Abstract
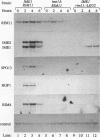
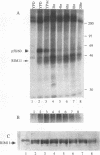
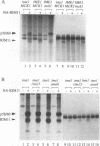
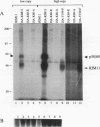
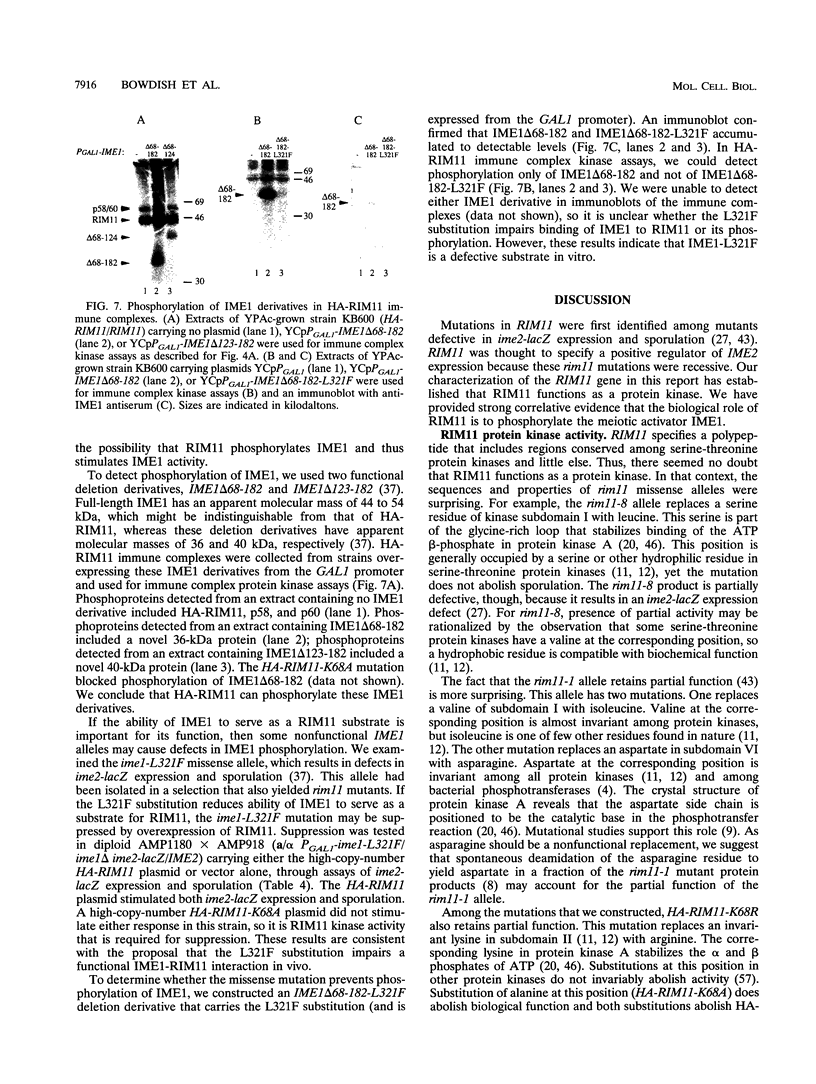
Many yeast genes that are essential for meiosis are expressed only in meiotic cells. Known regulators of early meiotic genes include IME1, which is required for their expression, and SIN3 and UME6, which prevent their expression in nonmeiotic cells. We report here the molecular characterization of the RIM11 gene, which we find is required for expression of several early meiotic genes. A close functional relationship between RIM11 and IME1 is supported by two observations. First, sin3 and ume6 mutations are epistatic to rim11 mutations; prior studies have demonstrated their epistasis to ime1 mutations. Second, overexpression of RIM11 can suppress an ime1 missense mutation (ime1-L321F) but not an ime1 deletion. Sequence analysis indicates that RIM11 specifies a protein kinase related to rat glycogen synthase kinase 3 and the Drosophila shaggy/zw3 gene product. Three partially defective rim11 mutations alter residues involved in ATP binding or catalysis, and a completely defective rim11 mutation alters a tyrosine residue that corresponds to the site of an essential phosphorylation for glycogen synthase kinase 3. Immune complexes containing a hemagglutinin (HA) epitope-tagged RIM11 derivative, HA-RIM11, phosphorylate two proteins, p58 and p60, whose biological function is undetermined. In addition, HA-RIM11 immune complexes phosphorylate a functional IME1 derivative but not the corresponding ime1-L321F derivative. We propose that RIM11 stimulates meiotic gene expression through phosphorylation of IME1.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianchi M. W., Plyte S. E., Kreis M., Woodgett J. R. A Saccharomyces cerevisiae protein-serine kinase related to mammalian glycogen synthase kinase-3 and the Drosophila melanogaster gene shaggy product. Gene. 1993 Nov 30;134(1):51–56. doi: 10.1016/0378-1119(93)90173-z. [DOI] [PubMed] [Google Scholar]
- Bowdish K. S., Mitchell A. P. Bipartite structure of an early meiotic upstream activation sequence from Saccharomyces cerevisiae. Mol Cell Biol. 1993 Apr;13(4):2172–2181. doi: 10.1128/mcb.13.4.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham L. E., Wang H. T., Elder R. T., McCarroll R. M., Slater M. R., Esposito R. E. Nucleotide sequence and promoter analysis of SPO13, a meiosis-specific gene of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9406–9410. doi: 10.1073/pnas.87.23.9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields S., Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989 Jul 20;340(6230):245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- Geiger T., Clarke S. Deamidation, isomerization, and racemization at asparaginyl and aspartyl residues in peptides. Succinimide-linked reactions that contribute to protein degradation. J Biol Chem. 1987 Jan 15;262(2):785–794. [PubMed] [Google Scholar]
- Gibbs C. S., Zoller M. J. Rational scanning mutagenesis of a protein kinase identifies functional regions involved in catalysis and substrate interactions. J Biol Chem. 1991 May 15;266(14):8923–8931. [PubMed] [Google Scholar]
