Abstract
Background
The development of alcohol dependence (AD) involves transitions through multiple stages of drinking behaviors and is shaped by both heritable and environmental influences. We attempted to capture this dynamic process by characterizing genetic and environmental contributions to the rate at which women progressed through 3 significant transitions along the pathway to AD: nonuse to initiation, initiation to onset of first alcohol-related problem, and first problem to onset of AD.
Methods
The sample consisted of 3,546 female twins from the Missouri Adolescent Female Twin Study. Participants ranged in age from 18 to 29 years. Retrospective reports of alcohol use histories were collected by telephone diagnostic interview and transition times between drinking milestones were coded ordinally. Standard genetic analyses were conducted in Mx to derive a trivariate model that provided estimates of genetic and environmental influences that were common as well as specific to the 3 transition times.
Results
Heritable influences were found for rate of progression across all 3 transitions, accounting for 30 to 47% of the variance in transition times. Shared environmental contributions were evident only in rate of progression from nonuse to initiation (i.e., age at first drink). Heritable contributions to the rate of movement through successive drinking milestones were attributable to a common factor, whereas environmental influences were transition-specific.
Conclusions
The current study is unique in its use of a genetically informative design to document the rate of movement between drinking milestones in a female sample and to examine genetic contributions to multiple transition times over the course of AD development. Results indicate that an earlier report of heritability for males in rate of progression from regular drinking to AD generalizes to women and to other alcohol stage transitions. Findings also suggest the need to consider stage-specific environmental contributions to alcohol outcomes in developing interventions.
Keywords: Alcohol Dependence, Genetics, Women, Transition, Course
TRANSITIONS IN THE COURSE OF ALCOHOL DEPENDENCE DEVELOPMENT
The development of alcohol dependence (AD) is a multistage process comprised transition points at which drinking behaviors increase in severity (e.g., initiation of alcohol use to onset of alcohol-related problems). Identifying factors that contribute to a lifetime diagnosis of AD, although important in assessing overall liability to the disorder, is of limited utility in predicting changes in vulnerability to alcohol-related problems over the developmental course of the disorder. In contrast, characterizing the sources of risk associated with the rate of progression between successive drinking milestones creates a more comprehensive picture of the course of AD development and facilitates identification of those junctures where alcohol outcomes may be most modifiable.
Evidence for developmental stage-specific predictors of drinking course is growing (Sartor et al., 2007; Sher et al., 2004), yet the course of alcohol use and dependence has only rarely been studied with respect to the series of stage transitions making up this pathway. A small number of studies have examined the predictors of progression to a more severe alcohol use status (e.g., increase in AD symptoms, movement from regular to hazardous use) at multiple points in drinking course (Bucholz et al., 2000; Lieb et al., 2002), but the outcomes in these investigations were whether, as opposed to how rapidly these transitions occurred. Initiation is the only stage of alcohol use that has been extensively studied in terms of the rate of progression to that drinking status (i.e., age at first drink). Early age at first drink has been tied to increased risk for AD development in numerous population-based studies (Dawson, 2000; DeWit et al., 2000; Nelson and Wittchen, 1998), including one by Grant and Dawson (1997), which reported that the odds of developing AD decreased by 5% for every year that initiation of alcohol use was delayed in a sample of 14- to 21-year olds (Grant and Dawson, 1997). Similarly, based on data from the National Epidemiological Survey on Alcohol and Related Conditions (NESARC), Hingson et al. (2006) reported that the lifetime prevalence of AD was 47% among individuals who had initiated alcohol use at age 14 years or younger versus 9% among those who began drinking at 21 years or older (Hingson et al., 2006). As a result of these and similar findings, delay of first alcohol use has been proposed to reduce risk for AD, but work by Prescott and Kendler (1999) suggests that, rather than reflecting a causal relationship, early age at first drink may be a marker for familial liability to AD.
THE HERITABILITY OF ALCOHOL USE AND DEPENDENCE
The heritability of AD, estimated at approximately 50 to 60% (Reed et al., 1996; True et al., 1996; van den Bree et al., 1998), has long been established in men (Hrubec and Omenn, 1981; Romanov et al., 1991) and has more recently been demonstrated in females as well (Heath et al., 1997; Kendler et al., 1994; Knopik et al., 2004; Prescott et al., 1999). Genetic influences on initiation of alcohol use have also been consistently reported, but are less robust (14 to 40%) (Hopfer et al., 2003) than those on AD and other later occurring alcohol use behaviors (Fowler et al., 2006; Rhee et al., 2003) and they account for a smaller proportion of variance in initiation of use relative to shared environmental influences (Fowler et al., 2006; Koopmans and Boomsma, 1996; Rose et al., 2001; Stallings et al., 1999). Among the few genetically informative studies to assess cross-stage influences on alcohol outcomes was Pagan et al.’s (2006) investigation of alcohol use initiation, frequency of use, and alcohol-related problems in a large Finnish twin sample. Shared environmental factors were reported to be the primary sources of variance for initiation, whereas frequency of use and alcohol-related problems were found to be largely attributable to a genetic source common to these 2 outcomes (Pagan et al., 2006). Like the epidemiologic investigations described earlier, these twin-based studies examined stages of alcohol use in terms of whether a given milestone was reached rather than the rate of progression between stages of use. The few that have incorporated timing of transitions into the research design provide the groundwork for the current study.
