Abstract
The antitumor effects of therapeutic mAbs may depend on immune effector cells that express FcRs for IgG. IL-12 is a cytokine that stimulates IFN-γ production from NK cells and T cells. We hypothesized that coadministration of IL-12 with a murine anti-HER2/neu mAb (4D5) would enhance the FcR-dependent immune mechanisms that contribute to its antitumor activity. Thrice-weekly therapy with IL-12 (1 μg) and 4D5 (1 mg/kg) significantly suppressed the growth of a murine colon adenocarcinoma that was engineered to express human HER2 (CT-26HER2/neu) in BALB/c mice compared with the result of therapy with IL-12, 4D5, or PBS alone. Combination therapy was associated with increased circulating levels of IFN-γ, monokine induced by IFN-γ, and RANTES. Experiments with IFN-γ–deficient mice demonstrated that this cytokine was necessary for the observed antitumor effects of therapy with IL-12 plus 4D5. Immune cell depletion experiments showed that NK cells (but not CD4+ or CD8+ T cells) mediated the antitumor effects of this treatment combination. Therapy of HER2/neu-positive tumors with trastuzumab plus IL-12 induced tumor necrosis but did not affect tumor proliferation, apoptosis, vascularity, or lymphocyte infiltration. In vitro experiments with CT-26HER2/neu tumor cells revealed that IFN-γ induced an intracellular signal but did not inhibit cellular proliferation or induce apoptosis. Taken together, these data suggest that tumor regression in response to trastuzumab plus IL-12 is mediated through NK cell IFN-γ production and provide a rationale for the coadministration of NK cell-activating cytokines with therapeutic mAbs.
HER2/neu is a member of the epidermal growth factor receptor family of receptor tyrosine kinases, which also includes HER1, HER3, and HER4 (1). HER2 is able to heterodimerize with other epidermal growth factor receptor family members to form high-affinity receptors for circulating ligands such as epidermal growth factor, amphiregulin, and neuregulin (2). HER2 overexpression is observed in multiple human malignancies, including breast, ovarian, and gastrointestinal tract cancers. In human breast cancer patients, HER2 overexpression is associated with decreased relapse-free and overall survival, increased incidence of lymph node metastasis, and altered sensitivity to chemotherapeutic regimens (3–5).
Trastuzumab (Herceptin) is a humanized mAb that binds to the extracellular domain of HER2. When combined with cytotoxic chemotherapy, trastuzumab induces clinical responses in 50–60% of women with metastatic disease and prolongs the survival of women who receive it as an adjuvant to surgery (6–8). The clinical activity of trastuzumab and other Abs directed against tumor Ags has largely been attributed to the direct, antiproliferative or proapoptotic effects of the Abs on the tumor cells. Possible mechanisms of action of trastuzumab include downregulation of HER2 protein expression (9), blockade of HER2 heterodimerization (10), initiation of G1 arrest, and induction of cyclin-dependent kinase inhibitors such as p27 (11). However, several studies have shown that the antitumor effects of trastuzumab were dependent on the presence of immune effector cells that bear FcγRs, such as NK cells (12, 13). These observations suggested that FcR-dependent immune mechanisms such as Ab-dependent cellular cytotoxicity (ADCC) and cytokine secretion might contribute to the antitumor activity of trastuzumab and implied that this activity could be enhanced by the coadministration of immune-enhancing adjuvants (14, 15).
IL-12 is produced by APCs and stimulates IFN-γ production from NK and T cells. In previous studies, we have shown that human NK cells costimulated with trastuzumab-coated tumor cells and IL-12 secreted >10-fold higher amounts of IFN-γ compared with those of NK cells stimulated with either agent alone (16). In a phase I trial where trastuzumab was administered with IL-12 to patients with HER2-overexpressing malignancies, favorable clinical outcomes were associated with NK cell production of IFN-γ and chemokines that could recruit CD8+ T cells (17). These results were confirmed in a follow-up phase I trial of trastuzumab, IL-12, and paclitaxel (18).
The goal of the current study was to elucidate further the role of NK cell-derived IFN-γ in the antitumor effects of combination therapy with IL-12 and an anti-HER2/neu Ab. We used a murine model of HER2-overexpressing adenocarcinoma to examine the mechanism of action of trastuzumab and IL-12 coadministration. We now demonstrate that the antitumor actions of trastuzumab are enhanced by IL-12 treatments and that this effect is dependent on NK cell production of IFN-γ.
Materials and Methods
Cytokines and Abs
Recombinant murine IL-12 was kindly provided by Wyeth Pharmaceuticals (Madison, NJ). 4D5, a murine mAb recognizing human HER2, was purchased from the National Cell Culture Center (Minneapolis, MN). Rabbit anti-asialo GM1 was purchased from Wako Pharmaceuticals (Richmond, VA). Rat anti-mouse CD4 (clone GK1.5) and CD8 (clone 2.43) depleting mAbs were purchased from the National Cell Culture Center.
Murine tumor model
Age-matched, female BALB/c mice (The Jackson Laboratory, Bar Harbor, ME) were injected s.c. on the right flank with 1 × 106 CT-26HER2/neu cells in 200 μl PBS. When the tumors had reached a volume of ~200 mm3 (5–7 d), mice were randomly allocated to treatment with PBS, 1 μg murine IL-12, 1 mg/kg 4D5, or 1 μg murine IL-12 plus 1 mg/kg 4D5 (n = 5 per group). All treatments were administered i.p. three times weekly. Tumor dimensions were measured daily with calipers, and tumor volume was calculated as follows: Tumor volume = 0.5 × [(large diameter) × (small diameter)2]. Treatment was continued until tumors had reached a diameter of 25 mm in any dimension (~3.5 wk) at which point the mice were euthanized in accordance with institutional policy. Mice were depleted of NK cells, CD8+ T cells, or CD4+ T cells via administration of specific Abs. Anti-asialo GM1 (for NK cell depletion), rat anti-mouse CD8 mAb, or rat anti-mouse CD4 mAb was administered i.p. on days −3, −1, +1, and +3 with respect to tumor inoculation and every 4 d thereafter. Mock-depleted mice received injections of isotype-matched control Abs. The efficiency of depletion was >98% as confirmed by flow cytometric analysis of peripheral blood and splenocytes. All protocols were approved by The Ohio State University Animal Care and Use Committee, and mice were treated in accordance with institutional guidelines for animal care. Of note, the growth of CT-26HER2/neu cells in BALB/c mice was identical to that of the CT-26 parent cell line, and their immunohistochemical analysis revealed no difference in immune cell infiltrate, indicating that host T cells did not respond to the presence of the human HER2 Ag on tumor cells (data not shown).
Proliferation assay
CT-26HER2/neu cells (2 × 105) were seeded in triplicate in 96-well plates with unsupplemented medium or medium containing 10 ng/ml murine IL-12, 100 μg/ml trastuzumab, or increasing concentrations of murine IFN-γ (1–100 ng/ml). Cells treated with DMSO served as a positive control. Cell proliferation was determined by the MTT assay as previously described (19).