- Gonzalez G. A., Montminy M. R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989 Nov 17;59(4):675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M. Protein kinase catalytic domain sequence database: identification of conserved features of primary structure and classification of family members. Methods Enzymol. 1991;200:38–62. doi: 10.1016/0076-6879(91)00126-h. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. Life cycle of the budding yeast Saccharomyces cerevisiae. Microbiol Rev. 1988 Dec;52(4):536–553. doi: 10.1128/mr.52.4.536-553.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth N. M., Goetsch L., Byers B. The HOP1 gene encodes a meiosis-specific component of yeast chromosomes. Cell. 1990 Apr 6;61(1):73–84. doi: 10.1016/0092-8674(90)90216-2. [DOI] [PubMed] [Google Scholar]
- Honigberg S. M., McCarroll R. M., Esposito R. E. Regulatory mechanisms in meiosis. Curr Opin Cell Biol. 1993 Apr;5(2):219–225. doi: 10.1016/0955-0674(93)90106-z. [DOI] [PubMed] [Google Scholar]
- Hughes K., Nikolakaki E., Plyte S. E., Totty N. F., Woodgett J. R. Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. EMBO J. 1993 Feb;12(2):803–808. doi: 10.1002/j.1460-2075.1993.tb05715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao G., Shah J. C., Clancy M. J. An RME1-independent pathway for sporulation control in Saccharomyces cerevisiae acts through IME1 transcript accumulation. Genetics. 1990 Dec;126(4):823–835. doi: 10.1093/genetics/126.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassir Y., Granot D., Simchen G. IME1, a positive regulator gene of meiosis in S. cerevisiae. Cell. 1988 Mar 25;52(6):853–862. doi: 10.1016/0092-8674(88)90427-8. [DOI] [PubMed] [Google Scholar]
- Knighton D. R., Zheng J. H., Ten Eyck L. F., Ashford V. A., Xuong N. H., Taylor S. S., Sowadski J. M. Crystal structure of the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991 Jul 26;253(5018):407–414. doi: 10.1126/science.1862342. [DOI] [PubMed] [Google Scholar]
- Koff A., Giordano A., Desai D., Yamashita K., Harper J. W., Elledge S., Nishimoto T., Morgan D. O., Franza B. R., Roberts J. M. Formation and activation of a cyclin E-cdk2 complex during the G1 phase of the human cell cycle. Science. 1992 Sep 18;257(5077):1689–1694. doi: 10.1126/science.1388288. [DOI] [PubMed] [Google Scholar]
- Law D. T., Segall J. The SPS100 gene of Saccharomyces cerevisiae is activated late in the sporulation process and contributes to spore wall maturation. Mol Cell Biol. 1988 Feb;8(2):912–922. doi: 10.1128/mcb.8.2.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luche R. M., Smart W. C., Marion T., Tillman M., Sumrada R. A., Cooper T. G. Saccharomyces cerevisiae BUF protein binds to sequences participating in DNA replication in addition to those mediating transcriptional repression (URS1) and activation. Mol Cell Biol. 1993 Sep;13(9):5749–5761. doi: 10.1128/mcb.13.9.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone R. E. Dual regulation of meiosis in yeast. Cell. 1990 May 4;61(3):375–378. doi: 10.1016/0092-8674(90)90517-i. [DOI] [PubMed] [Google Scholar]