Prescott and Kendler (1999) estimated genetic, shared environmental, and unique environmental sources of co-variance in early initiation of alcohol use and AD in a large sample of male and female twin pairs and found that 29% of variation in risk for AD in women and 19% in men was shared with factors contributing to age at onset of first drink, nearly all of which was genetic. They concluded that familial sources fully accounted for the shared variance between early first alcohol use and AD (Prescott and Kendler, 1999). In contrast, an investigation of common vulnerability to early onset of regular drinking and AD development in an all-male twin sample, which also revealed genetic and shared environmental sources of risk, concluded that the association could not be fully explained by familial factors common to the 2 alcohol outcomes (Grant et al., 2006). Stallings et al. (1999) assessed 2 alcohol outcomes as transition times, age at onset of first alcohol use and latency between first use and onset of regular drinking, and found that shared environmental factors accounted for the most variance in age at initiation, whereas genetic influences were more prominent in rate of progression to regular drinking (Stallings et al., 1999). The only known genetically informative study to address the rate of progression in AD development was conducted by Liu et al. (2004), in which age at onset of regular alcohol use and of AD as well as the length of time between these 2 transition points were examined in an all-male twin sample. Approximately 25% of the variability in transition time was accounted for by genetic factors (Liu et al., 2004).
Although significant steps in addressing the heritability of AD development, these studies leave critical questions regarding progression through drinking milestones unanswered. First, do results from this body of literature generalize to other stage transitions not addressed in these studies? Second, do Liu et al.’s findings apply to women? Finally, to what degree do the genetic and environmental influences impacting rate of progression overlap across alcohol stage transitions? The present study aims to address these issues by examining the lag times between successive drinking milestones along the pathway to AD using a large all-female twin sample. We attempt to capture the dynamic course of AD development by focusing on 3 significant transitions: nonuse to initiation, initiation to onset of first alcohol-related problem, and first problem to onset of AD. Common as well as transition-specific genetic and environmental contributions to the rate of progression across these alcohol stage transitions are estimated.
MATERIALS AND METHODS
Participants
The sample consisted of 3,546 female twins [954 monozygotic (MZ) pairs and 819 dizygotic (DZ) pairs] from the Missouri Adolescent Female Twin Study (MOAFTS), a longitudinal study of alcohol-related problems and associated psychopathology in female adolescents and young adults (Heath et al., 2002). Birth records were used to recruit female twin pairs born in Missouri to Missouri-resident parents between July 1, 1975 and June 30, 1985 using a cohort-sequential design. Cohorts of 13-, 15-, 17-, and 19-year-old female twin pairs and their families were ascertained in the first 2 years, with new cohorts of 13-year-old twins and their families added in the subsequent 2 years. Recruitment was conducted from 1995 to 1999. A total of 2,369 families were initially targeted for inclusion in the study. Parental diagnostic interviews were completed by at least 1 parent in 1,819 families, representing 77% of eligible families. (For further details on ascertainment, see Heath et al., 2002). Data for the present study were drawn from the fourth wave of data collection, when participants had a mean age of 21.6 years (range = 18 to 29 years). Approximately 86% of the sample identified as Caucasian and 14% as African-American.
Procedure
Data were collected over the telephone by trained interviewers. At baseline, an initial screening to determine zygosity of the twin pair was conducted with one of the twin’s parents and, when permission was granted, parental diagnostic interviews were scheduled. Interviews with the twins were scheduled after obtaining verbal consent (and, for those under the age of 18, the consent of parents), consistent with procedures approved by the Institutional Review Board at Washington University. Wave 4 data were collected approximately 5 years later.
Assessment Battery
The Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) was adapted for interview via telephone and used to collect detailed histories of alcohol use and to obtain DSM-IV diagnoses of alcohol abuse and dependence. The SSAGA is a highly reliable (Bucholz et al., 1994) and well-validated (Hesselbrock et al., 1999) instrument designed to assess alcohol use disorders (AUDs) and related psychiatric disorders in adults. [Telephone administration of similar diagnostic assessments has yielded good reliability estimates for AUD diagnoses (Slutske et al., 1998)].
Alcohol Use
Age at initiation of alcohol use was defined as the age at which the first full alcoholic drink, i.e., a standard can or bottle of beer, a glass of wine or a shot of liquor, was consumed. Consumption of one or more alcoholic drinks over the lifetime was reported by 85.3% of participants.