Intracellular staining for tyrosine phosphorylation (p-STAT1) and flow cytometry for HER2/neu
CT-26HER2/neu cells were resuspended in 100 μl RPMI 1640 medium, treated with various doses of IFN-γ (1–100 ng/ml), fixed with Fix & Perm Reagent A (Caltag Laboratories, Burlingame, CA) for 2–3 min at room temperature, and then incubated at 4°C for 10 min in 3 ml cold methanol. Cells were then washed in flow buffer (PBS supplemented with 5% FBS) and permeabilized with 100 μl Fix & Perm Reagent B (Caltag Laboratories). Cells were incubated for 60 min at room temperature in Fix & Perm Reagent B containing Alexa Fluor 488 STAT1 (pTyr 701) mAb (BD Pharmingen) or an appropriate isotype control Ab. Cells were then washed with flow buffer, fixed in 1% formalin, and stored at 4°C until flow cytometric analysis. Analyses were performed as previously described using a Becton Dickinson FACSCalibur flow cytometer (Becton-Dickinson, San Jose, CA) (20). The binding of the 4D5 Ab to HER2/neu on the surface of CT-26HER2/neu tumor cells was confirmed by flow cytometry using 4D5 and a FITC-labeled rabbit anti-mouse secondary Ab.
Analysis of apoptosis via annexin V–propidium iodide staining
Phosphatidylserine exposure was assessed in CT-26HER2/neu tumor cells by flow cytometry using allophycocyanin–annexin V and propidium iodide (PI) (BD Pharmingen) as previously described (21).
Histopathologic and immunohistochemical analysis of tumor
Harvested tumors were immediately divided in half and snap frozen in liquid nitrogen with rapid transfer to −80°C or placed in PBS and transferred to 10% neutral buffered formalin for a minimum of 24 h. Fixed tumors were processed by routine methods, embedded in paraffin wax, sectioned into 4-μm slices using a standard microtome, attached to lysine-coated slides, and stained with H&E. Replicate sections were deparaffinized in xylene (two times, 10 min each at room temperature) and rehydrated by stepwise washes at decreasing ethanol/H2O ratios. Frozen sections were embedded in OCT medium, sectioned into 3-μm sections using a standard cryostat, and attached to lysine-coated slides. For immunohistochemistry of unstained paraffin or frozen tumor sections, endogenous peroxidase activity was blocked with dH2O (diH2O) containing 3% hydrogen peroxide for 5 min, followed by repeated rinses in diH2O. Ag retrieval was achieved in Dako’s target retrieval solution (Dako S1699) by heating slides in a steamer at 94°C for 30 min and cooling at room temperature for 15 min. After rinsing in diH2O, slides were incubated for 60 min with Abs specific for CD3 (DakoCytomation A0452), CD4 (BD Pharmingen clone H129.19), CD8 (BD Pharmingen clone 53-6.7), CD45 (BD Pharmingen clone 30-F11), CD68 (Serotec MCA 1957), CD31 (BD Pharmingen clone MEC 13.3), and Ki67 (Dako clone MIB-1) or appropriate isotype control Abs. Detection was achieved with the Vectastain Elite ABC system and Novared Chromogen (Vector, Burlingame, CA). Evaluation of apoptosis in formalin-fixed, paraffin-embedded tumor xenografts was conducted using TUNEL as recommended by the manufacturer (Chemicon). All samples were examined in a blinded fashion by an experienced pathologist (K.M.L. or T.L.P.) using an Olympus BX45 light microscope with an attached DP25 digital camera (B & B Microscopes limited, Center Valley, PA). For each tumor, the percentage of necrosis for the entire section and the number of mitotic figures in three random ×40 fields were assessed in H&E slides. The binding of each Ab to target Ag was determined in viable areas of the tumor based on the following semiquantitative scoring system: 0, none; 1, few (<25%); 2, many (25–75%); 3, majority (>75%). Tumor samples were compared with the appropriate positive control slides as well as with internal controls. Immunofluorescence microscopy of tumor sections was performed using 10 μg/ml of an anti-CD335/NKp46 (BD Pharmingen) primary Ab and 1:2500 goat anti-rat–Alexa Fluor 647 secondary Ab (Invitrogen) using a technique adapted from Eisenring et al. (22). Tumor sections were counterstained with DAPI for nuclear identification. Stained sections were analyzed on an Olympus Fluoview 1000 Laser Scanning Confocal microscope.
Electron microscopy
Harvested tumors were fixed in 3% glutaraldehyde and 4% paraformaldehyde, postfixed in 4% osmium tetroxide, and embedded in EPON. Thin sections were stained with uranyl acetate and lead citrate and examined in an FEI Tecnai G2 Spirit transmission electron microscope.
Cytotoxicity assay
Splenocytes were harvested from the spleens of treated mice and mixed with CT-26HER2/neu target cells at an E:T ratio of 4:1 for 4 h. Target cell viability was assayed by flow cytometry for HER2 and PI with double-positive cells being classified as nonviable [modified from Cho et al. (23)].
Statistics
Changes in tumor volume over time were assessed via a longitudinal model. Tumor values were first log-transformed, and then a mixed effects model was applied to the data. Estimated slopes (changes in tumor volume over time) were calculated with 95% confidence intervals, and estimated differences in tumor volume were calculated at baseline and at the end of each study.
Results
Inhibition of tumor growth by IL-12 and an anti-HER2 Ab is associated with IFN-γ production
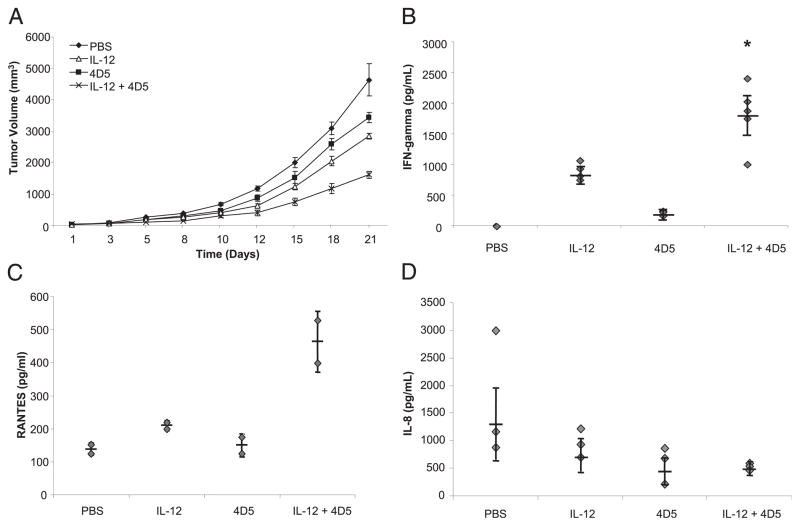
A murine tumor model was used to determine whether IL-12 could enhance the effect of an anti-tumor mAb in vivo. Mice bearing s.c. tumors of the CT-26HER2/neu cell line were treated three times weekly with PBS, 1 μg murine IL-12, 1 mg/kg 4D5 (a murine mAb recognizing human HER2), or IL-12 and 4D5 combined (Fig. 1A). These doses were chosen on the basis of titration experiments examining the efficacy of IL-12 or 4D5 alone against CT-26HER2/neu tumors (data not shown). Based on a longitudinal model using log-transformed values, no significant differences in tumor volume were found between the four groups at baseline. However, by day 21 of treatment, the average tumor volumes of mice receiving either IL-12 or 4D5 alone were significantly smaller than those of the PBS-treated mice (p <0.0001). Furthermore, the average tumor volumes for mice receiving the combination of IL-12 and 4D5 were significantly less than those for mice receiving IL-12 alone (p <0.005) or 4D5 alone (p <0.0001). Twenty-four hours after the final administration of IL-12 and 4D5, serum was harvested from each of the mice, and the levels of IFN-γ, RANTES, and IL-8 were measured by ELISA. As shown in Fig. 1B, mice receiving both IL-12 and 4D5 produced significantly higher amounts of IFN-γ than those of mice receiving IL-12 or 4D5 alone. Similarly, levels of RANTES in the peripheral blood were higher in the dual-therapy group compared with those of mice receiving either treatment alone (Fig. 1C). In contrast, the levels of IL-8 in the serum of mice receiving IL-12 and 4D5 were actually lower than those of mice receiving IL-12 or 4D5 alone (Fig. 1D), whereas the circulating levels of MIP-1α was minimal in all groups (data not shown). These data demonstrate that IL-12 can enhance the effects of a therapeutic mAb in a murine solid tumor model and that this effect was associated with increased production of IFN-γ and RANTES and reduced levels of IL-8. Notably, mice receiving IL-12 and/or 4D5 exhibited normal behavior, maintained their weight, and showed no evidence of organ injury as determined by pathologic examination of normal tissues (data not shown).