- Mitchell A. P., Bowdish K. S. Selection for early meiotic mutants in yeast. Genetics. 1992 May;131(1):65–72. doi: 10.1093/genetics/131.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A. P. Control of meiotic gene expression in Saccharomyces cerevisiae. Microbiol Rev. 1994 Mar;58(1):56–70. doi: 10.1128/mr.58.1.56-70.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A. P., Driscoll S. E., Smith H. E. Positive control of sporulation-specific genes by the IME1 and IME2 products in Saccharomyces cerevisiae. Mol Cell Biol. 1990 May;10(5):2104–2110. doi: 10.1128/mcb.10.5.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neigeborn L., Mitchell A. P. The yeast MCK1 gene encodes a protein kinase homolog that activates early meiotic gene expression. Genes Dev. 1991 Apr;5(4):533–548. doi: 10.1101/gad.5.4.533. [DOI] [PubMed] [Google Scholar]
- Puziss J. W., Hardy T. A., Johnson R. B., Roach P. J., Hieter P. MDS1, a dosage suppressor of an mck1 mutant, encodes a putative yeast homolog of glycogen synthase kinase 3. Mol Cell Biol. 1994 Jan;14(1):831–839. doi: 10.1128/mcb.14.1.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach P. J. Multisite and hierarchal protein phosphorylation. J Biol Chem. 1991 Aug 5;266(22):14139–14142. [PubMed] [Google Scholar]
- Rose M. D., Novick P., Thomas J. H., Botstein D., Fink G. R. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60(2-3):237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- Shah J. C., Clancy M. J. IME4, a gene that mediates MAT and nutritional control of meiosis in Saccharomyces cerevisiae. Mol Cell Biol. 1992 Mar;12(3):1078–1086. doi: 10.1128/mcb.12.3.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shero J. H., Hieter P. A suppressor of a centromere DNA mutation encodes a putative protein kinase (MCK1). Genes Dev. 1991 Apr;5(4):549–560. doi: 10.1101/gad.5.4.549. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. E., Driscoll S. E., Sia R. A., Yuan H. E., Mitchell A. P. Genetic evidence for transcriptional activation by the yeast IME1 gene product. Genetics. 1993 Apr;133(4):775–784. doi: 10.1093/genetics/133.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. E., Mitchell A. P. A transcriptional cascade governs entry into meiosis in Saccharomyces cerevisiae. Mol Cell Biol. 1989 May;9(5):2142–2152. doi: 10.1128/mcb.9.5.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. E., Su S. S., Neigeborn L., Driscoll S. E., Mitchell A. P. Role of IME1 expression in regulation of meiosis in Saccharomyces cerevisiae. Mol Cell Biol. 1990 Dec;10(12):6103–6113. doi: 10.1128/mcb.10.12.6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns T., Hoyt M. A., Botstein D. Yeast mutants sensitive to antimicrotubule drugs define three genes that affect microtubule function. Genetics. 1990 Feb;124(2):251–262. doi: 10.1093/genetics/124.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strich R., Slater M. R., Esposito R. E. Identification of negative regulatory genes that govern the expression of early meiotic genes in yeast. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10018–10022. doi: 10.1073/pnas.86.24.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strich R., Surosky R. T., Steber C., Dubois E., Messenguy F., Esposito R. E. UME6 is a key regulator of nitrogen repression and meiotic development. Genes Dev. 1994 Apr 1;8(7):796–810. doi: 10.1101/gad.8.7.796. [DOI] [PubMed] [Google Scholar]
