulin-like growth factor-1 and insulin-like growth factor binding protein-3.
Results:
As of September 2012, 1837 patients were enrolled in the study from 184 sites in 10 European countries. To date, efficacy data are reassuring and consistent with previous studies. In addition, there have been no confirmed cases of diabetes occurring under Omnitrope® treatment, no reports of malignancy and no safety issues in PWS patients.
Conclusions:
The efficacy and safety profile of Omnitrope® in the PATRO Children study so far are as expected. The ongoing study will extend the safety database for Omnitrope®, and rhGH products more generally, in paediatric indications. Of particular interest, PATRO Children will add important information on the diabetogenic potential of rhGH in children born SGA, the risk of malignancies in children receiving rhGH, and AEs with a possible causal relationship to rhGH treatment in children with PWS.
Keywords: children, Omnitrope®, paediatric, recombinant human growth hormone
Introduction
Since the introduction of the first recombinant human growth hormone (rhGH), a substantial body of data has been collected, from randomised controlled trials as well as observational studies, to establish the efficacy and safety of these treatments. rhGH products are used in the treatment of several paediatric indications, including growth hormone deficiency (GHD), Turner syndrome (TS), children born small for gestational age (SGA), chronic renal insufficiency (CRI) and Prader–Willi syndrome (PWS) [American Society of Clinical Endocrinologists, 2003; Kirk and Butler, 2006; GH Research Society, 2000; Clayton et al. 2005; Bondy, 2007; Lee et al. 2000, 2003; Mahan and Warady, 2006].
A number of rhGH products are available for the treatment of paediatric growth disturbances, including somatropin (Omnitrope®, Sandoz, Kundl, Austria). Somatropin is a rhGH approved by the European Medicines Agency (EMA) in 2006; it was the first product ever to be approved via the biosimilar regulatory pathway, with approval granted on the basis of comparable quality, safety and efficacy to the reference product (Genotropin®, Pfizer) [EMA, 2008]. Somatropin is licensed for use to treat growth disturbances in the following paediatric indications: GHD, TS, CRI, SGA and PWS [EMA, 2011].
This paper describes the design of, and first data from, PATRO Children, a postmarketing surveillance (noninterventional study) of the long-term efficacy and safety of somatropin for the treatment of infants, children and adolescents with growth disturbances. The study is part of the Risk Management Plan for Omnitrope®, and therefore fulfils the regulatory obligations of the study sponsor. It will also extend the safety database for somatropin, as well as contributing to the available data for all rhGH products.
Methods
PATRO Children is a multicentre, open, longitudinal, noninterventional study. The study is being conducted in children’s hospitals and specialised endocrinology clinics across various countries in which somatropin has been approved. All physicians prescribing somatropin in participating countries are invited to enrol patients into the study. The primary objective is to collect and analyse data on the long-term safety of somatropin in infants, children and adolescents treated in routine clinical practice, with particular emphasis on the following: diabetogenic potential (both type 1 and type 2 diabetes) of rhGH therapy in children born SGA and treated for growth disturbance; occurrence of malignancies in rhGH-treated patients; respiratory and diabetogenic side-effects of rhGH treatment in PWS patients; occurrence and clinical implications of anti-GH antibodies. A secondary objective is to collect and analyse data on the long-term efficacy of somatropin treatment. The study protocol has been approved by all relevant Independent Ethics Committees.
Study population
Eligible patients are infants, children and adolescents (male or female) who are receiving treatment with somatropin and who have provided informed consent. All diagnoses were made by investigators. Patients who have been treated with another hGH product before starting somatropin therapy are also eligible for inclusion. The minimum enrolment target is 1500 patients; based on the ‘rule of three’, this target will allow for detection of adverse events (AEs) occurring at an incidence of 1/500 person-years [Eypasch et al. 1995; Jovanovic and Levy, 1997]. It is estimated that the median duration of treatment will be at least 5 years, therefore an AE occurring with an incidence of 1/2500 person-years will be detected. Within this enrolment target, a minimum of 30 patients with PWS will be included and analysed separately (at the request of the regulatory authorities).
