Abstract
Rationale
Alcohol tolerance is observed as a diminished response to a given dose as a function of repeated administrations of the drug. Research has consistently shown that heavier drinkers display reduced reactions to alcohol (i.e., tolerance) compared with lighter drinkers. However, the majority of this work has focused primarily on measures of motor performance, whereas the development of tolerance to alcohol’s impairing effects on cognitive processes, such as inhibitory mechanisms of behavioral control, remains relatively unexplored.
Objective
The purpose of the present study was to examine the relationship between drinking habits and the degree to which alcohol affects drinkers’ inhibitory control and motor coordination.
Methods
Fifty-two non-dependent drinkers reported their recent drinking patterns. Their inhibitory control and motor coordination were measured in response to placebo and 0.65 g/kg alcohol.
Results
Alcohol significantly impaired inhibitory control and motor coordination compared with placebo. Moreover, greater quantity and frequency of recent consumption predicted less alcohol impairment of motor coordination. However, there was no relationship between recent drinking habits and the degree of impairment of inhibitory control.
Conclusions
These findings suggest that tolerance to the disinhibiting effects of alcohol might not readily develop as a result of recent, heavy drinking.
Keywords: Alcohol, Tolerance, Inhibitory control, Motor coordination
Alcohol tolerance is observed as a diminished response to a given dose as a function of repeated administrations of the drug. As tolerance develops, higher doses of alcohol are needed to reinstate the initial effect. Thus, alcohol tolerance has become recognized as a factor that may contribute to alcohol abuse and dependence by encouraging the use of higher doses (American Psychiatric Association 1994). It is generally assumed that heavier drinkers should display reduced reactions to a dose of alcohol (i.e., tolerance), whereas lighter drinkers should be more affected by the same dose. Indeed, these assumptions are supported by laboratory research. Early studies compared alcohol responses in healthy adults to those displayed by alcohol-dependent individuals who were in treatment (Goldberg 1943; Mendelson and Mello 1966; Nathan et al. 1971). These studies found that alcohol-dependent drinkers displayed less behavioral impairment to alcohol compared with healthy controls. More recent studies have also shown that tolerance can be observed in relation to the drinking habits of non-dependent, –social drinkers,–such that those who drink frequently and engage in binge drinking display less impairment than lighter, infrequent drinkers (e.g., Fillmore and Vogel-Sprott 1995; Holdstock et al. 2000; Townshend and Duka 2005; Brumback et al. 2007). Taken together, the evidence suggests that tolerance may be readily influenced by recent drinking patterns which do not necessarily reflect alcohol abuse or dependence.
Although such evidence might suggest that tolerance is a ubiquitous phenomena produced by a history of heavy consumption, the evidence to date has focused almost exclusively on measures of motor performance, such as body sway, hand steadiness, and visuo-motor tracking. However, in recent years, there have been major advancements in the identification of specific behavioral and cognitive processes by which alcohol impairs self-regulation, and comparatively little is known about the development of tolerance to alcohol impairment of these mechanisms. Human laboratory studies have employed stop-signal and cued go/no-go models to evaluate behavioral control as the ability to activate and inhibit prepotent (i.e., instigated) responses (Logan and Cowan 1984; Miller et al. 1991; Logan 1994). These models are based on reaction time tasks that require individuals to quickly activate a response to a go-signal and inhibit a response when a stop or no-go signal occurs. Studies show that these mechanisms of behavioral control are sensitive to the disruptive effects of alcohol. Following administration, alcohol increases inhibitory failures and slows response activation in a dose-dependent manner (Fillmore et al. 2005; Fillmore and Weafer 2004). Moreover, alcohol-induced impairments in inhibitory control have been linked to abuse potential. Studies suggest that acute impairments of inhibition might reduce the ability to terminate drinking behavior during a drinking episode, thus resulting in excessive, binge drinking (Fillmore 2003a, b; 2007). As such, it is important to determine if recent drinking patterns might also affect tolerance to alcohol impairment of inhibitory control.
The purpose of the present study was to examine the relationship between recent drinking habits and the degree to which alcohol impairs drinkers’ inhibitory mechanisms of behavioral control. The study examined a large group of non-dependent adult drinkers who reported a wide range of drinking habits (both quantity and frequency). Behavioral effects were tested in response to a moderate dose of alcohol (0.65 g/kg) and a placebo (0.0 g/kg). In addition to examining inhibitory control, the study also included a measure of motor coordination, which has shown tolerance as a function drinking habits in previous research (e.g., Fillmore and Vogel-Sprott 1996; Schweizer et al. 2004; Fillmore et al. 2005).