Alcohol-Related Problem
Age that first alcohol-related problem was experienced was defined as the age at onset of the first alcohol abuse or dependence symptom. One or more alcohol use disorder symptoms were reported by 40.6% of the sample (47.6%of drinkers).
Alcohol Dependence
Age at onset of AD was defined as the age at which full DSM-IV criteria for AD (i.e., 3 or more AD symptoms occurring during the same 12-month period) were first met. AD criteria were met by 7.3% of the sample (8.6% of drinkers).
Operationalizing Rate of Transition
Categories of “slow” and “rapid” rate of transition between successive drinking milestones were created by dividing distributions of continuous indicators of transition times into 2 approximately equal groups. For nonuse to initiation, slow was defined as 17 years or more, rapid as 16 or fewer years. In the initiation to first AUD symptom transition, slow was defined as 3 or more years and rapid was 2 years or fewer. For first AUD symptom to onset of AD, slow was defined as 2 or more years, rapid as 1 year or less.
Data Analysis
Twin Modeling
Data from MZ and DZ twins can be utilized to parse out the extent to which additive genetic (A), shared environmental (C) and nonshared environmental (E) factors influence population variation in speed of transitions between drinking milestones. Nonadditive (including dominance, and thus denoted as D) genetic influences can be estimated in place of shared environment when the correlation between members of DZ twin pairs is less than half the correlation between their MZ counterparts for a given transition time. However, both C and D cannot be jointly estimated when data from twins alone are used. In most instances, such as ours, where the DZ correlations are greater than half the MZ correlations, there is preliminary evidence in favor of C and an ACE model is used.
Univariate Twin Models
We fit univariate twin models to raw categorical data on the 3 transition times: nonuse to initiation, initiation to first AUD symptom and first symptom to AD. We created categories for each transition measure to represent did not transition (as “0”) and early-, and late-onset transitions (as “2” and “1” respectively) to account for the potential skewness in the continuous forms of these variables. Initiation to first AUD symptom was missing in those who had never used alcohol. Likewise, the transition from first AUD symptom to AD was missing in those who were lifetime abstainers and in those who had used alcohol but never reported any AUD symptoms. In the statistical software package Mx, we used a maximum likelihood estimator to examine the extent to which A, C, and E shaped transition times. The thresholds for each transition time, which were expressed as z-scores on the underlying standard normal distribution, were adjusted for age at interview (dummy coded as “0” for 22 years or older and “1” for 21 years or younger). A series of submodels examining the statistical significance of A, C, and E were then compared to the full model using the difference between the −2 log likelihood fit of the full model and nested submodel, which was distributed as chi-squared for the given degrees of freedom.
Trivariate Hierarchical Model
Whereas the univariate models estimated the extent to which the rate of progression to each stage was influenced by genetic and environmental influences, they did not address the important question of the extent to which common and specific genetic and environmental influences act across all 3 transitions. A trivariate triangular decomposition model (also known as a Cholesky decomposition model) can disentangle the extent to which the genetic, shared and nonshared environmental influences on rate of transition between drinking milestones overlap across alcohol stages and the extent to which they are specific to each transition. In addition, the 3 phenotypes under investigation here are contingent on each other. That is to say, the time from initiation to first AUD symptom can only be measured in those who reported either early- or late-onset transition from nonuse to initiation but is structurally missing in those who never used alcohol. Therefore, any individual coded as “0” for the transition from nonuse to initiation was structurally missing on the latter 2 dimensions of initiation to first AUD symptom and first symptom to AD. Additionally, rate of transition from first AUD symptom to DSM-IV AD could not be assessed in those who did not endorse any AUD symptoms. Therefore, the final transition from first symptom to AUD was structurally missing in those who were coded as “0” on the transition from initiation to first AUD symptom. This imposed a hierarchy across the Cholesky, such that genetic and environmental influences on each transition time were contingent on having made the prior transition (i.e., being coded as 1 or 2, but not 0, on the prior transition). Heath et al. (2004) have demonstrated the applicability of such a hierarchical model for contingent phenotypes, including stages of substance use behavior.
Trivariate hierarchical models were fit in Mx. Raw categorical data were used and thresholds for all 3 transitions were simultaneously adjusted for age at interview. As with the univariate models, submodels, which tested the statistical significance of the genetic, shared and nonshared environmental overlap, were tested against the fit of the full model using the likelihood ratio chi-squared statistic.