Treatment
Included patients receive somatropin treatment in accordance with the recommendations in the Summary of Product Characteristics [EMA, 2011] and/or the prescribing information of the respective countries. Once included in the study, it is anticipated that children will continue to receive treatment with somatropin until they achieve final height. However, treatment may be interrupted or discontinued prematurely, or patients may withdraw their informed consent. All data generated up to the time of consent withdrawal or study discontinuation (including all periods of treatment interruption) will be analysed and the reasons for each interruption or definite discontinuation will be recorded. All concomitant therapy (dose, date of administration and reason for use) is recorded in the electronic case report form (eCRF).
Visit schedule and assessments
The frequency of visits is at the discretion of the treating physician; no additional or specific visits, tests or assessments are required as part of the study. Data are collected at each routine visit during treatment with somatropin. For all patients included in the study, all available data (visits, laboratory data, findings from examinations, etc.) are recorded in a CRF at least once a year. Table 1 lists the tests and assessments that will be performed at visit 1 (the start of somatropin treatment), and at subsequent visits if performed as part of routine clinical practice.
Table 1.
Visit schedule and assessments.
| Parameter (if appropriate performed) | Visit 1 (baseline start) | Subsequent visits (intervals corresponding to routine clinical practice) |
|---|---|---|
| Informed consent | X | |
| Patient code | X | X |
| Date of visit | X | X |
| Patient data (gender, date of birth) | X | |
| Primary diagnosis (date, evidenced indication, indication-specific characteristics) | X | |
| Family history | X | |
| Pregnancy | X | |
| Birth history | X | |
| Previous auxological data | X | |
| Physical examination | X | |
| Relevant medical history and concomitant diseases | X | |
| Concomitant medication/therapies | X | X |
| Previous clinical trial participation | X | |
| Previous GH treatment | X | |
| Omnitrope® treatment | X | X |
| Current auxological data | X | X |
| Vital signs | X | X |
| Pubertal development | X | X |
| Bone age | X | X |
| Body composition | X | X |
| Haematology | X | X |
| Blood chemistry | X | X |
| Glucose metabolism/OGTT | X | X |
| Fasting lipid profile | X | X |
| Urinalysis | X | X |
| Hormones (thyroid, gonadal, adrenal function) | X | X |
| IGF-I, IGFBP-3 determinations | X | X |
| Anti-HGH antibody determination | X | X |
| Adverse events | X | |
| Pregnancy reporting | X | |
| Medical device vigilance reports | X | |
| Discontinuation (reasons) | X |
IGF, insulin-like growth factor; IGFBP, insulin-like growth factor binding protein; OGTT, oral glucose tolerance test.
Safety assessments
All AEs, including serious AEs (SAEs), are monitored and recorded irrespective of causality. Particular emphasis is being placed on: long-term safety; the recording of malignancies; the occurrence and clinical impact of anti-hGH antibodies; the development of diabetes during treatment with somatropin in SGA patients; safety issues in patients with PWS.
Efficacy assessments
The following auxological parameters are being assessed: height velocity (HV, cm/year); height velocity standard deviation score (HVSDS); height standard deviation score (HSDS). The growth hormone parameters, insulin-like growth factor 1 (IGF-1) and insulin-like growth factor-binding protein 3 (IGFBP-3), will also be assessed; values will be classified (low, normal, high) according to their normal ranges. The impact of somatropin treatment on body mass index as a surrogate of body composition (total fat mass, lean body mass, total body water) is also evaluated.