Methods
Participants
Fifty-two adult drinkers (25 men and 27 women) between the ages of 21 to 33 (mean age = 23.6, SD = 3.3) participated in this study. The sample was comprised of 7 African- American, 1 Asian, and 45 Caucasian participants. Volunteers who self-reported head trauma, a diagnosis of a psychiatric disorder, or current substance abuse disorder were excluded from participation. Volunteers who reported alcohol dependence, as determined by a score of 5 or higher on the Short-Michigan Alcoholism Screening Test (S-MAST; Seltzer et al. 1975) were also excluded.
Potential volunteers had to report drinking at least once per month in an amount of at least two drinks to participate. With regard to other drug use, the majority of the sample reported using caffeine (n = 44). Those who use caffeine reported drinking caffeinated beverages an average of 4.9 (SD = 2.4) days per week. Twelve participants reported smoking cigarettes. Out of those who reported smoking, one participant reported smoking more than a pack of cigarettes (i.e., 20 cigarettes) a day, while the others (n = 11) reported smoking less than a pack of cigarettes a day. Eight participants reported some past month use of marijuana. No other drug use in the past month, including stimulants, opiates, or cocaine, was reported. All participants were in good health with no contraindications to alcohol consumption. The University of Kentucky Medical Institutional Review Board approved the study, and participants received $85.
Materials and measures
Cued go/no-go task
Response inhibition was measured using a cued go/no-go task that has been used in previous research (e.g., Fillmore et al. 2005; Marczinski and Fillmore 2003). E-Prime experiment generation software (Schneider et al. 2002) was used to operate the task, which was performed on a computer. Cues provide preliminary information regarding the type of imperative target stimulus (i.e., go or no-go) that is likely to follow, and the cues have an 80 % probability of signaling the correct target (thus on 20 % of these trials, the cue will precede an incorrect target). Participants were instructed to press the forward (/) slash key on the keyboard as soon as a go (green) target appeared and to suppress the response when a no-go (blue) target was presented. Key presses were made with the right index finger. To encourage quick and accurate responding, feedback was presented to the participant during the inter-trial interval by displaying the words correct or incorrect along with the RT in milliseconds. A test required approximately 15 min to complete.
Grooved pegboard task
Motor coordination was measured by a grooved pegboard task (Lafayette Instruments). The task consists of a 5×5 in. board that contains 25 holes arranged in five rows of five holes each. The holes are –keyhole–shaped and the pegs fit into them as a key would fit into a lock. Using their dominant hand, participants take pegs from a tray one at a time and fit them in the holes, filling in each row at a time from left to right. Extra pegs are available to replace any dropped pegs during the trial. A trial is complete after all holes are filled. The time to complete a trial (in seconds) is the measure of interest. A test consists of four trials.
Timeline follow-back
The timeline follow-back (TLFB) (Sobell and Sobell 1992) provides an assessment of drinking habits over the past 3 months. Four measures of drinking habits for the past 3 months were obtained: (1) total number of drinking days for that period (drinking days), (2) total number of drinks consumed in that period (total drinks), (3) total number of days in which participants reported feeling drunk (drunk days), and (4) total number of days on which binge drinking occurred (binge days). A binge was defined as drinking an amount of alcohol sufficient to elevate a subject’s BAC to 0.08 % (80 mg/100 ml) or higher (NIAAA 2004). To determine the number of binge days, an estimate of BAC was calculated based on the number of drinks consumed, the type of alcohol consumed, the time span in hours spent drinking, and gender and body weight. This was done using well-established, valid anthropometric-based BAC estimation formulae which assume an average clearance rate of 15 mg/dl per hour of the drinking episode (Watson et al. 1981; McKim 2007).
Procedure
Volunteers were told that the purpose of the experiment was to study the effects of alcohol on cognitive and behavioral tasks. Sessions were conducted in the Behavioral Pharmacology Laboratory of the Department of Psychology, and participants were tested individually. Before test sessions, participants were instructed to fast for 4 h and to abstain from alcohol for 24 h. Prior to sessions, participants provided urine samples that were tested for drug metabolites, including amphetamine, barbiturates, benzodiazepines, cocaine, opiates, and tetrahydrocannabinol (On Trak TesTstiks, Roche Diagnostics Corporation, Indianapolis, IN) and pregnancy in the female participants (Mainline Confirms HGL, Mainline Technology, Ann Arbor, MI). A zero BAC was verified for participants from breath samples. Participants completed an intake session to provide background information and to become acquainted with laboratory procedures and the behavioral tasks.