FIGURE 1.
IL-12 enhances the effects of an anti-HER2 mAb in a murine tumor model. A, Mice with s.c. CT-26HER2/neu tumors were treated i.p. with PBS, 1 μg IL-12, 1 mg/kg 4D5 (a murine mAb recognizing human HER2), or IL-12 plus 4D5 (same doses). Tumor volumes were calculated as described in Materials and Methods. Standard error was <5% for each data point shown. This experiment was repeated five times with similar results. B–D, Serum was harvested from each of the mice 24 h after the final administration of IL-12 and 4D5, and an ELISA technique was used to measure levels of the following cytokines: (B) IFN-γ, (C) RANTES, and (D) IL-8. *p <0.01 versus all conditions shown.
The antitumor effects of IL-12 and 4D5 are dependent on endogenous IFN-γ production
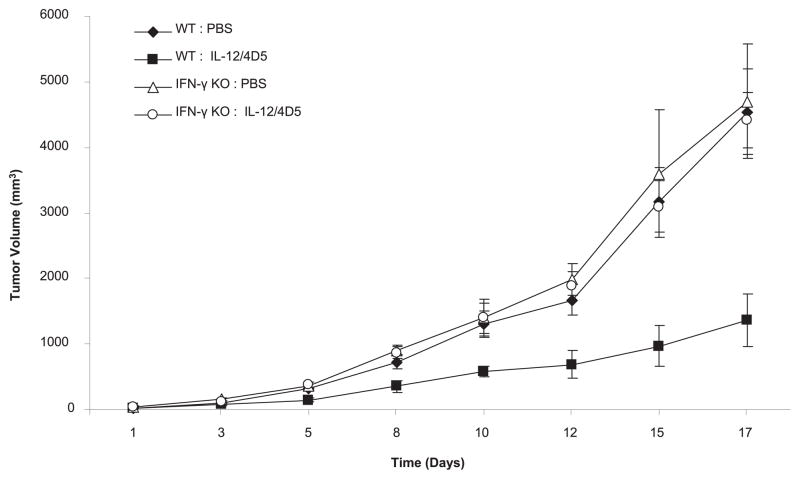
To determine whether the therapeutic efficacy of 4D5/IL-12 was dependent on the endogenous secretion of IFN-γ, wild-type and IFN-γ–deficient mice bearing CT-26HER2/neu tumors were treated with PBS or with IL-12 plus 4D5 (Fig. 2). By day 17, wild-type mice receiving IL-12 and 4D5 exhibited an approximate 70% reduction in tumor volume compared with that of wild-type mice receiving PBS, consistent with previous experiments (p <0.0001). In contrast, tumor growth in the IFN-γ–deficient mice receiving IL-12 plus 4D5 was not significantly different from tumor growth in the IFN-γ–deficient mice receiving PBS (p = 0.5481). Analysis of serum from each group of mice revealed IFN-γ production in wild-type mice receiving combination therapy but not in IFN-γ–deficient mice (data not shown). These data demonstrate that the therapeutic effects of IL-12 and 4D5 in a murine solid tumor model are dependent on the endogenous production of IFN-γ.
FIGURE 2.
Antitumor effects of IL-12 and 4D5 are dependent on IFN-γ. Wild-type or IFN-γ–deficient mice (IFN-γ−/−) bearing CT-26HER2/neu tumors were treated with PBS or the combination of IL-12 (1 μg) and 4D5 (1 mg/kg). Tumor volumes were calculated as described in Materials and Methods. Standard error was <5% for each data point shown. This experiment was repeated twice with similar results.
NK cells are the source of the IFN-γ required for the antitumor effect of IL-12 and 4D5
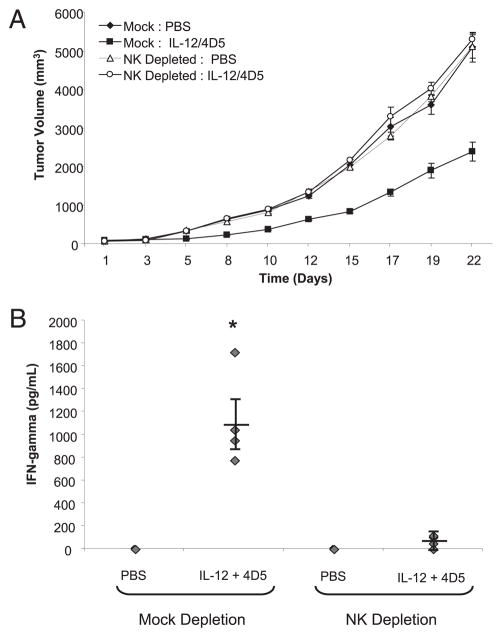
We next wanted to confirm that NK cells were the source of the IFN-γ that was detected in the serum of mice receiving IL-12 and 4D5. Tumor-bearing mice were depleted of NK cells via administration of an anti-asialo GM1 Ab and then treated with PBS or IL-12 plus 4D5. As observed previously, tumor growth was significantly inhibited in mock-depleted mice receiving IL-12 and 4D5. However, tumor growth in NK cell-depleted mice treated with IL-12 plus 4D5 was equivalent to that of mice receiving PBS (Fig. 3A). Twenty-four hours after the final administration of IL-12 and 4D5, serum IFN-γ levels were measured by ELISA. As expected, mock-depleted mice receiving IL-12 and 4D5 exhibited elevated levels of IFN-γ (p <0.001). However, little IFN-γ was detected in the serum of NK cell-depleted mice receiving IL-12 and 4D5 (Fig. 3B). Taken together, these data suggest that the antitumor effect of IL-12 and 4D5 is dependent on NK cells and their secretion of IFN-γ.