Data collection and statistical analysis
Investigators enter data from source documents into a Web-based electronic data collection (EDC) system. All eCRFs are reviewed by data management, and centralised monitoring is performed by a contract research organisation (CRO). The EDC system is state of the art, being simple to use and easy to navigate. Entered data are saved automatically, and the system contains automatic data checks to ensure the quality of the collected data. Any entered data that is outside of the expected range for a particular category should automatically be queried by the system. The system also ensures that users provide auxological data from historical height measurements to allow efficacy parameters to be calculated. Another useful feature is the ability of the system to generate custom reports, such as growth charts for individual patients that map their growth against national reference data.
All enrolled patients who have received at least one dose of somatropin will be included in the safety analysis. All children with at least one documented height measurement at baseline/start of somatropin treatment, plus one measure of height under somatropin treatment with the corresponding dates of measurements, will be included in the efficacy analysis. Data from patients who discontinue treatment before reaching final height are retained and included in all analyses. Descriptive statistics will be used to describe continuous parameters (e.g. age, height, weight) and categorical parameters (e.g. gender). These will be displayed overall and by somatropin indication if appropriate. Continuous parameters will be compared, if appropriate, by t-test or by Wilcoxon nonparametric test (when normality of distribution cannot be assumed). Categorical data will be compared using chi-squared or Fischer’s exact test for 2 × 2 tables with small samples. Statistical tests will be two-sided at the 0.05 significance level.
The Poisson distribution will be used to assess the diabetogenic potential of GH in short children born SGA and the occurrence of malignancies. The AE profile of children with PWS will be compared with those of other indications. For analysis of BMI and fat mass, PWS children will be analysed separately. If more than 30 children develop antibodies to hGH, growth velocity, HSDS and final height SDS will be compared between the following groups: children with antibodies detected before starting somatropin treatment, those with antibodies detected after starting somatropin treatment, and those without antibodies to hGH.
Results
As of September 2012, 1837 patients had been enrolled in the study in Europe from 184 sites in 10 countries. Baseline characteristics of these patients are presented in Table 2. Overall, 19.7% of these patients had GH treatment before enrolment; the majority received doses in accordance with the prescribing information. The mean (SD) duration of pretreatment by indication is as follows: GHD, 3.78 (2.38) years; TS, 4.04 (2.47) years; SGA, 4.20 (2.44); PWS, 2.04 (1.83); other, 3.83 (2.22).
Table 2.
Baseline characteristics of patients enrolled as of September 2012 (n=1837).
| Indication | n (% of total) | Male/female (%) | Chronological age, years (mean ± SD) | Bone age, years (mean ± SD) | Median BMI (kg/m2) | Naive/pretreated (%) | Median dose (mg/kg/day) |
|---|---|---|---|---|---|---|---|
| GHD | 1078 (58.7) | 64.8/35.2 | 9.81±3.81 (Nmiss=3) | 8.22±4.00 (Nmiss=739) | 16.10 (Nmiss=259) | 81.1/18.9 | 0.030 |
| SGA | 475 (25.9) | 48.8/51.2 | 8.40±3.35 (Nmiss=2) | 6.55±3.66 (Nmiss=364) | 15.20 (Nmiss=135) | 78.9/21.1 | 0.035 |
| TS | 81 (4.4) | 0/100 | 9.91±4.36 | 8.74±4.34 (Nmiss=63) | 17.40 (Nmiss=22) | 66.7/33.3 | 0.041 |
| PWS | 42 (2.3) | 42.9/57.1 | 3.87±4.24 | 8.08±5.54 (Nmiss=40) | 16.05 (Nmiss=22) | 90.5/9.5 | 0.030 |
| CRI | 12 (0.7) | 41.7/58.3 | 7.17±5.01 | 7.33±4.19 (Nmiss=9) | 16.45 | 83.3/16.7 | 0.047 |
| Other | 111 (6.0) | 60.4/39.6 | 10.39±3.89 | 8.18±4.07 (Nmiss=74) | 16.62 (Nmiss=26) | 81.1/18.9 | 0.035 |
| Not yet recorded | 38 (2.1) | 63.2/36.8 | 9.90±3.45 | 6.25±2.47 (Nmiss=36) | 15.85 (Nmiss=30) | 92.1/7.9 | 0.031 |
| Total | 1837 (100) | 56.9/43.1 | 9.33±3.89 (Nmiss=11) | 7.84±3.99 (Nmiss=1352) | 15.90 (Nmiss=494) | 80.3/19.7 | 0.032 |
GHD, growth hormone deficiency; SGA, small for gestational age; TS, Turner syndrome; PWS, Prader–Willi syndrome; CRI, chronic renal insufficiency; Nmiss, number of patients with data missing.