Test sessions
Task performance was tested under two doses of alcohol: 0.0 g/kg (placebo) and 0.65 g/kg. Each dose was administered during a separate test session, and dose order was counterbalanced across participants. Sessions were separated by a minimum of 1 day and a maximum of 1 week. Alcohol doses were calculated on the basis of body weight and administered as absolute alcohol mixed with three parts carbonated soda. The placebo dose (0.0 g/kg) consisted of a volume of carbonated mix that matched the total volume of the 0.65 g/kg alcohol drink. A small amount (3 ml) of alcohol was floated on the surface of the beverage. It was sprayed with an alcohol mist that resembled condensation and provided a strong alcoholic scent as the beverage was consumed. Previous research has shown that individuals report that this beverage contains alcohol (Fillmore and Vogel-Sprott 1998). Drinks were consumed in 6 min. Following 0.65 g/kg alcohol, a peak BAC of 80 mg/ 100 ml was expected to occur approximately 60 min after drinking (Fillmore and Vogel-Sprott 1998).
Participants were tested on the ascending limb of the BAC curve. Thirty minutes after drinking began, they performed the 20-min test battery, which consisted of the cued go/no-go and grooved pegboard tasks. BACs were measured at 30 and 50 min post-drinking (i.e., before and after testing occurred). Breath samples were also obtained at these times during the placebo session ostensibly to measure BACs. After testing, participants received a meal and were released once their BAC fell below 20 mg/100 ml.
Criterion measures
Failures of response inhibition were measured as the proportion of no-go targets in which a participant failed to inhibit a response. The measure of interest was the proportion (p) of inhibition failures in the go cue (i.e., prepotent) condition. Greater p-inhibition failures indicate poorer inhibitory control (i.e., disinhibition). The pegboard task measured motor coordination as the number of seconds required to fit all of the pegs into the board averaged across the four trials. Longer mean completion times indicated poorer motor coordination.
Data analyses
Dependent measures were analyzed by one-way repeated measures analyses of variance (ANOVA) testing the main effect of dose (0.0 vs. 0.65 g/kg) on performance. Initially, all analyses were conducted with gender and dose order (placebo first vs. alcohol first) as between-subjects factors. There were no significant main effects or significant interactions involving gender or dose order for any of the dependent measures of interest. Therefore, all subsequent analyses presented were collapsed across gender and dose order.
The relationship of each drinking habit measure to the degree of alcohol impairment on inhibitory control and motor coordination was tested by regression analyses. For each regression, the individual drinking habit measure from the TLFB was entered as the independent (i.e., predictor) variable, and the magnitude of the alcohol effect on each behavioral measure (motor coordination, inhibitory control) was the dependent measure. To determine the magnitude of alcohol effects, impairment scores were calculated by subtracting each participant’s performance score following placebo from his or her score following 0.65 g/kg alcohol. The impairment score for motor coordination was calculated by subtracting the task completion time following placebo from the completion time following alcohol such that larger scores indicated greater impairment. For inhibitory control, impairment scores were calculated by subtracting p failures following placebo from p failures following alcohol such that larger impairment scores indicated greater impairment of inhibitory control.
Results
Blood alcohol concentrations
BACs in the active dose condition were analyzed by a 2 (gender)×2 (time) mixed-design ANOVA. No main effect or interaction involving gender was observed (ps>0.10). There was a main effect of time, F (1, 50) = 20.5, p<0.01, owing to an increase in BAC on the ascending limb of the BAC curve when testing occurred. For the entire sample, the mean BAC was 76.2 mg/100 ml (SD = 21.2) at the beginning of the test (30 min after drinking) and 84.9 mg/100 ml (SD = 14.4) at the conclusion of the test (50 mins after drinking). No detectable BACs were observed under the placebo condition.
Alcohol effects
Alcohol increased the mean p-inhibition failures from 0.088 (SD = 0.12) to 0.132 (SD = 0.11). For motor coordination, alcohol increased the mean time needed to complete the task from 52.2 s (SD = 6.77) to 55.5 s (SD = 8.00). The effects of alcohol on inhibitory control and motor coordination were analyzed by individual, one-way repeated measures ANOVAs. Significant main effects of dose on performance were found for both p-inhibition failures, F (1, 51) = 13.1, p<0.01 and pegboard performance, F (1, 51) = 26.1, p<0.001.