RESULTS
Rates of Movement Across Alcohol Stages
Table 1 displays the rates of transition to the next drinking milestone by rate at which the previous milestone was reached. A larger number of rapid (age 16 or younger) than slow (age 17 or older) initiators of alcohol use went on to develop alcohol-related problems (59.9% vs. 32.5%) (χ2(1) = 224.75; p < 0.001), but as seen in Table 1, individuals in the rapid initiation group were about equally likely to transition slowly (in 3 or more years) as quickly (in 2 or fewer years) to the symptom development stage. In contrast, a larger proportion of the slow initiators who developed AUD symptoms transitioned rapidly than slowly (22.8% vs. 9.7%). A similar pattern was observed for the transition from first AUD symptom to onset of AD. A higher percentage of individuals in the rapid versus slow symptom onset groups went on to develop AD (22.0 and 13.5%, respectively) (χ2(1) = 17.46; p < 0.001), but those who transitioned quickly to symptomatic drinking were about equally likely to make the transition to AD in 1 year or less (12.8%) as in 2 years or more (9.2%). By comparison, those who developed symptoms slowly were nearly 3 times as likely to transition to AD rapidly (9.9%) than slowly (3.6%).
Table 1.
Distribution of Transition Times by Rate of Transition Through Previous Stage of Alcohol Use
| Nonuse to Initiation | Initiation to 1st AUD Symptom | Initiation to 1st AUD Symptom | 1st AUD Symptom to AD | ||
|---|---|---|---|---|---|
| Never n = 522 | – | Never n = 1,583 | – | ||
| Slow (≥17 years) | Never | 67.5% | Slow (≥3 years) n = 673 | Never | 86.5% |
| n = 1,353 | Slow (≥3 years) | 9.7% | Slow (≥2 years) | 3.6% | |
| Rapid (≤2 years) | 22.8% | Rapid (≤1 year) | 9.9% | ||
| Rapid (≤16 years) | Never | 40.1% | Rapid (≤2 years) n = 768 | Never | 78.0% |
| n = 1,671 | Slow (≥3 years) | 32.4% | Slow (≥2 years) | 9.2% | |
| Rapid (≤2 years) | 27.5% | Rapid (≤1 year) | 12.8% | ||
Within-twin cross-trait polychoric correlations revealed modest associations between transition times: 0.27 for transitions 1 and 2 (nonuse to initiation and initiation to first AUD symptom), 0.20 for transitions 2 and 3 (initiation to first AUD symptom and first AUD symptom to AD onset), and 0.31 for transitions 1 and 3 (nonuse to initiation and first AUD symptom to AD onset).
Univariate Models
Significant heritable influences (30 to 37%) on rate of progression were noted for all 3 transitions (Table 2). In addition, for the first transition from nonuse to initiation of alcohol use, evidence for shared environmental influences (43%) was found, even after adjustment for age at interview. For the transitions from initiation to first symptom and from the first symptom to AD, the remaining variance in rate of progression was explained by individual-specific environmental factors (Δχ2(1) = 0.00 for significance of C).
Table 2.
Magnitude of Additive Genetic (A), Shared Environmental (C), and Nonshared Environmental (E) Influences on Speed of Transitions Through Alcohol Stages: Univariate Models
| Proportion of total variance in phenotype attributable to A, C, and E (CI) | |||
|---|---|---|---|
| Transition | A | C | E |
| 1. Nonuse to initiation (adjusted for age group) | 0.30 (0.15–0.46) | 0.43 (0.28–0.56) | 0.27 (0.23–0.32) |
| 2. Initiation to 1st AUD symptom (adjusted for age group & early vs. late initiation) | 0.36 (0.19–0.44) | 0.00 (0.00–0.14) | 0.64 (0.56–0.72) |
| 3. 1st AUD symptom to AD (adjusted for age group & rapid vs. slow transition from 1st drink to 1st AUD symptom) | 0.37 (0.00–0.58) | 0.03 (0.00–0.47) | 0.60 (0.42–0.80) |
Trivariate Hierarchical Model
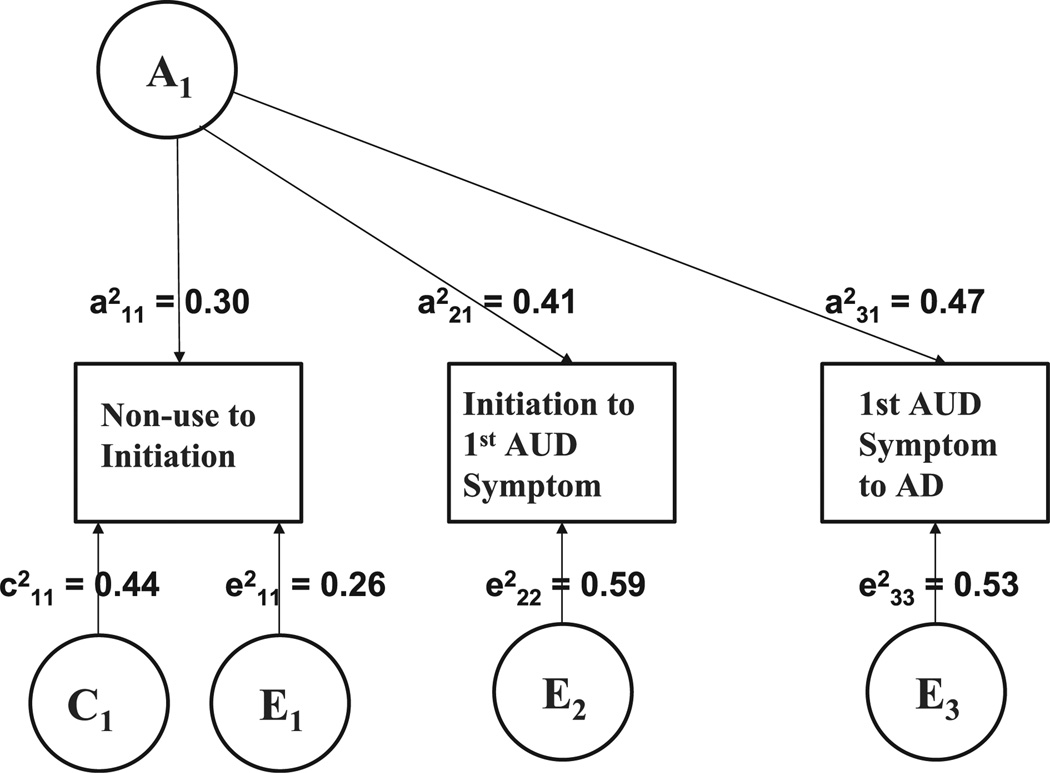