- Su S. S., Mitchell A. P. Identification of functionally related genes that stimulate early meiotic gene expression in yeast. Genetics. 1993 Jan;133(1):67–77. doi: 10.1093/genetics/133.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S. S., Mitchell A. P. Molecular characterization of the yeast meiotic regulatory gene RIM1. Nucleic Acids Res. 1993 Aug 11;21(16):3789–3797. doi: 10.1093/nar/21.16.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. S., Knighton D. R., Zheng J., Sowadski J. M., Gibbs C. S., Zoller M. J. A template for the protein kinase family. Trends Biochem Sci. 1993 Mar;18(3):84–89. doi: 10.1016/0968-0004(93)80001-r. [DOI] [PubMed] [Google Scholar]
- Treinin M., Simchen G. Mitochondrial activity is required for the expression of IME1, a regulator of meiosis in yeast. Curr Genet. 1993 Mar;23(3):223–227. doi: 10.1007/BF00351500. [DOI] [PubMed] [Google Scholar]
- Tyers M., Tokiwa G., Futcher B. Comparison of the Saccharomyces cerevisiae G1 cyclins: Cln3 may be an upstream activator of Cln1, Cln2 and other cyclins. EMBO J. 1993 May;12(5):1955–1968. doi: 10.1002/j.1460-2075.1993.tb05845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vershon A. K., Hollingsworth N. M., Johnson A. D. Meiotic induction of the yeast HOP1 gene is controlled by positive and negative regulatory sites. Mol Cell Biol. 1992 Sep;12(9):3706–3714. doi: 10.1128/mcb.12.9.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal M., Gaber R. F. RPD3 encodes a second factor required to achieve maximum positive and negative transcriptional states in Saccharomyces cerevisiae. Mol Cell Biol. 1991 Dec;11(12):6317–6327. doi: 10.1128/mcb.11.12.6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal M., Strich R., Esposito R. E., Gaber R. F. RPD1 (SIN3/UME4) is required for maximal activation and repression of diverse yeast genes. Mol Cell Biol. 1991 Dec;11(12):6306–6316. doi: 10.1128/mcb.11.12.6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. T., Frackman S., Kowalisyn J., Esposito R. E., Elder R. Developmental regulation of SPO13, a gene required for separation of homologous chromosomes at meiosis I. Mol Cell Biol. 1987 Apr;7(4):1425–1435. doi: 10.1128/mcb.7.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Clark I., Nicholson P. R., Herskowitz I., Stillman D. J. The Saccharomyces cerevisiae SIN3 gene, a negative regulator of HO, contains four paired amphipathic helix motifs. Mol Cell Biol. 1990 Nov;10(11):5927–5936. doi: 10.1128/mcb.10.11.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodgett J. R. A common denominator linking glycogen metabolism, nuclear oncogenes and development. Trends Biochem Sci. 1991 May;16(5):177–181. doi: 10.1016/0968-0004(91)90071-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. K., Gonzalez G. A., Menzel P., Rivier J., Montminy M. R. Characterization of a bipartite activator domain in transcription factor CREB. Cell. 1990 Feb 23;60(4):611–617. doi: 10.1016/0092-8674(90)90664-z. [DOI] [PubMed] [Google Scholar]
- Yang X., Hubbard E. J., Carlson M. A protein kinase substrate identified by the two-hybrid system. Science. 1992 Jul 31;257(5070):680–682. doi: 10.1126/science.1496382. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Elledge S. J. DUN1 encodes a protein kinase that controls the DNA damage response in yeast. Cell. 1993 Dec 17;75(6):1119–1127. doi: 10.1016/0092-8674(93)90321-g. [DOI] [PubMed] [Google Scholar]