Drinking habits and alcohol impairment
Table 1 presents participants’ drinking habits as reported on the TLFB. As the table shows, there was substantial variation with regard to the frequency and quantity of consumption reported. Some participants reported infrequent, light drinking (e.g., drinking less than twice a month over the past 3 months, and never binge drinking). Others reported drinking frequently (e.g., consuming alcohol on 86 out of the past 90 days) and in consistently large amounts (e.g., binge drinking on nearly one third of the past 90 days).
Table 1.
Descriptive statistics for drinking habits over the past 90 days as reported on the timeline follow-back (TLFB), N = 52
| Mean | SD | Minimum | Maximum | |
|---|---|---|---|---|
| Measure | ||||
| Drinking days | 26.9 | 17.7 | 5.0 | 86.0 |
| Total drinks | 120.5 | 94.3 | 20.0 | 371.0 |
| Binge days | 10.3 | 10.0 | 0.0 | 34.0 |
| Drunk days | 9.1 | 7.6 | 0.0 | 27.0 |
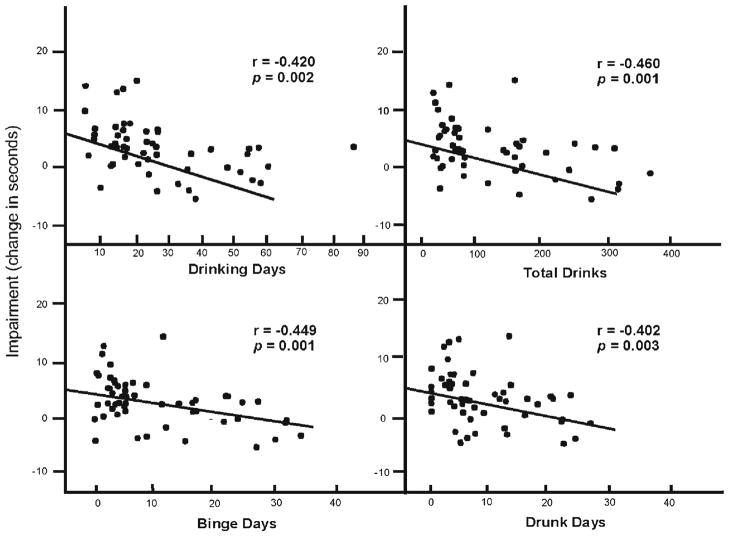
To test the relationship between drinking habits and alcohol impairment, zero-order regression analyses of each drinking habit measure onto the drinkers’ alcohol impairment scores for motor coordination and for inhibitory control were performed. For motor coordination, each drinking habit measure bore a significant negative relationship to the degree of motor impairment observed in response to alcohol (ps<0.01). Figure 1 plots the relationships between alcohol impairment of motor coordination and subjects’ drinking habits. The figure shows that impairment was inversely related to the drinkers’ levels of recent alcohol consumption. Specifically, those who reported drinking the most days and in the greatest quantities were the least impaired and those who reported drinking less frequently and in lower quantities were the most impaired.
Fig. 1.
Scatter plot of the relationship between drinking habit measures and the change in performance on the grooved pegboard task under placebo to 0.65 g/kg alcohol. Impairment is expressed as an increase in the time (in seconds) to complete the test under alcohol versus placebo. The least-squares regression lines are derived from a simple linear regression of each drinking habit measure and the change in performance score. Pearson r correlation coefficients and p values are presented for each relationship
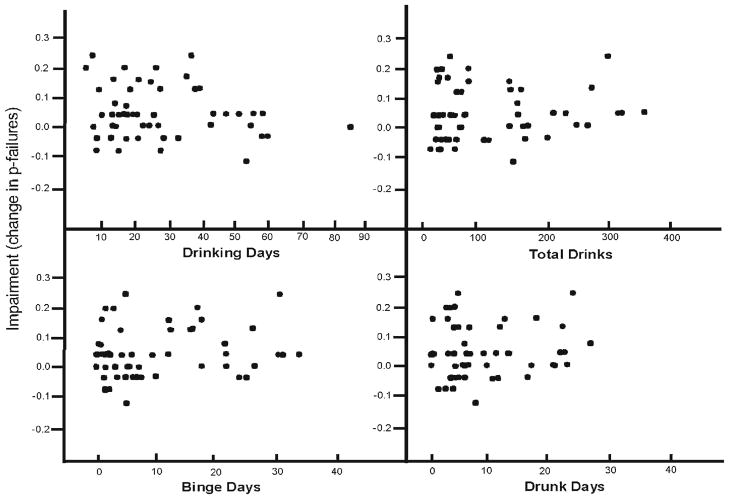
In contrast to motor impairment, none of the drinking habits bore a significant relationship with the degree of impaired inhibitory control displayed by participants (ps> 0.21). Figure 2 plots the individual differences in impaired inhibitory control. Some drinkers displayed considerable increases in inhibitory failures under alcohol, whereas others showed little or no impairment. However, unlike motor coordination, the figure shows that recent heavy drinking was not associated with reduced impairment of inhibitory control.