FIGURE 3.
Antitumor effects of IL-12 and 4D5 are dependent on NK cells. A, PBS or the combination of IL-12 and 4D5 was administered to CT-26HER2/neu tumor-bearing mice that had been depleted of NK cells via administration of anti-asialo GM1 (see Materials and Methods for depletion protocol). Mock-treated mice received an isotype-matched control Ab. This treatment resulted in >95% depletion of NK cells from the peripheral blood and spleen, as determined by flow cytometry (data not shown). B, Serum was harvested from each of the mice 24 h after the final dose of IL-12 and 4D5 and analyzed for IFN-γ content by ELISA. *p <0.001 versus all conditions shown. This experiment was repeated twice with similar results.
IFN-γ does not inhibit proliferation or induce apoptosis of CT-26HER2/neu tumor cells
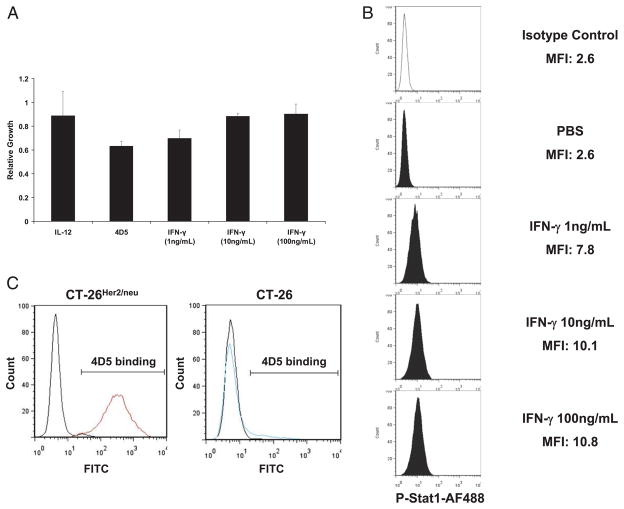
IFN-γ can inhibit proliferation and induce apoptosis in tumor cell lines in a STAT1-dependent manner (24). We were therefore interested in determining whether the IFN-γ secreted in response to IL-12 and 4D5 inhibited tumor growth via its direct effects on tumor cells or via its ability to activate host immune effector cells. Proliferation of CT-26HER2/neu tumor cells was assessed in vitro after treatment with trastuzumab, murine IL-12, or increasing concentrations of IFN-γ (1–100 ng/ml). As shown in Fig. 4A, proliferation of this cell line was not affected by these treatments. Flow cytometric analysis of CT-26HER2/neu cells stained with annexin V and PI also revealed that these manipulations failed to induce apoptosis (data not shown). Failure of IFN-γ to inhibit proliferation or induce apoptosis was not due to a defect in IFN-γ signal transduction, as treatment of CT-26HER2/neu cells with IFN-γ led to significant activation of STAT1 as determined by intracellular flow cytometry for p-STAT1 (Fig. 4B). The binding of 4D5 to HER2/neu on the surface of CT-26HER2/neu cells was confirmed by flow cytometry (Fig. 4C, left panel). Parental CT-26 cells did not express HER2/neu (Fig. 4C, right panel).
FIGURE 4.
IFN-γ does not inhibit proliferation of CT-26HER2/neu cells. A, CT-26HER2/neu cells were treated in vitro with IL-12, trastuzumab, or increasing concentrations of IFN-γ. Proliferation was determined by the MTT assay. B, CT-26HER2/neu cells were stimulated for 15 min in vitro with increasing concentrations of IFN-γ or PBS. The percentage of cells containing activated STAT1 was assessed by flow cytometry. The x-axis of each histogram represents the specific fluorescence of p-STAT1 on a four-decade logarithmic scale, and the y-axis represents the total number of events. C, CT-26HER2/neu cells and parental CT-26 cells were analyzed for the binding of 4D5 to cell surface HER2/neu by flow cytometry using the 4D5 Ab and an FITC-labeled rabbit anti-murine Ab.
The antitumor effects of IL-12 and 4D5 are not dependent on CD8+ or CD4+ T cells and are not associated with increased infiltration of tumors by lymphocytes
We were interested in determining whether T cells were involved in mediating the antitumor effects of IL-12 and 4D5. To address this question, we examined the effects of 4D5 and IL-12 in CT-26HER2/neu tumor-bearing BALB/c mice that had been depleted of either CD4+ T cells or CD8+ T cells via administration of anti-CD4+ and anti-CD8+ mAbs. However, there was no apparent difference in average tumor volumes between mice receiving 4D5 and IL-12 or PBS in this study (data not shown). In a previous phase I trial of trastuzumab and IL-12 conducted by our group, the response to therapy was associated with infiltration of tumor tissue by CD8+ T cells (17). Therefore, we examined tumor sections for the presence of tumor-infiltrating CD4+ and CD8+ T cells at varying times in CT-26HER2/neu tumor-bearing wild-type mice receiving PBS, IL-12, 4D5, or the combination of IL-12 and 4D5. All tumor sections examined exhibited minimal infiltration of tumor tissue by CD4+ or CD8+ T cells (Fig. 5A, 5B). Similar results were obtained with stains for CD3 (T cells), CD45 (lymphocytes), and CD68 (monocyte/macrophage). Also, depletion of CD4+ T cells and CD8+ T cells did not significantly inhibit the actions of 4D5 Ab when given in combination with IL-12 (data not shown). These data suggest that the regression of CT-26HER2/neu tumors after administration of IL-12 in combination with 4D5 was likely not the direct result of T cell-mediated immunity. We also explored whether the antitumor effect of combination therapy involved a more efficient cross-talk between NK cells and dendritic cells. However, flow cytometric analysis of splenocytes did not reveal a marked change in the number of CD11c+ dendritic cells or their activation status as measured by expression of CD80 and CD86 (data not shown).
FIGURE 5.

Lymphocyte infiltration of tumors is similar in the four treatment groups. CT-26HER2/neu tumors from mice treated with PBS, IL-12, 4D5, or a combination of both agents were stained for (A) CD4+ lymphocytes and (B) CD8+ lymphocytes. Representative tumor sections from each treatment group are shown with arrows depicting Novared positive CD4 and CD8 stained T cells. Original magnification ×40 (all fields).
The antitumor effect of combination therapy is associated with necrosis of tumor tissue
The absence of any differences between the treatment groups with respect to the infiltration of the tumor tissue with T cells, CD45+ lymphocytes (e.g., NK cells), and monocytes/macrophages, along with our experiments in IFN-γ–deficient mice suggested that the effects of this treatment might be primarily the result of some other antitumor action of IFN-γ. Therefore, we hypothesized that high levels of IFN-γ might exert a direct antitumor effect. However, an analysis of tumors from mice receiving PBS, IL-12, 4D5, or the combination treatment over time revealed that there was no statistical difference between these four conditions with respect to staining for a marker of proliferation (Ki67), apoptosis (TUNEL assay), or angiogenesis (CD31) (data not shown).