Within the first year of somatropin treatment, the overall incidence of AEs was 910 events in 380 patients; the incidence of adverse drug reactions was 129 events in 88 patients. Adverse drug reactions occurring with an incidence >0.001 up to September 2012 are shown in Table 3. A total of 66 SAEs in 38 patients were reported. Two SAEs in two patients were reported and assessed to be related to somatropin treatment. One patient (primary diagnosis: isolated GHD) experienced benign intracranial hypertension, which required hospitalisation, and somatropin treatment was discontinued; the event resolved completely. The second patient (primary diagnosis: SGA), with a medical history of heart injury, severe pulmonary artery atresia and hypothyroidism, was hospitalised for acute cardiac failure. Somatropin treatment was interrupted several times before the patient underwent heart transplantation. After transplantation somatropin treatment was permanently stopped. The outcome was reported as resolved.
Table 3.
Adverse drug reactions occurring with an incidence >0.001 up to September 2012.
| Adverse drug reaction*,** | Events (N = 129), n (%) | Patients (N = 88), n | Incidence (patient-years† = 2851.16) |
|---|---|---|---|
| Headache | 30 (23.3) | 25 | 0.00877 |
| Hypothyroidism | 11 (8.5) | 10 | 0.00351 |
| Arthralgia | 7 (5.4) | 7 | 0.00246 |
| Pain in extremity | 9 (7.0) | 7 | 0.00246 |
| Injection-site haematoma | 5 (3.0) | 5 | 0.00175 |
| Decreased glucose tolerance | 12 (9.3) | 5 | 0.00175 |
| Asthenia | 3 (2.3) | 3 | 0.00105 |
| Injection-site pain | 3 (2.3) | 3 | 0.00105 |
| Increased blood creatine phosphokinase | 4 (3.1) | 3 | 0.00105 |
| Myalgia | 3 (2.3) | 3 | 0.00105 |
Preferred term/MeDRA dictionary.
Mild or moderate.
Until cut-off date.
To date there have been no confirmed cases of type 1 or type 2 diabetes that occurred under somatropin treatment, and no malignancy related to somatropin treatment has been reported. A total of 69 tests for anti-hGH antibodies have been performed in 30 patients; no antibodies have been detected so far.
A total of 285 patients discontinued treatment, for the following reasons: patient completed treatment successfully (n = 87; one patient was referred to an adult endocrinologist); slow down of HV to <1 cm/year (n = 15); patient was satisfied with current height (n = 23); patient did not wish to continue with injections (n = 23); noncompliance (n = 17); patient was switched to another GH product (n = 31); patient withdrew consent (n = 1); AE (n = 6); other/unknown (n = 82).