Fig. 2.
Scatter plot of the relationship between drinking habits and the changes in p failures under placebo and 0.65 g/kg alcohol. Impairment is expressed as an increase in p failures under alcohol versus placebo
The possibility that drinking history is related to baseline performance of motor coordination and inhibitory control was also tested. Separate regression analyses were performed by regressing each drinking habit measure on the mean p-inhibition and motor coordination score following placebo. The analyses revealed no significant relationships between any of the drinking habit measures and motor coordination (ps>0.08) or inhibitory control (ps>0.46) following placebo.
Drinking habits and BAC
Extended periods of heavy drinking can activate additional enzymes, such as the microsomal ethanol oxidizing system, to hasten the metabolism of alcohol resulting in faster elimination and lower BACs. The possibility that such metabolic tolerance could account for the observed relationship between heavy drinking and reduced motor impairment was examined by determining the degree to which individual differences in drinkers’ BACs during testing were related to their drinking habits. Tests of zero-order correlations showed that neither BAC at the beginning of testing (30 min) or at the conclusion of testing (50 min) bore a significant relationship with any drinking habit measure (ps >0.27).
Discussion
This study examined the development of chronic tolerance to alcohol’s impairing effects on motor coordination and inhibitory control as a function of recent drinking habits. The results revealed significant relationships between drinkers’ recent patterns of alcohol consumption and the degree to which alcohol impaired their motor coordination. These were negative relationships, such that heavier, more frequent drinking and more binge drinking episodes predicted less alcohol impairment of motor coordination. This is in accord with the notion that the quantity and frequency of recent alcohol consumption can contribute to the development of chronic tolerance. However, drinking habits bore no relationship to the degree of alcohol impairment of inhibitory control. These results suggest that heavy alcohol use can lead to tolerance to the drug’s motor impairing effects, but not to its disinhibiting effects.
A failure to observe a relationship between drinking habits and the degree to which alcohol impaired subjects’ inhibitory control cannot be due to limited range in drinking habits or to a lack of sensitivity of inhibitory control to the impairing effects of alcohol in this study. Participants’ drinking habits were carefully assessed over a sustained period of time using a 90-day assessment tool, the timeline follow-back. A wide range of drinking behavior both in terms of typical quantity and frequency of consumption was observed. Indeed, this range of consumption was sufficient to display a relationship with the degree to which alcohol impaired the subjects’ motor coordination. With regard to the sensitivity of our behavioral assessments, we observed significant alcohol impairment of both motor coordination and inhibitory control in response to the dose tested. Moreover, we observed substantial variation in the degree to which subjects were impaired on both tasks.
It is not clear why inhibitory control might fail to develop tolerance to the impairing effects of alcohol as a function of recent heavy drinking. There is a growing body of research that suggests that inhibitory control might be especially vulnerable to the disruptive effects of alcohol compared with other behavioral functions. For example, studies show that inhibitory control is impaired at BACs that are insufficient to impair other behavioral functions, such as reaction time (Fillmore and Vogel-Sprott 1999; de Wit et al. 2000). Alcohol-induced impairment of inhibitory control also fails to show acute recovery (i.e., acute tolerance) within a single drinking session. Studies have shown that impaired motor coordination and reaction time display acute tolerance while response inhibition remains impaired from the ascending to the descending limb (e.g., Fillmore et al. 2005; Ostling and Fillmore 2010). The current findings build upon this earlier work by suggesting that the lack of acute tolerance to alcohol observed in inhibitory control might contribute to the lack of chronic tolerance found in the present study.