Microscopic analysis of murine tumors treated with 4D5 and IL-12
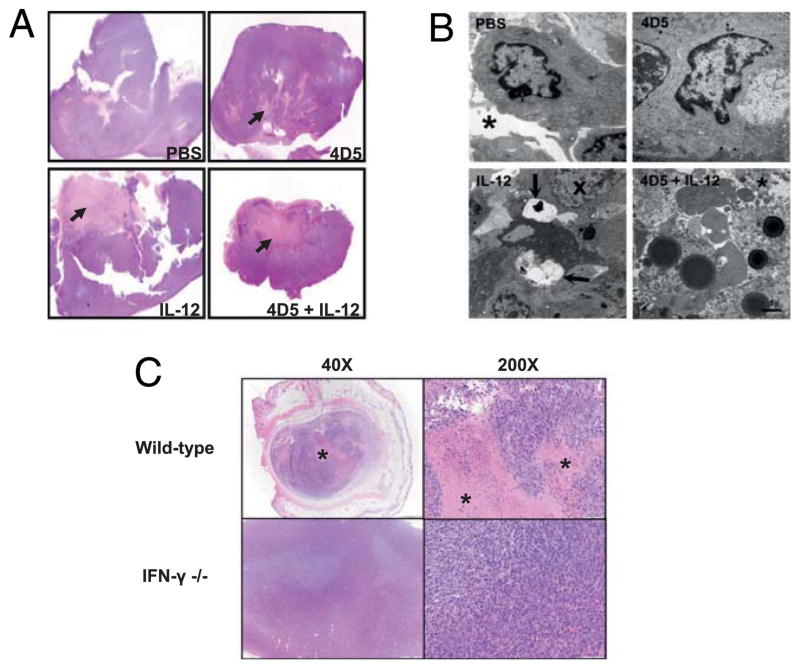
Electron microscopy of tumors from animals treated with PBS, 4D5, IL-12, or 4D5 plus IL-12 was performed to investigate the fine structure of tumors at 21 d. Analysis of these tumor tissues showed that the combination treatment group exhibited a significantly higher degree of necrosis compared with that of control conditions. In PBS-treated animals, tumors cells appeared healthy, and occasional dividing cells were observed. The extracellular space was clear of debris (Fig. 6B, asterisk). Tumors from mice treated with 4D5 had an appearance that was similar to that of tumors from the PBS-treated animals. The IL-12–treated tumors had many nuclei with distorted chromatin patterns and some cells with large clear auto-phagocytic vesicles. The tumors in animals treated with both 4D5 and IL-12 exhibited considerable extracellular debris, cell fragmentation, and accumulation of dense lipid vesicles. The overall appearance of these tumors suggested that a large amount of cell death had occurred prior to fixation with few intact cells remaining. These data indicated that tumor regression after combined treatment was associated with the onset of cell death at an early time point (Fig. 6A, 6B). Notably, administration of combination therapy to IFN-γ–deficient mice led to a marked reduction in the level of necrosis seen on histologic examination compared with that of wild-type mice (2 versus 15% necrosis, respectively; Fig. 6C). This finding is consistent with the work of other groups that have identified IFN-γ–induced necrosis as an important antitumor mechanism after IL-12 administration (25–27).
FIGURE 6.
Histopathology and electron microscopy analysis of tumor tissues. Tumor sections obtained on day 21 of the study were analyzed for necrosis by H&E staining and electron microscopy as described in Materials and Methods. A, A representative H&E-stained tumor section from mice treated with PBS, IL-12, 4D5, or IL-12 plus 4D5 is shown with arrows indicating regions of necrosis. Original magnification ×40 (all fields). B, Electron micrographs of tumors treated with PBS, IL-12, 4D5, or IL-12 plus 4D5. PBS-treated tumors contain dividing tumor cells surrounded by a clear extracellular space (asterisk). 4D5-treated tumors had similar characteristics. Tumor cells from IL-12–treated mice had nuclei with dispersed heterochromatin (X) and membrane containing autophagocytic vesicles (arrows). Tumors from mice receiving dual therapy exhibited high levels of extracellular debris, swollen mitochondria, and fragments of dead cells. Scale bar, 1 μm. C, Photomicrographs of H&E-stained day 15 tumor sections from wild-type and IFN-γ–deficient mice treated with IL-12 plus 4D5. Xenografts in treated knockout mice were smaller and exhibited minimal coagulation necrosis (asterisks).
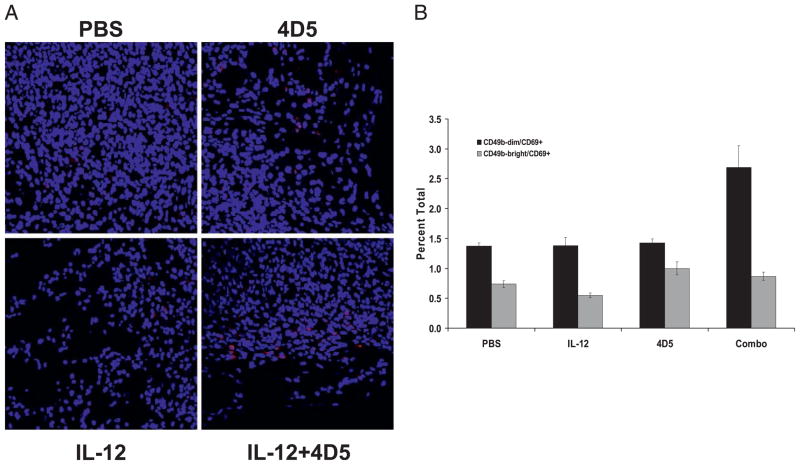
Analysis of NK cell activation status
A series of experiments was conducted to determine whether the combination treatment had led to increased activation of intra-tumoral NK cells and increased cytotoxic activity. Tumors were evaluated for the presence of NK cell activation markers (CD69, CD94, NKG2D, Ly49G2) by immunofluorescence microscopy. The expression of CD335 (an NK cytotoxicity receptor expressed exclusively on NK cells) (28) was increased in day 13 tumors from mice treated with IL-12 and anti-HER2 Ab (Fig. 7A). These markers were also measured in splenocytes from treated mice by flow cytometry. Mice receiving combination therapy exhibited a 2-fold increase in the number of CD69+ cells in the CD49bdim NK cell subset (Fig. 7B). A cytotoxicity assay was performed in which CT-26HER2/neu tumor cells were mixed with splenocytes from treated mice (4:1 E:T ratio). Target cell viability was assayed by flow cytometry for HER2/neu and PI with double-positive cells being classified as nonviable. The cytotoxic activity of splenocytes isolated from combination-treated mice against CT-26HER2/neu tumor cells was consistently elevated above that of the control conditions (27.2 versus 20.9% for the 4D5-treated group; 24.5% for IL-12; and 20.7% for PBS).
FIGURE 7.
Analysis of NK cell activation status. A, Immunofluorescent microscopy for CD335 (NKp46, an NK cell activation marker, in red) in tumors of treated mice. Tumor sections counterstained with DAPI for nuclear identification. Original magnification ×40. B, Flow cytometry for CD49b and CD69 in the splenocytes of treated mice.