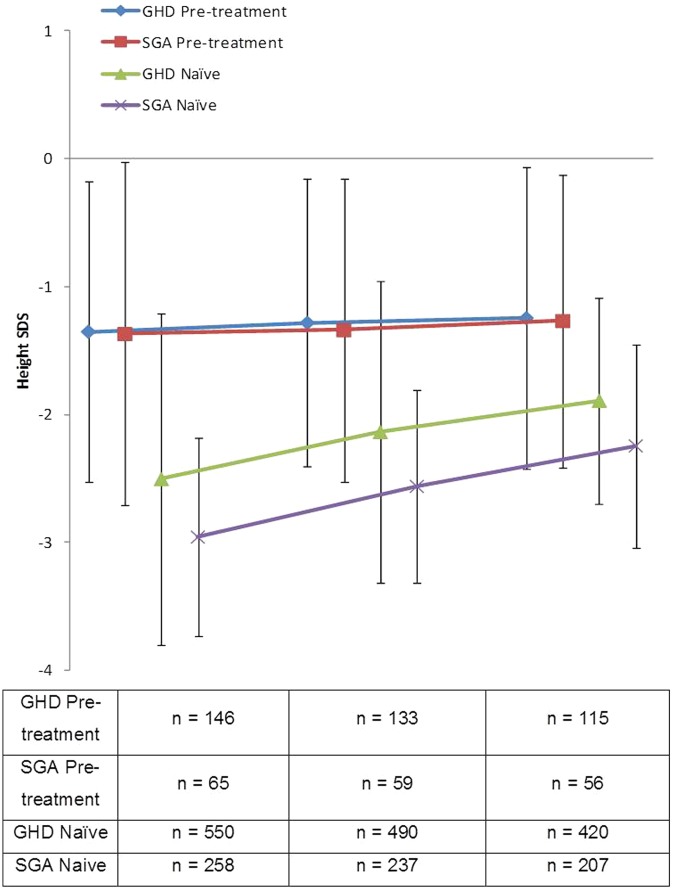
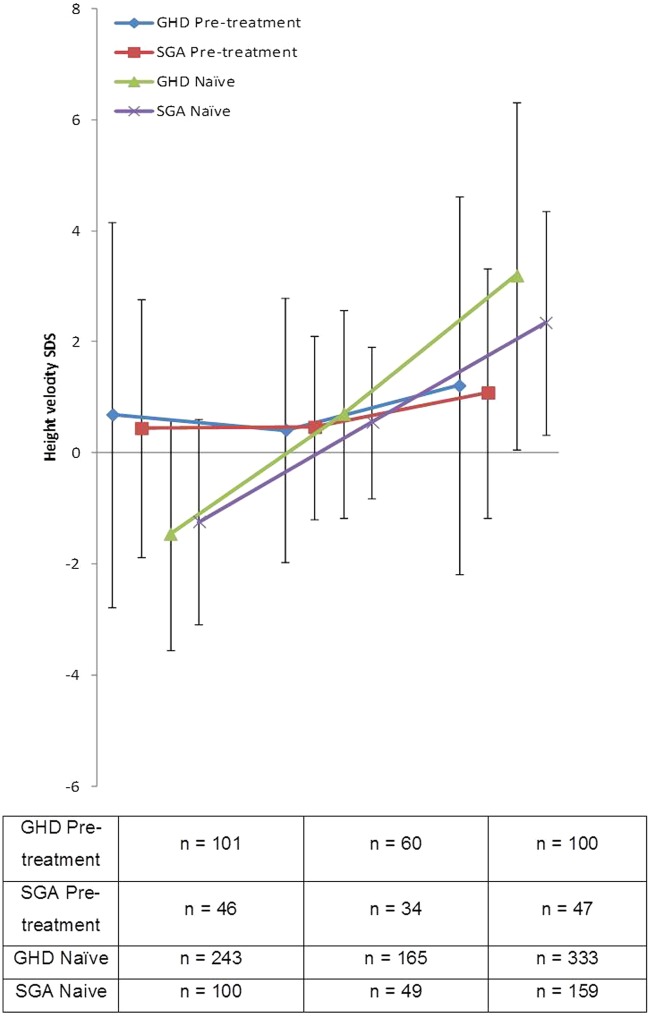
Figures 1 and 2 show the change in HSDS and HVSDS, respectively, from baseline to 12 months for the GHD and SGA populations. As expected, somatropin provided benefits in both patient groups; treatment-naïve patients showed a larger response to somatropin during the first 6 months compared with pretreated patients.