The best-fitting model is shown in Fig. 1 and summarized with 95% confidence limits in Table 3. When all 3 transition times were analyzed in the same model, heritable influences explained 30 to 47% of the total variance in speed of transition through alcohol stages. There was also considerable overlap across these genetic factors. Genetic correlations for rate of progression across stages ranged from 0.83 to 0.93, with the upper confidence limit reaching 1.00, thus suggesting little evidence in favor of transition-specific genetic factors. We were able to allow for a single genetic factor acting on all 3 transition times, without a significant deterioration of model fit (Δχ2(3) = 3.66, constraining any specific genetic variance on the second and third transition, and the genetic covariance between them to zero). In contrast, we found no support for correlated individual-specific environmental factors (Δχ2(3) = 1.26, constraining the individual-specific environmental covariance between the transitions to zero). Thus, while nonshared environment (E) explained between 26 and 59% of the total variance in rate of progression across alcohol stages, these factors were entirely specific to each transition. As with the univariate models, shared environmental factors were significant only for speed of transition from nonuse to initiation and hence, shared environmental correlations across the 3 stage transitions were undefined.
Fig. 1.
Trivariate Model: genetic and environmental influences on speed of progression through alcohol stages.
Table 3.
Best-Fitting Trivariate Model
| Source of variance |
Proportion of total variance in phenotype (CI) |
|
|---|---|---|
| A1 | Nonuse to initiation | 0.30 (0.23–0.31) |
| Initiation to 1st AUD symptom | 0.41 (0.34–0.48) | |
| 1st AUD symptom to AD | 0.47 (0.31–0.62) | |
| C1 | Nonuse to initiation | 0.44 (0.36–0.51) |
| E1 | Nonuse to initiation | 0.26 (0.22–0.31) |
| E2 | Initiation to 1st AUD symptom | 0.59 (0.52–0.66) |
| E3 | 1st AUD symptom to AD | 0.53 (0.38–0.69) |
DISCUSSION
Building on evidence for heritable influences on various stages of alcohol use and the association of age at first drink with likelihood of developing alcohol-related problems, we examined genetic and environmental contributions to the rate at which women progressed through 3 major transitions in the course of AD development. Analyses were conducted with a multiple stage modeling approach aimed at reducing potential biases and adjusting for age at the time of assessment. The current study is unique in its use of a genetically informative design to document the rate of movement between drinking milestones in a female sample and to examine genetic contributions to multiple transition times over the course of AD development. Our focus on indicators of change in alcohol-related behaviors over time offers a novel perspective on heritable risk for alcohol outcomes in women.
Consistent with the well-documented finding that early initiators of alcohol use are at elevated risk for AD (Grant and Dawson, 1997; Hingson et al., 2006), women in the current sample who had transitioned rapidly to a given milestone were at increased risk for moving to the next stage of alcohol use. However, they were not more likely than others to progress to the next stage at a faster rate. In fact, we found that a higher proportion of late than early initiators transitioned quickly to symptomatic status. Though seemingly counterintuitive, other studies of substance use have documented the tendency of early initiators to progress more slowly to later stages of use (Anthony and Petronis, 1995; Breslau et al., 1993; Sartor et al., 2007; Sung et al., 2004). One possible interpretation is that although those at highest risk for later alcohol-related problems are likely to begin drinking at a young age, the relatively greater difficulty they have in acquiring alcohol slows down the progression of drinking behaviors. Importantly, this pattern of results underscores the need to consider years of exposure to alcohol in the context of the developmental stage when exposure occurs. It also suggests the value of dividing the course of AD development into stages to capture changes in the rate of progression that could not be detected in a design aimed at characterizing the trajectory as a whole.