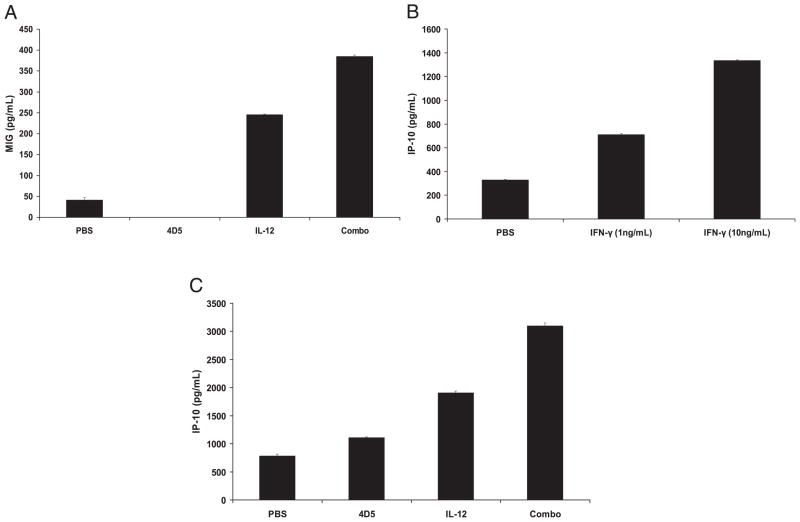
Expression of monokine induced by IFN-γ and IFN-γ–inducible protein 10
IFN-γ exerts anti-angiogenic effects via its ability to induce the secretion of IFN-γ–inducible protein 10 (IP-10) and monokine induced by IFN-γ (MIG). Circulating levels of MIG were significantly elevated in mice receiving the combination treatment compared with those of control treatments (p <0.05, Fig. 8A). It was believed that tumor cells were partially responsible for the production of these factors, as IFN-γ treatment of CT-26HER2/neu tumor cells led to increased production of IP-10 (Fig. 8B). Additionally, 48-h coculture of tumor cells with day 13 splenocytes isolated from combination-treated mice led to increased production of IP-10 and MIG compared with that using splenocytes from control-treated mice (Fig. 8C and data not shown). Taken together, these results indicate that combined therapy with IL-12 and 4D5 Ab was able to generate an anti-angiogenic environment characterized by the release of IP-10 and MIG.
FIGURE 8.
Expression of IFN-γ–induced anti-angiogenic factors. A, Circulating levels of MIG were measured by ELISA in the plasma of treated mice on day 13. B, Overnight culture of 2 × 105 CT-26HER2/neu tumor cells with IFN-γ (1 or 10 ng/ml) led to increased production of IP-10 in supernatants as measured by ELISA. C, Forty-eight hour coculture of tumor cells (5 × 104 cells) with day 13 splenocytes (2 × 105 cells) isolated from combination-treated mice led to increased production of IP-10 compared with that using splenocytes from control-treated mice.
Discussion
The goal of the current study was to elucidate further the role of NK cell-derived IFN-γ and the antitumor effects of combination therapy with IL-12 and an anti-HER2/neu Ab. We demonstrated that the antitumor effect of coadministration of IL-12 and 4D5 was associated with IFN-γ production by NK cells. Specifically, depletion of NK cells or elimination of IFN-γ abrogated the anti-tumor effects of the combined 4D5–IL-12 therapy, whereas depletion of CD4+ or CD8+ T cells had little effect. In vitro studies suggested that the antitumor effects of IFN-γ were not mediated via direct antiproliferative or proapoptotic effects on the CT-26HER2/neu tumor cell line, and careful analysis of tumors did not reveal a clear mechanism for the growth inhibition. However, increased tumor necrosis was observed in mice receiving the combination of 4D5 and IL-12. These results lend support to the concept that coadministration of cytokines could enhance the anti-tumor effects of mAb therapy.
Although it is now well accepted that FcR-dependent effects contribute to the antitumor actions of trastuzumab, most studies have attributed tumor regression to ADCC. Although NK cells mediate ADCC against HER2-positive cell lines in vitro, a role for trastuzumab-mediated ADCC in vivo has been difficult to prove (29). Furthermore, conflicting results have been obtained in studies that examined the correlation between ADCC and clinical outcome in patients receiving trastuzumab. Gennari et al. (30) reported that the ability of patient PBMCs to mediate ADCC against HER2-positive cell lines in vitro correlated with clinical outcome. Musolino et al. (31) showed that in trastuzumab-treated patients with metastatic breast cancer, the FcγRIIIa V/V polymorphism correlated with higher overall clinical responses, responses of longer duration, and more robust ADCC than those in patients who did not have the V/V genotype. In contrast, clinical outcome did not correlate with ADCC in patients enrolled in a phase I clinical trial of trastuzumab and IL-2 (32). We have also reported that ADCC did not correlate with clinical outcome in a phase I trial of trastuzumab and IL-12 (33) or in a follow-up phase I trial of trastuzumab and IL-12 with paclitaxel (18). This finding might reflect the inability of in vitro assays to capture the true extent of NK cell cytotoxic activity taking place at the level of the tumor microenvironment in human subjects. Of note, clinical outcome did correlate with serum IFN-γ levels of patients in the latter two studies. These findings suggest that NK cell IFN-γ production and ADCC might both be important antitumor mechanisms of anti-HER2 mAb therapy when administered in combination with cytokines such as IL-12.
The secretion of IFN-γ by NK cells in vivo after the administration of IL-12 and an anti-HER2/neu mAb led to tumor suppression, indicating that this cytokine is important to the antitumor effects of this treatment combination. The potential antitumor effects of IFN-γ in vivo fall into three broad categories that include 1) antiproliferative and proapoptotic effects; 2) inhibition of angiogenesis; and 3) stimulatory actions on innate and adaptive immune systems. IFN-γ–induced activation of the JAK–STAT signaling pathway directly stimulates transcription of p21WAF1/CIP1 and p27Kip1, two potent inhibitors of cell cycle progression that inactivate cyclin-dependent kinases CDK-2 and CDK-4 (34). The anti-angiogenic effects of IFN-γ stem from its ability to induce the secretion of three CXC family chemokines IP-10, MIG, and I-TAC by tumor cells and also possibly by non-hematopoietic cells of the host. These chemokines bind to the CXCR3 chemokine receptor that is expressed on endothelial cells and inhibit neovascularization (35). IP-10 and MIG were upregulated in the current model, but additional experiments will be required to confirm the anti-angiogenic effects of IL-12 and 4D5 Ab. Importantly, Strasly and colleagues have shown that IL-12 activates a lymphocyte-dependent anti-angiogenic program in Con A-activated mouse spleen cells and human PBMCs that is characterized by cell cycle arrest and reduced adherence of endothelial cells along with reduced production of matrix metalloproteinase-9. These effects resulted from continuous interaction between activated lymphoid cells and endothelial cells and were dependent on the actions of IP-10 and MIG (36).