Figure 1.
Height SDS for pretreated and hormone-naïve GHD and SGA patients following 1 year of treatment with Omnitrope®.
Figure 2.
Height velocity SDS for pretreated and hormone-naïve GHD and SGA patients following 1 year of treatment with Omnitrope®.
Discussion
PATRO Children is a noninterventional postmarketing surveillance designed to record the safety and efficacy of long-term somatropin treatment in infants, children and adolescents in routine clinical practice. Recombinant hGH treatment has been shown to induce transient resistance to the actions of insulin in children, raising concern over the diabetogenic potential of this therapy in individuals predisposed to metabolic abnormalities (such as children born SGA); epidemiological data suggest that children born SGA may indeed be at increased risk of insulin resistance and type 2 diabetes in later life [McCance et al. 1994; Curhan et al. 1996; Phillips, 1996; Lithell et al. 1996; Reynolds and Phillips, 1998; Rich-Edwards et al. 1999; Boyko, 2000]. Other studies point to a possible increased risk of type 2 diabetes in GH-treated children more generally [Cutfield et al. 2000; Child et al. 2011]. Another potential concern with GH therapy, given that both GH and IGF-1 have mitogenic properties, is the risk of malignant disease. The PATRO Children study, with its large patient cohort and extended duration of follow up, will provide important information on the diabetogenic potential of rhGH in children born SGA, as well as the risk of malignancies in all patients.
Another important aspect of PATRO Children will be its contribution to data in patients with PWS. This is a relatively rare condition and it is anticipated that the study will enrol a sufficient number of patients with PWS to provide useful information on AEs with a possible causal relationship to rhGH treatment and which are unexpected and/or unique to this population.
The development of anti-hGH antibodies in response to rhGH treatment that cause therapeutic failure is very rare. Still, the evaluation of the occurrence and clinical implications of anti-hGH antibodies in a large population that includes patients with different indications will further contribute to the understanding of the relevance of these antibodies in the treatment of growth disorders.
Interventional studies have already demonstrated that somatropin is efficacious and safe in infants, children and adolescents with GHD [Romer et al. 2009; López-Siguero et al. 2011]. Based on our preliminary data, the efficacy of somatropin in PATRO Children is consistent with that observed in previous phase III studies with somatropin in patients with GHD, and the product also appears to be effective in other indications that were not required to be studied as part of the product registration clinical trial programme (such as TS, CRI, SGA and PWS). Further, there has been no signal of increased diabetogenic potential, increased risk of malignancies or increased risk of developing anti-hGH antibodies. Comparison of PATRO Children data with 1-year registry data for the reference product is not straightforward, as a result of the large number of different cohorts studied and different analyses conducted. However, based on available data the 1-year data from the two registries appear to be similar [Ranke and Guilbaud, 1990; Ranke and Lindberg, 2003; Ranke et al. 2012; Westphal and Lindberg, 2008].
In summary, the efficacy and safety profile of somatropin in the PATRO Children study so far are as expected. The ongoing study will extend the safety database for somatropin, and rhGH products more generally, in paediatric indications. Of particular interest, PATRO Children will provide important information on the diabetogenic potential of rhGH in children born SGA, the risk of malignancies in children receiving rhGH, and AEs with a possible causal relationship to rhGH treatment in children with PWS.
Acknowledgments
Medical writing assistance in the preparation of this paper was provided by Tony Reardon of Spirit Medical Communications Ltd and funded by Sandoz Biopharmaceuticals (Sandoz International GmbH).
Footnotes
Funding: This study is funded by Sandoz Biophar- maceuticals.
Conflict of interest statement: Ellen Schuck and Markus Zabransky are employees of Sandoz.
Contributor Information
Roland Pfäffle, Department of Paediatric Endocrinology, University of Leipzig Medical School, Leipzig, Germany.