Heritable influences were apparent in all 3 transition times, consistent with previously reported estimates of modest genetic influences on age at alcohol use initiation (Stallings et al., 1999) and age at onset of regular alcohol use (Liu et al., 2004). The best-fitting model incorporating all 3 alcohol outcomes was one that assumed no transition-specific genetic liability. Heritable contributions to the rate of progression through all 3 stage transitions along the pathway to AD were attributable to a common genetic factor. This is in keeping with findings from prior twin-based investigations that focused specifically on early alcohol use as it relates to risk for AD. Prescott and Kendler (1999), for example, found that 98% of the association between early age at first drink and AD in women was attributable to common genetic sources of variance (Prescott and Kendler, 1999) and Grant et al. (2006) reported that the strong link between alcohol use at a young age and later alcohol-related problems was due in large part to common genetic influences (Grant et al., 2006).
A substantial effect of shared environmental influences was evident in the transition from nonuse to initiation of alcohol use, but was absent in the other alcohol stage transitions. Given that first alcohol use typically occurs in early to mid adolescence, it may be that shared environmental influences reflect parental control over teens’ behavior, which translates into suppression of genetic liability, or that shared peer influences are important in this age group. Although the mechanisms underlying this finding have not been clearly articulated, our results are in line with previous genetically informative studies on alcohol use initiation (Koopmans and Boomsma, 1996; Rose et al., 2001; Stallings et al., 1999), despite the fact that much of the earlier work in this area has used binary indicators (use vs. nonuse) and we distinguished early versus late onset in the nonuse to initiation transition.
Unique environmental factors (i.e., factors that make members of a twin pair different on a given outcome) accounted for the majority of stage-specific variance. Furthermore, unlike genetic factors, these individual-specific environmental factors were uncorrelated across the 3 measures of transition times, suggesting that environmental exposures impacting movement to the next stage in the course of AD development differ by phase of involvement in drinking behaviors. Rapid movement from first drink to onset of alcohol-related problems may, for example, be influenced in part by accessibility of alcohol, whereas the rate of transition from problem drinking to AD onset may not, as there is likely to be less variation in accessibility in later stages of problem use.
In sum, heritable influences across all 3 transitions appeared to be traceable to a common genetic factor. Environmental influences, in contrast, were stage-transition specific, with shared environmental variance emerging only in the nonuse to initiation transition. Findings confirm that Liu et al.’s (2004) report of a modest heritable influence on rate of movement between regular alcohol use and AD in an adult male sample holds for women. Furthermore, Stallings et al.’s (1999) finding of higher heritability in transition times later in the course of alcohol use (compared with the nonuse to first use transition) also applied in our study to the rate of progression from first use to onset of first problem. Results highlight the unique aspect of the course of AD development captured by using lag times between drinking milestones, especially for late stages of drinking behaviors.
Although not directly tested in the current investigation, results have implications for the ongoing debate regarding causality in the relationship between early initiation of alcohol use and later alcohol-related problems. Our finding that genetic contributions to speed of transitions across drinking milestones were attributable to a common source of variance supports the noncausal association posed by Prescott and Kendler (1999). Our results are consistent with their argument that the link between early use and AUDs is explained by shared familial influences, but, given the phenotypic distinction between transition times and AUDs, comparisons should be made with caution. In addition, findings from the present study indicate that genetic liability contributes to rapid transitions throughout the pathway to AD, reinforcing the notion that intervention efforts at all stages of alcohol use should consider individuals with family histories of AUDs to be especially vulnerable. However, genetic factors accounted for the minority of variance in the rate at which alcohol stage transitions occurred, suggesting that changes in the environment (e.g., adopting a different peer group) can be implemented that may significantly slow the progression of alcohol-related problems. Given the stage-specific environmental influences on rate of progression, consideration of the changing nature of environmental contributions to problem drinking behaviors is clearly essential for creating developmentally appropriate interventions.
Limitations and Future Directions
The current study has some limitations that suggest possible directions for further work in this area. First, although most participants had passed through the age of risk for first drink, not all had reached the peak age range for developing alcohol-related problems. Further follow-up of this sample should confirm whether age adjustments sufficiently addressed potential age-related biases in our sample. Second, the assumption of underlying normality (on which the creation of ordinal variables for the genetic analyses were premised) was met for rate of progression from first AUD symptom to AD but not for the other 2 stage transitions, so results should be interpreted with that in mind. Third, data on alcohol use was based on retrospective reports and, although drinking histories spanned a relatively short period of time, prospective accounts would likely be more reliable. Fourth, our goal was to characterize heritable and environmental risk for alcohol stage transitions in women, so we do not purport that our results will generalize to men, but consider the examination of potential gender differences to be an important next step in this line of research. Finally, the present study focused on the rate of progression through milestones leading up to AD onset, but the course of AD includes a number of other intriguing transitions, such as remission and relapse, that remain to be explored in a genetically informative design.