The identification of necrosis in tumors from mice receiving combination therapy is of great interest, given previous work with IL-12. Cavallo et al. (37) found that necrosis was a prominent feature of established tumors that had been treated with IL-12–secreting murine mammary adenocarcinoma cells. Morphologic analysis of these tumors by this group revealed major anomalies in the tumor microvasculature that were associated with damaged endothelial cells, vessel thrombosis, and microhemorrhages within the tumor stroma. Tumor regression was attributed to the altered delivery of oxygen to the tumor microenvironment. As in the current model, neutralization of IFN-γ or the depletion of NK cells impaired the efficacy of this treatment. Thus, the production of IFN-γ in response to IL-12 can lead to overwhelming cell death and the appearance of tumor necrosis. Notably, administration of IFN-γ–secreting tumor cells was markedly less effective than the administration of IL-12–secreting tumor cells (37). This finding indicates that co-secreted factors may potentiate the actions of IFN-γ in the setting of IL-12 administration with mAbs.
Increased CD8+ T cell infiltration of tumor deposits was not detected in mice receiving IL-12 and 4D5, and elimination of CD8+ T cells via Ab treatments had a modest and statistically non-significant inhibitory effect on the antitumor effects of combined therapy with IL-12 and 4D5 Ab. In humans, therapy with IL-12 and trastuzumab led to the production of chemotactic factors by NK cells and increased infiltration of T cells into patient tumor tissues and had minimal toxicity at physiological doses (17, 38). The dependence of this model on the NK cell compartment may reflect the fact that combination treatment is specifically targeted to FcR-bearing cells rather than cytolytic T cells. It is possible that increased participation of effector T cell populations might be observed in a transgenic model of breast cancer that more closely mimics the natural history of human breast cancer. These experiments are currently under way.
The combination of IL-12 and an anti-HER2/neu mAb mediated superior antitumor activity compared with that of either agent alone. The mechanism of action appeared to require the actions of NK cells and IFN-γ. Further investigation in the setting of a well-planned neoadjuvant clinical trial of IL-12 and trastuzumab is an appropriate next step.
Acknowledgments
This work was supported by National Institutes of Health Grants P01 CA95426, K24 CA93670 (to W.E.C.), T32 CA90338-27, T32 GM068412 (to B.L.M.-B.), P30 CA16058, and T32 GM068412 (to A.C.J.-R.). A.M. was a National Research Service Award T32 fellow.
We greatly appreciate the work of Florinda Jaynes, Deborah Devor-Henneman, and Alan Flechtner of the Comparative Pathology and Mouse Phenotyping Shared Resource at The Ohio State University, Department of Veterinary Biosciences and Comprehensive Cancer Center. We thank The Ohio State University Campus Microscopy and Imaging Facility for help with electron micrograph imaging.
Abbreviations used in this article
- ADCC
Ab-dependent cellular cytotoxicity
- IP-10
IFN-γ–inducible protein 10
- MIG
monokine induced by IFN-γ
- PI
propidium iodide
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Tzahar E, Yarden Y. The ErbB-2/HER2 oncogenic receptor of adenocarcinomas: from orphanhood to multiple stromal ligands. Biochim Biophys Acta. 1998;1377:M25–M37. doi: 10.1016/s0304-419x(97)00032-2. [DOI] [PubMed] [Google Scholar]
- 2.Pinkas-Kramarski R, Soussan L, Waterman H, Levkowitz G, Alroy I, Klapper L, Lavi S, Seger R, Ratzkin BJ, Sela M, Yarden Y. Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J. 1996;15:2452–2467. [PMC free article] [PubMed] [Google Scholar]
- 3.Baselga J, Tripathy D, Mendelsohn J, Baughman S, Benz CC, Dantis L, Sklarin NT, Seidman AD, Hudis CA, Moore J, et al. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol. 1996;14:737–744. doi: 10.1200/JCO.1996.14.3.737. [DOI] [PubMed] [Google Scholar]
- 4.Révillion F, Bonneterre J, Peyrat JP. ERBB2 oncogene in human breast cancer and its clinical significance. Eur J Cancer. 1998;34:791–808. doi: 10.1016/s0959-8049(97)10157-5. [DOI] [PubMed] [Google Scholar]
- 5.Ross JS, Fletcher JA. The HER-2/neu oncogene in breast cancer: prognostic factor, predictive factor, and target for therapy. Oncologist. 1998;3:237–252. [PubMed] [Google Scholar]
- 6.Baselga J, Albanell J, Molina MA, Arribas J. Mechanism of action of trastuzumab and scientific update. Semin Oncol. 2001;28(5, Suppl 16):4–11. doi: 10.1016/s0093-7754(01)90276-3. [DOI] [PubMed] [Google Scholar]
- 7.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 8.Seidman AD, Fornier MN, Esteva FJ, Tan L, Kaptain S, Bach A, Panageas KS, Arroyo C, Valero V, Currie V, et al. Weekly trastuzumab and paclitaxel therapy for metastatic breast cancer with analysis of efficacy by HER2 immunophenotype and gene amplification. J Clin Oncol. 2001;19:2587–2595. doi: 10.1200/JCO.2001.19.10.2587. [DOI] [PubMed] [Google Scholar]
- 9.Drebin JA, V, Link C, Stern DF, Weinberg RA, Greene MI. Down-modulation of an oncogene protein product and reversion of the transformed phenotype by monoclonal antibodies. Cell. 1985;41:697–706. doi: 10.1016/s0092-8674(85)80050-7. [DOI] [PubMed] [Google Scholar]
- 10.Klapper LN, Vaisman N, Hurwitz E, Pinkas-Kramarski R, Yarden Y, Sela M. A subclass of tumor-inhibitory monoclonal antibodies to ErbB-2/HER2 blocks crosstalk with growth factor receptors. Oncogene. 1997;14:2099–2109. doi: 10.1038/sj.onc.1201029. [DOI] [PubMed] [Google Scholar]
- 11.Sliwkowski MX, Lofgren JA, Lewis GD, Hotaling TE, Fendly BM, Fox JA. Nonclinical studies addressing the mechanism of action of trastuzumab (Herceptin) Semin Oncol. 1999;26(4, Suppl 12):60–70. [PubMed] [Google Scholar]
- 12.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 13.Spiridon CI, Guinn S, Vitetta ES. A comparison of the in vitro and in vivo activities of IgG and F(ab′)2 fragments of a mixture of three monoclonal anti-Her-2 antibodies. Clin Cancer Res. 2004;10:3542–3551. doi: 10.1158/1078-0432.CCR-03-0549. [DOI] [PubMed] [Google Scholar]
- 14.Carson WE, III, Liang MI. Current immunotherapeutic strategies in breast cancer. Surg Oncol Clin N Am. 2007;16:841–860. ix. doi: 10.1016/j.soc.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Lieberman MD, Sigal RK, Williams NN, II, Daly JM. Natural killer cell stimulatory factor (NKSF) augments natural killer cell and antibody-dependent tumoricidal response against colon carcinoma cell lines. J Surg Res. 1991;50:410–415. doi: 10.1016/0022-4804(91)90211-4. [DOI] [PubMed] [Google Scholar]