A lack of tolerance to alcohol’s disinhibiting effects might also contribute to heavy, binge drinking. Many drinkers report intentions to limit their alcohol consumption only to fail and drink excessively, fueling the notion that alcohol reduces control over consumption in some individuals (Collins 1993). Thus, impairment of inhibitory mechanisms from an initial dose of alcohol could compromise the ability to stop subsequent administrations of alcohol in a drinking situation, resulting in a binge episode. A failure of inhibitory control to adapt to the impairing effects of alcohol even after sustained heavy use might pose a potential risk for impulsive, disinhibited behavior.
The present study focused on one aspect of behavioral control (i.e., the ability to inhibit a prepotent response). However, the findings raise questions about how tolerance might fail to develop to alcohol impairment on a broad range of inhibitory functions. For instance, another component of disinhibition is attentional control, which refers to the ability to ignore irrelevant information. Researchers have identified inhibitory mechanisms that gate the influence of irrelevant information (Houghton and Tipper 1994). Alcohol has been shown to impair these inhibitory mechanisms, resulting in a decreased ability to direct attention from distractions (Fillmore et al. 2000; Abroms and Fillmore 2004). Moreover, such impairments in mechanisms of attentional control have also been implicated as a factor that might contribute to alcohol abuse (Tarter et al. 2004; Blume et al. 2005). As such, it is important to examine whether alcohol-induced impairments of attentional control also fail to develop tolerance as a function of frequent, heavy drinking. Such a possibility remains to be examined.
As with all correlational studies, this study cannot demonstrate a specific causal relationship between recent, heavy consumption and tolerance to alcohol’s impairing effects on motor coordination. It is reasonable to suggest that the observed relationship between drinking habits and the degree to which alcohol impaired motor coordination is evidence of the physiological development of tolerance as a function of more frequent, heavy consumption. Indeed, there was wide variation in the drinking patterns in this sample, with heavier drinkers consuming alcohol in vastly greater amounts and with more frequency than lighter drinkers. As such, it is likely that the reduced impairment or motor coordination by heavier drinkers was observed as tolerance. However, other possible explanations for the observed results remain. For instance, it might be the case that for some, a reduced response to alcohol might actually precede heavy drinking. In fact, it has been suggested that differences in the degree to which alcohol impairs behavior represent –behavioral markers–that are related to other factors, such as a family risk of alcoholism that contribute to the development of alcohol-related problems (e.g., Eng et al. 2005; Chung and Martin, 2009). Specifically, it might be the case that a low level of responding to alcohol might predict later alcohol abuse and dependence, as the drinker must consume more alcohol to achieve the desirable effects (Shuckit 2009). The present findings are consistent with this notion, in that the low level of impairment shown by some of the drinkers in the sample might promote the heavy drinking that they report.
Finally, it is also possible that other factors might contribute to the degree to which individuals display tolerance. Some factors that differentiate heavier from lighter drinkers include demographics such as age, gender, and socioeconomic status, with those who drink more heavily and use drugs being more likely to be male, younger, and of a lower socioeconomic status than lighter, social drinkers (e.g., Stinson et al. 2005). Illicit drug use is another factor that might be related to alcohol tolerance. Many drinkers, particularly those who report heavy, problematic consumption, also engage in regular illicit drug use, such as cocaine and methamphetamine (i.e., Kandel and Yamaguchi 2001; Degenhardt et al. 2001; Wagner and Anthony 2002). Studies suggest that alcohol tolerance also might be enhanced by a history of co-administration with stimulant drugs (e.g., Fillmore 2003a, b). The sample in our study was comprised of relatively young drinkers who reported little to no use of other drugs. Thus, we could not examine the relation between of alcohol impairment and history of illicit drug use. Given that alcohol tolerance may contribute to abuse by encouraging the use of escalating doses, it is important to determine how alcohol tolerance might be affected by a history of coadministration with other drugs.
Acknowledgments
This research was supported by National Institute on Alcohol Abuse and Alcoholism Grants R01 AA018274 and R01 AA012895, and by National Institute on Drug Abuse Grants P50 DA005312 and T32 DA07304. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism, National Institute on Drug Abuse, or the National Institutes of Health.