Supplementary Material
ACKNOWLEDGMENTS
This study was funded by grants AA017010, AA009022, AA007728, and AA011998 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA), grant HD049024 from the National Institute of Child Health and Human Development (NICHD), and grants DA18660 and DA023668 from the National Institute on Drug Abuse (NIDA).
REFERENCES
- Anthony JC, Petronis KR. Early-onset drug use and risk of later drug problems. Drug Alcohol Depend. 1995;40:9–15. doi: 10.1016/0376-8716(95)01194-3. [DOI] [PubMed] [Google Scholar]
- Breslau N, Fenn N, Peterson EL. Early smoking initiation and nicotine dependence in a cohort of young adults. Drug Alcohol Depend. 1993;33:129–137. doi: 10.1016/0376-8716(93)90054-t. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Reich T, Schmidt I, Schuckit MA. A new semi-structured psychiatric interview for use in genetic linkage studies: A report of the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Heath AC, Madden PAF. Transitions in drinking in adolescent females: evidence from the Missouri Adolescent Female Twin Study. Alcohol Clin Exp Res. 2000;24:914–923. [PubMed] [Google Scholar]
- Dawson DA. The link between family history and early onset alcoholism: earlier initiation of drinking or more rapid development of dependence? J Stud Alcohol. 2000;61:637–646. doi: 10.15288/jsa.2000.61.637. [DOI] [PubMed] [Google Scholar]
- DeWit D, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry. 2000;157:745–750. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- Fowler T, Lifford K, Shelton K, Rice F, Thapar A, Neale MC, McBride A, van den Bree MBM. Exploring the relationship between genetic and environmental influences on initiation and progression of substance use. Addiction. 2006;101:413–422. doi: 10.1111/j.1360-0443.2006.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the Longitudinal Alcohol Epidemiological Survey. J Adolesc Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Grant JD, Scherrer JF, Lynskey MT, Lyons MJ, Eisen SA, Tsuang MT, True WR, Bucholz KK. Adolescent alcohol use is a risk factor for adult alcohol and drug dependence: evidence from a twin design. Psychol Med. 2006;35:1–10. doi: 10.1017/S0033291705006045. [DOI] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut RJ, Statham DJ, Dunne NP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychol Med. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Heath AC, Howells W, Bucholz KK, Glowinski AL, Nelson EC, Madden PA. Ascertainment of a mid-western US female adolescent twin cohort for alcohol studies: assessment of sample representativeness using birth record data. Twin Res. 2002;5:107–112. doi: 10.1375/1369052022974. [DOI] [PubMed] [Google Scholar]
- Heath AC, Martin NG, Lynskey MT, Todorov AA, Madden PAF. Estimating two-stage models for genetic influences on alcohol, tobacco or drug use initiation and dependence vulnerability in twin and family data. Twin Res. 2004;5:113–124. doi: 10.1375/1369052022983. [DOI] [PubMed] [Google Scholar]
- Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA – A comparison with the SCAN. Addiction. 1999;94:1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch Pediatr Adolesc Med. 2006;160:739–746. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- Hopfer CJ, Crowley TJ, Hewitt JK. Review of twin and adoption studies of adolescent substance use. J Am Acad Child Adolesc Psychiatry. 2003;42:710–719. doi: 10.1097/01.CHI.0000046848.56865.54. [DOI] [PubMed] [Google Scholar]
- Hrubec Z, Omenn GS. Evidence of genetic predisposition to alcoholic cirrhosis and psychosis: twin concordance for alcoholism and its biological end points by zygosity among male veterans. Alcohol Clin Exp Res. 1981;5:207–215. doi: 10.1111/j.1530-0277.1981.tb04890.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Heath AC, Kessler RC, Eaves LC. A twin-family study of alcoholism in women. Am J Psychiatry. 1994;151:707–715. doi: 10.1176/ajp.151.5.707. [DOI] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Madden PA, Bucholz KK, Slutske WS, Nelson EC, Statham D, Whitfield JB, Martin NG. Genetic effects on alcohol dependence risk: re-evaluating the importance of psychiatric and other heritable risk factors. Psychol Med. 2004;34:1519–1530. doi: 10.1017/s0033291704002922. [DOI] [PubMed] [Google Scholar]