- 16.Parihar R, Dierksheide J, Hu Y, Carson WE. IL-12 enhances the natural killer cell cytokine response to Ab-coated tumor cells. J Clin Invest. 2002;110:983–992. doi: 10.1172/JCI15950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roda JM, Parihar R, Magro C, Nuovo GJ, Tridandapani S, Carson WE., III Natural killer cells produce T cell-recruiting chemokines in response to antibody-coated tumor cells. Cancer Res. 2006;66:517–526. doi: 10.1158/0008-5472.CAN-05-2429. [DOI] [PubMed] [Google Scholar]
- 18.Bekaii-Saab TS, Roda JM, Guenterberg KD, Ramaswamy B, Young DC, Ferketich AK, Lamb TA, Grever MR, Shapiro CL, Carson WE., III A phase I trial of paclitaxel and trastuzumab in combination with interleukin-12 in patients with HER2/neu-expressing malignancies. Mol Cancer Ther. 2009;8:2983–2991. doi: 10.1158/1535-7163.MCT-09-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guenterberg KD, V, Grignol P, Raig ET, Zimmerer JM, Chan AN, Blaskovits FM, Young GS, Nuovo GJ, Mundy BL, Lesinski GB, Carson WE., III Interleukin-29 binds to melanoma cells inducing Jak-STAT signal transduction and apoptosis. Mol Cancer Ther. 2010;9:510–520. doi: 10.1158/1535-7163.MCT-09-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lesinski GB, Kondadasula SV, Crespin T, Shen L, Kendra K, Walker M, Carson WE., III Multiparametric flow cytometric analysis of inter-patient variation in STAT1 phosphorylation following interferon Alfa immunotherapy. J Natl Cancer Inst. 2004;96:1331–1342. doi: 10.1093/jnci/djh252. [DOI] [PubMed] [Google Scholar]
- 21.Lesinski GB, Raig ET, Guenterberg K, Brown L, Go MR, Shah NN, Lewis A, Quimper M, Hade E, Young G, et al. IFN-alpha and bortezomib overcome Bcl-2 and Mcl-1 overexpression in melanoma cells by stimulating the extrinsic pathway of apoptosis. Cancer Res. 2008;68:8351–8360. doi: 10.1158/0008-5472.CAN-08-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisenring M, vom Berg J, Kristiansen G, Saller E, Becher B. IL-12 initiates tumor rejection via lymphoid tissue-inducer cells bearing the natural cytotoxicity receptor NKp46. Nat Immunol. 2010;11:1030–1038. doi: 10.1038/ni.1947. [DOI] [PubMed] [Google Scholar]
- 23.Cho D, Shook DR, Shimasaki N, Chang YH, Fujisaki H, Campana D. Cytotoxicity of activated natural killer cells against pediatric solid tumors. Clin Cancer Res. 2010;16:3901–3909. doi: 10.1158/1078-0432.CCR-10-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chin YE, Kitagawa M, Kuida K, Flavell RA, Fu XY. Activation of the STAT signaling pathway can cause expression of caspase 1 and apoptosis. Mol Cell Biol. 1997;17:5328–5337. doi: 10.1128/mcb.17.9.5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tannenbaum CS, Hamilton TA. Immune-inflammatory mechanisms in IFNgamma-mediated anti-tumor activity. Semin Cancer Biol. 2000;10:113–123. doi: 10.1006/scbi.2000.0314. [DOI] [PubMed] [Google Scholar]
- 26.Tannenbaum CS, Wicker N, Armstrong D, Tubbs R, Finke J, Bukowski RM, Hamilton TA. Cytokine and chemokine expression in tumors of mice receiving systemic therapy with IL-12. J Immunol. 1996;156:693–699. [PubMed] [Google Scholar]
- 27.Weiner LM, Dhodapkar MV, Ferrone S. Monoclonal antibodies for cancer immunotherapy. Lancet. 2009;373:1033–1040. doi: 10.1016/S0140-6736(09)60251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elboim M, Gazit R, Gur C, Ghadially H, Betser-Cohen G, Mandelboim O. Tumor immunoediting by NKp46. J Immunol. 2010;184:5637–5644. doi: 10.4049/jimmunol.0901644. [DOI] [PubMed] [Google Scholar]
- 29.Spector NL, Blackwell KL. Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2009;27:5838–5847. doi: 10.1200/JCO.2009.22.1507. [DOI] [PubMed] [Google Scholar]
- 30.Gennari R, Menard S, Fagnoni F, Ponchio L, Scelsi M, Tagliabue E, Castiglioni F, Villani L, Magalotti C, Gibelli N, et al. Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin Cancer Res. 2004;10:5650–5655. doi: 10.1158/1078-0432.CCR-04-0225. [DOI] [PubMed] [Google Scholar]
- 31.Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, Laccabue D, Zerbini A, Camisa R, Bisagni G, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26:1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 32.Repka T, Chiorean EG, Gay J, Herwig KE, Kohl VK, Yee D, Miller JS. Trastuzumab and interleukin-2 in HER2-positive metastatic breast cancer: a pilot study. Clin Cancer Res. 2003;9:2440–2446. [PubMed] [Google Scholar]
- 33.Parihar R, Nadella P, Lewis A, Jensen R, De Hoff C, Dierksheide JE, VanBuskirk AM, Magro CM, Young DC, Shapiro CL, Carson WE., III A phase I study of interleukin 12 with trastuzumab in patients with human epidermal growth factor receptor-2-overexpressing malignancies: analysis of sustained interferon gamma production in a subset of patients. Clin Cancer Res. 2004;10:5027–5037. doi: 10.1158/1078-0432.CCR-04-0265. [DOI] [PubMed] [Google Scholar]
- 34.Chin YE, Kitagawa M, Su WC, You ZH, Iwamoto Y, Fu XY. Cell growth arrest and induction of cyclin-dependent kinase inhibitor p21 WAF1/CIP1 mediated by STAT1. Science. 1996;272:719–722. doi: 10.1126/science.272.5262.719. [DOI] [PubMed] [Google Scholar]
- 35.Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13:95–109. doi: 10.1016/s1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 36.Mitola S, Strasly M, Prato M, Ghia P, Bussolino F. IL-12 regulates an endothelial cell-lymphocyte network: effect on metalloproteinase-9 production. J Immunol. 2003;171:3725–3733. doi: 10.4049/jimmunol.171.7.3725. [DOI] [PubMed] [Google Scholar]
- 37.Cavallo F, Signorelli P, Giovarelli M, Musiani P, Modesti A, Brunda MJ, Colombo MP, Forni G. Antitumor efficacy of adenocarcinoma cells engineered to produce interleukin 12 (IL-12) or other cytokines compared with exogenous IL-12. J Natl Cancer Inst. 1997;89:1049–1058. doi: 10.1093/jnci/89.14.1049. [DOI] [PubMed] [Google Scholar]
- 38.Eisenbeis CF, Lesinski GB, Anghelina M, Parihar R, Valentino D, Liu J, Nadella P, Sundaram P, Young DC, Sznol M, et al. Phase I study of the sequential combination of interleukin-12 and interferon alfa-2b in advanced cancer: evidence for modulation of interferon signaling pathways by interleukin-12. J Clin Oncol. 2005;23:8835–8844. doi: 10.1200/JCO.2005.02.1691. [DOI] [PubMed] [Google Scholar]