Contributor Information
Melissa A. Miller, Department of Psychology, University of Kentucky, Lexington, KY 40506-0044, USA
Lon R. Hays, Department of Psychology, University of Kentucky, Lexington, KY 40506-0044, USA
Mark T. Fillmore, Email: Fillmore@uky.edu, Department of Psychology, University of Kentucky, Lexington, KY 40506-0044, USA
References
- Abroms BD, Fillmore MT. Alcohol-induced impairment of inhibitory mechanisms involved in visual search. Exp Clin Psychopharmacol. 2004;12:243–250. doi: 10.1037/1064-1297.12.4.243. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: 1994. [Google Scholar]
- Blume AW, Schmaling KB, Marlatt GA. Memory, executive cognitive function, and readiness to change drinking behavior. Addict Behav. 2005;30:301–314. doi: 10.1016/j.addbeh.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Brumback T, Cao D, King A. Effects of alcohol on psychomotor performance and perceived impairment in heavy binge social drinkers. Drug Alcohol Depend. 2007;91:10–17. doi: 10.1016/j.drugalcdep.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung T, Martin CS. Subjective stimulant and sedative effects of alcohol during early drinking experiences predict alcohol involvement in treated adolescents. J Stud Alcohol Drugs. 2009;70:660–667. doi: 10.15288/jsad.2009.70.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins RL. Drinking restraint and risk for alcohol abuse. Exp Clin Psychopharm. 1993;1:44–54. [Google Scholar]
- De Wit H, Crean J, Richards JB. Effects of d-amphetamine and ethanol on a measure of behavioral inhibition in humans. Beh Neurosci. 2000;114:830–837. doi: 10.1037//0735-7044.114.4.830. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Hall W, Lynskey M. Alcohol, cannabis and tobacco use among Australians: a comparison of their associations with other drug use disorders, affective and anxiety disorders and psychosis. Addiction. 2001;96:1603–1614. doi: 10.1046/j.1360-0443.2001.961116037.x. [DOI] [PubMed] [Google Scholar]
- Eng MY, Schuckit MA, Smith TL. The level of response to alcohol in daughters of alcoholics and controls. Drug Alcohol Depend. 2005;79:83–93. doi: 10.1016/j.drugalcdep.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Fillmore MT. Alcohol tolerance in humans is enhanced by prior caffeine-antagonism of alcohol-induced impairment. Exp Clin Psychoparm. 2003a;11:9–17. doi: 10.1037//1064-1297.11.1.9. [DOI] [PubMed] [Google Scholar]
- Fillmore MT. Drug abuse as a problemof impaired control: current approaches and findings. Behav Cog Neurosci Rev. 2003b;2:179–197. doi: 10.1177/1534582303257007. [DOI] [PubMed] [Google Scholar]
- Fillmore MT. Acute alcohol-induced impairment of cognitive functions; past and present findings. Int J Dis Hum Dev. 2007;6:115–125. [Google Scholar]
- Fillmore MT, Vogel-Sprott M. Behavioral effects of alcohol in novice and experienced drinkers: alcohol expectancies and impairment. Psychopharmacology. 1995;122:175–181. doi: 10.1007/BF02246092. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. Social drinking history, behavioral tolerance and the expectation of alcohol. Psychopharmacology (Berl) 1996;127:359–364. doi: 10.1007/s002130050098. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. Behavioral impairment under alcohol: cognitive and pharmacokinetic factors. Alcohol Clin Exp Res. 1998;22:1476–1482. [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. An alcohol model of impaired inhibitory control and its treatment in humans. Exp Clin Psychoparm. 1999;7:49–55. doi: 10.1037//1064-1297.7.1.49. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Weafer J. Alcohol impairment of behavior in men and women. Addiction. 2004;99:1237–1246. doi: 10.1111/j.1360-0443.2004.00805.x. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Dixon MJ, Schweizer TA. Alcohol affects processing of ignored stimuli in a negative priming paradigm. J Stud Alcohol. 2000;61:571–278. doi: 10.15288/jsa.2000.61.571. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Marczinski CA, Bowman AM. Acute tolerance to alcohol effects on inhibitory and activational mechanisms of behavioral control. J Stud Alcohol. 2005;33:663–672. doi: 10.15288/jsa.2005.66.663. [DOI] [PubMed] [Google Scholar]
- Goldberg L. Quantitative studies on alcohol tolerance in man: the influence of ethyl alcohol on sensory, motor, and psychological functions referred to blood alcohol in normal and habituated individuals. Acta Physiol Scand. 1943;5:1–128. [Google Scholar]