- Koopmans JR, Boomsma DI. Familial resemblance in alcohol use: genetic or cultural transmission? J Stud Alcohol. 1996;57:19–28. doi: 10.15288/jsa.1996.57.19. [DOI] [PubMed] [Google Scholar]
- Lieb R, Merikangas KR, Hofler M, Pfister H, Wittchen HU. Parental alcohol use disorders and alcohol use and disorders in offspring: a community study. Psychol Med. 2002;32:63–78. doi: 10.1017/s0033291701004883. [DOI] [PubMed] [Google Scholar]
- Liu IC, Blacker DL, Xu R, Fitzmaurice G, Lyons MJ, Tsuang MT. Genetic and environmental contributions to the development of alcohol dependence in male twins. Arch Gen Psychiatry. 2004;61:897–903. doi: 10.1001/archpsyc.61.9.897. [DOI] [PubMed] [Google Scholar]
- Nelson CB, Wittchen H-U. DSM-IV alcohol disorders in a general population sample of adolescents and young adults. Addiction. 1998;93:1065–1077. doi: 10.1046/j.1360-0443.1998.937106511.x. [DOI] [PubMed] [Google Scholar]
- Pagan JL, Rose RJ, Viken RJ, Pulkkinen L, Kaprio J, Dick DM. Genetic and environmental influences on stages of alcohol use across adolescence and into young adulthood. Behav Genet. 2006;36:483–497. doi: 10.1007/s10519-006-9062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott CA, Aggen SH, Kendler KS. Sex differences in the sources of genetic liability to alcohol abuse and dependence in a population-based sample of U.S. twins. Alcohol Clin Exp Res. 1999;23:1136–1144. doi: 10.1111/j.1530-0277.1999.tb04270.x. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Age at first drink and risk for alcoholism: a noncausal association. Alcohol Clin Exp Res. 1999;23:101–107. [PubMed] [Google Scholar]
- Reed T, Page WF, Viken RJ, Christian JC. Genetic predisposition to organ-specific endpoints of alcoholism. Alcohol Clin Exp Res. 1996;23:1528–1533. doi: 10.1111/j.1530-0277.1996.tb01695.x. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Genetic and environmental influences on substance initiation use problem use in adolescents. Arch Gen Psychiatry. 2003;60:1256–1264. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- Romanov K, Kaprio J, Rose RJ, Koskenvuo M. Genetics of alcoholism: effects of migration on concordance rates among male twins. Alcohol Alcohol Suppl. 1991;1:137–140. [PubMed] [Google Scholar]
- Rose RJ, Dick DM, Viken RJ, Pulkkinen L, Kaprio J. Drinking or abstaining at age 14? A genetic epidemiological study. Alcohol Clin Exp Res. 2001;25:1594–1604. [PubMed] [Google Scholar]
- Sartor CE, Lynskey MT, Heath AC, Jacob T, True W. The role of childhood risk factors in initiation of alcohol use and progression to alcohol dependence. Addiction. 2007;102:216–225. doi: 10.1111/j.1360-0443.2006.01661.x. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Gotham HJ, Watson AL. Trajectories of dynamic predictors of disorder: their meaning and implications. Dev Psychopathol. 2004;16:825–856. doi: 10.1017/s0954579404040039. [DOI] [PubMed] [Google Scholar]
- Slutske WS, True WR, Scherrer JF, Goldberg J, Bucholz KK, Heath AC, Henderson WG, Eisen SA, Lyons MJ, Tsuang MT. Long-term reliability and validity of alcoholism diagnoses and symptoms in a large national telephone survey. Alcohol Clin Exp Res. 1998;22:553–558. doi: 10.1111/j.1530-0277.1998.tb04292.x. [DOI] [PubMed] [Google Scholar]
- Stallings MC, Hewitt JK, Beresford T, Heath AC, Eaves LJ. A twin study of drinking and smoking onset and latencies from first to regular use. Behav Genet. 1999;6:409–421. doi: 10.1023/a:1021622820644. [DOI] [PubMed] [Google Scholar]
- Sung M, Erkanli A, Angold A, Costello EJ. Effects of age at first substance use and psychiatric comorbidity on the development of substance use disorders. Drug Alcohol Depend. 2004;75:287–299. doi: 10.1016/j.drugalcdep.2004.03.013. [DOI] [PubMed] [Google Scholar]
- True WR, Heath AC, Bucholz KK, Slutske W, Romeis JC, Scherrer JF, Lin N, Eisen SA, Goldberg J, Lyons MJ, Tsuang MT. Models of treatment seeking for alcoholism: the role of genes and environment. Alcohol Clin Exp Res. 1996;20:1577–1581. doi: 10.1111/j.1530-0277.1996.tb01702.x. [DOI] [PubMed] [Google Scholar]
- van den Bree MBM, Johnson EO, Neale MC, Svikis DS, McGue M, Pickens RW. Genetic analysis of diagnostic systems of alcoholism in males. Biol Psychiatry. 1998;43:139–145. doi: 10.1016/S0006-3223(97)00225-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.