Karl Otfried Schwab, Department of Paediatric Diabetology and Endocrinology, University Clinic Freiburg, Freiburg, Germany.
Otilia Marginean, Department of Paediatrics, University of Medicine and Pharmacy “Victor Babes”, Timisoara, Romania.
Mieczyslaw Walczak, Department of Paediatric Endocrinology and Diabetology, Pomeranian Medical University, Szczecin, Poland.
Mieczyslaw Szalecki, Department of Endocrinology and Diabetology, Children’s Health Research Institute, Warsaw, Poland, and Faculty of Health Science, UJK, Kielce, Poland.
Ellen Schuck, Sandoz International GmbH, Holzkirchen, Germany.
Markus Zabransky, Sandoz International GmbH, Industriestr. 25, D-83607 Holzkirchen, Germany.
Stefano Zucchini, Department of Paediatrics, S. Orsola-Malpighi Hospital, University of Bologna, Bologna, Italy.
References
- American Society of Clinical Endocrinologists (2003) Medical guidelines for clinical practice for growth hormone use in adults and children. 2003 update. Endocr Pract 9: 64–76 [DOI] [PubMed] [Google Scholar]
- Bondy C. for the Turner Syndrome Consensus Study Group (2007) Clinical practice guidelines – care of girls and women with Turner Syndrome: a guideline of the Turner Syndrome Study Group. J Clin Endocrinol Metab 92: 10–25 [DOI] [PubMed] [Google Scholar]
- Boyko E. (2000) Proportion of type 2 diabetes cases resulting from impaired fetal growth. Diabetes Care 23: 1260–1264 [DOI] [PubMed] [Google Scholar]
- Child C., Zimmermann A., Scott R., Cutler G., Jr, Battelino T., Blum W. for the GeNeSIS International Advisory Board (2011) Prevalence and incidence of diabetes mellitus in GH-treated children and adolescents: analysis from the GeNeSIS observational research program. J Clin Endocrinol Metab 96: E1025–E1034 [DOI] [PubMed] [Google Scholar]
- Clayton P., Cuneo R., Juul A., Monson J., Shalet S., Tauber M. for the European Society of Paediatric Endocrinology (2005) Consensus statement on the management of the GH-treated adolescent in the transition to adult care. Eur J Endocrinol 152: 165–170 [DOI] [PubMed] [Google Scholar]
- Curhan G., Willett W., Rimm E., Spiegelman D., Ascherio A., Stampfer M. (1996) Birth weight and adult hypertension, diabetes mellitus and obesity in US men. Circulation 94: 3246–3250 [DOI] [PubMed] [Google Scholar]
- Cutfield W., Wilton P., Bennmarker H., Albertsson-Wikland K., Chatelain P., Ranke M., et al. (2000) Incidence of diabetes mellitus and impaired glucose tolerance in children and adolescents receiving growth-hormone treatment. Lancet 355: 610–613 [DOI] [PubMed] [Google Scholar]
- EMA (2008) Omnitrope® European Public Assessment Report. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000607/WC500043692.pdf [accessed 4th December 2012].
- EMA (2011) Omnitrope® Summary of Product Characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000607/WC500043695.pdf [accessed 4th December 2012].