- Holdstock L, King AC, de Wit H. Subjective and objective responses to ethanol in moderate/heavy and light social drinkers. Alcohol Clin Exp Res. 2000;24:789–794. [PubMed] [Google Scholar]
- Houghton G, Tipper SP. A model of inhibitory mechanisms of selective attention. In: Dagbach D, Carr TH, editors. Inhibitory processes in attention, memory, and language. Academic Press; San Diego: 1994. pp. 53–112. [Google Scholar]
- Kandel D, Yamaguchi K. From beer to crack: developmental patterns of drug involvement. Am J Public Health. 2001;83:851–855. doi: 10.2105/ajph.83.6.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD. On the ability to inhibit thought and action: a user’s guide to the stop signal paradigm. In: Dagenbach D, Carr TH, editors. Inhibitory processes in attention, memory, and language. Academic Press; San Diego: 1994. pp. 189–239. [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: a theory of an act of control. Psychol Rev. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT. Preresponse cues reduce the impairing effects of alcohol on the execution and suppression of responses. Exp Clin Psychopharmacol. 2003;11:110–117. doi: 10.1037//1064-1297.11.1.110. [DOI] [PubMed] [Google Scholar]
- McKim WA. Drugs and behavior: an introduction to behavioral pharmacology. 6. Prentice Hall; New Jersey: 2007. [Google Scholar]
- Mendelson JH, Mello NK. Experimental analysis of drinking behavior of chronic alcoholics. Ann NY Acad Sci. 1966;133:828–845. doi: 10.1111/j.1749-6632.1966.tb50930.x. [DOI] [PubMed] [Google Scholar]
- Miller J, Schaffer R, Hackley SA. Effects of preliminary information in a go versus no-go task. Acta Psychol. 1991;76:241–292. doi: 10.1016/0001-6918(91)90022-r. [DOI] [PubMed] [Google Scholar]
- Nathan PE, O’Brien JS, Lowenstein LM. Operant studies of chronic alcoholism: interaction of alcohol and alcoholics. In: McIsaac W, Creaven P, editors. Biological aspects of alcohol. University of Texas Press; Austin: 1971. pp. 341–370. [Google Scholar]
- NIAAA (National Institute on Alcohol Abuse and Alcoholism) . Winter) NIAAA council approves definition of binge drinking. Newsletter. 2004;3:3. [Google Scholar]
- Ostling EW, Fillmore MT. Tolerance to the impairing effects of alcohol on the inhibition and activation of behavior. Psychopharmacology. 2010;212:465–473. doi: 10.1007/s00213-010-1972-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime user–s guide. Psychology Software Tools; Pittsburgh: 2002. [Google Scholar]
- Schuckit MA. An overview of genetic influences in alcoholism. J Subst Abuse Treat. 2009;36:S5–S14. [PubMed] [Google Scholar]
- Schweizer TA, Jolicoeur P, Vogel-Sprott M, Dixon MJ. Fast, but error-prone, responses during acute alcohol intoxication: effects of stimulus-response mapping complexity. Alcohol Clin Exp Res. 2004;28:643–649. doi: 10.1097/01.alc.0000121652.84754.30. [DOI] [PubMed] [Google Scholar]
- Seltzer ML, Vinokur A, Rooijen L. A self-administered Short Michigan Alcoholism Screening Test (SMAST) J Stud Alcohol. 1975;36:117–126. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- Sobell L, Sobell M. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring alcohol consumption: psychosocial and biochemical methods. Human Press; New Jersey: 1992. pp. 41–72. [Google Scholar]
- Stinson FS, Grant BF, Dawson DA, Ruan WJ, Huang B, Saha T. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: results from the National Epidemiological Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2005;80:105–116. doi: 10.1016/j.drugalcdep.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Habeych M, Reynolds M, Vanyukov M. Neurobehavior disinhibition in childhood predisposes boys to substance use disorder by young adulthood: direct and mediated etiologic pathways. Drug Alcohol Depend. 2004;73:121–132. doi: 10.1016/j.drugalcdep.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Towhshend JM, Duka T. Binge drinking, cognitive performance and mood in a population of young social drinkers. Alcohol Clin Exp Res. 2005;29:317–325. doi: 10.1097/01.alc.0000156453.05028.f5. [DOI] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC. Into the world of illegal drug use: exposure opportunity and other mechanisms linking the use of alcohol, tobacco, marijuana, cocaine. Am J Epidemiol. 2002;155:918–925. doi: 10.1093/aje/155.10.918. [DOI] [PubMed] [Google Scholar]
- Watson PE, Watson ID, Batt RD. Prediction of blood alcohol concentrations in human subjects: updating the Widmark equation. J Stud Alcohol. 1981;42:547–556. doi: 10.15288/jsa.1981.42.547. [DOI] [PubMed] [Google Scholar]