- Eypasch E., Lefering R., Kum C., Troidl H. (1995) Probability of adverse events that have not yet occurred: a statistical reminder. Br Med J 311: 619–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GH Research Society (2000) Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: summary statement of the GH Research Society. J Clin Endocrinol Metab 85: 3990–3993 [DOI] [PubMed] [Google Scholar]
- Jovanovic B., Levey P. (1997) A look at the rule of three. Am Stat 51: 137–139 [Google Scholar]
- Kirk J., Butler G. on behalf of the British Society for Paediatric Endocrinology and Diabetes (BSPED) (2006) Treatment of children with recombinant human growth hormone (r-HGH): shared care guidelines, April 2006 [Google Scholar]
- Lee P., Allen D., Angulo M., Cappa M., Carrel A., Castro-Magana M., et al. (2000) Consensus statement – Prader–Willi Syndrome: growth hormone (GH)/insulin-like growth factor axis deficiency and GH treatment. Endocrinologist 10 (Suppl1): 71–74 [Google Scholar]
- Lee P., Chernausek S., Hokken-Koelega A., Czernichow P. for the International Small for Gestational Age Advisory Board (2003) International Small for Gestational Age Advisory Board consensus development conference statement: management of short children born small for gestational age, April 24–October 1, 2001. Pediatrics 111: 1253–1261 [DOI] [PubMed] [Google Scholar]
- Lithell H., McKeigue P., Berglund L., Mohsen R., Lithell U., Leon D. (1996) Relation of size at birth to non-insulin dependent diabetes and insulin concentration in men aged 50–60 years. Br Med J 312: 406–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Siguero J., Borrás Pérez M., Balser S., Khan-Boluki J. (2011) Long-term safety and efficacy of the recombinant human growth hormone Omnitrope® in the treatment of Spanish growth hormone deficient children: results of a phase III study. Adv Ther 28: 879–893 [DOI] [PubMed] [Google Scholar]
- Mahan J., Warady B. for the Consensus Committee (2006) Assessment and treatment of short stature in pediatric patients with chronic kidney disease: a consensus statement. Pediatr Nephrol 21: 917–930 [DOI] [PubMed] [Google Scholar]
- McCance D., Pettitt D., Hanson R., Jacobsson L., Knolwer W., Bennett P. (1994) Birth weight and non-insulin dependent diabetes: thrifty genotype, thrifty phenotype, or surviving small baby genotype? Br Med J 308: 942–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. (1996) Insulin resistance as a programmed response to fetal undernutrition. Diabetologia 39: 1119–1122 [DOI] [PubMed] [Google Scholar]
- Ranke M., Guilbaud O. (1990) Growth response in prepubertal children with idiopathic growth hormone deficiency during the first year of treatment with human growth hormone. Analysis of the Kabi International Growth Study. Acta Paediatr Scand (Suppl) 370: 122–130 [DOI] [PubMed] [Google Scholar]
- Ranke M., Lindberg A. (2003) Early-onset idiopathic growth hormone deficiency within KIGS. Horm Res 60(Suppl. 1): 18–21 [DOI] [PubMed] [Google Scholar]
- Ranke M., Lindberg A., Brosz M., Kaspers S., Loftus J., Wollmann H., et al. (2012) Accurate long-term prediction of height during the first four years of growth hormone treatment in prepubertal children with growth hormone deficiency or Turner Syndrome. Horm Res Paediatr 78: 8–17 [DOI] [PubMed] [Google Scholar]
- Reynolds R., Phillips D. (1998) Long-term consequences of intrauterine growth retardation. Horm Res 49(Suppl. 2): 28–31 [PubMed] [Google Scholar]
- Rich-Edwards J., Colditz G., Stampfer M., Willett W., Gillman M., Hennekens C., et al. (1999) Birth weight and the risk for type 2 diabetes mellitus in adult women. Ann Intern Med 130: 278–284 [DOI] [PubMed] [Google Scholar]
- Romer T., Saenger P., Peter F., Walczak M., Le Bouc Y., Khan-Boluki J., et al. (2009) Seven years of safety and efficacy of the recombinant human growth hormone Omnitrope in the treatment of growth hormone deficient children: results of a phase III study. Horm Res 72: 359–369 [DOI] [PubMed] [Google Scholar]
- Westphal O., Lindberg A. (2008) final height in Swedish children with idiopathic growth hormone deficiency enrolled in KIGS treated optimally with growth hormone. Acta Paediatrica 97: 1698–1706 [DOI] [PubMed] [Google Scholar]