Abstract
Fibroblast growth factor-21 (FGF21) is a potential metabolic regulator with multiple beneficial effects on metabolic diseases. FGF21 is mainly expressed in the liver, but is also found in other tissues including the intestine, which expresses β-klotho abundantly. The intestine is a unique organ that operates in a physiologically hypoxic environment, and is responsible for the fat absorption processes including triglyceride breakdown, re-synthesis and absorption into the portal circulation. In the present study, we investigated the effects of hypoxia and the chemical hypoxia inducer, cobalt chloride (CoCl2), on FGF21 expression in Caco-2 cells and the consequence of fat accumulation. Physical hypoxia (1% oxygen) and CoCl2 treatment decreased both FGF21 mRNA and secreted protein levels. Gene silence and inhibition of hypoxia-inducible factor-α (HIFα) did not affect the reduction of FGF21 mRNA and protein levels by hypoxia. However, CoCl2 administration caused a significant increase in oxidative stress. The addition of n-acetylcysteine (NAC) suppressed CoCl2-induced reactive oxygen species (ROS) formation and completely negated CoCl2-induced FGF21 loss. mRNA stability analysis demonstrated that the CoCl2 administration caused a remarkable reduction in FGF21 mRNA stability. Furthermore, CoCl2 increased intracellular triglyceride (TG) accumulation, along with a reduction in mRNA levels of lipid lipase, hormone sensitive lipase (HSL) and adipose triglyceride lipase (ATGL), and an increase of sterol regulatory element-binding protein-1c (SREBP1c) and stearoyl-coenzyme A (SCD1). Addition of both NAC and recombinant FGF21 significantly attenuated the CoCl2-induced TG accumulation. In conclusion, the decrease of FGF21 in Caco-2 cells by chemical hypoxia is independent of HIFα, but dependent on an oxidative stress-mediated mechanism. The regulation of FGF21 by hypoxia may contribute to intestinal lipid metabolism and absorption.
Keywords: Fibroblast growth factor-21, Hypoxia, Cobalt chloride, Hypoxia-inducible factor, Caco-2
Introduction
Fibroblast growth factor (FGF)-21 (FGF21) is a member of FGF superfamily and has been identified as a potent metabolic regulator. FGF21 levels have been correlated to responses to fasting metabolism, which regulates hepatic gluconeogenesis, adipose lipolysis in adipocytes, provides fatty acids to the liver for ketogenesis, and torpor (Badman et al., 2007; Inagaki et al., 2007). Pharmacological administration of FGF21 produces glucose and lipid lowering effects in rodents and rhesus monkeys with diet-induced or genetic obesity and diabetes (Kharitonenkov et al., 2005; Kharitonenkov et al., 2007). FGF21 has broad metabolic actions that include enhancing insulin sensitivity, decreasing plasma glucose and triglyceride concentration, lowering LDL cholesterol, increasing HDL cholesterol and reducing body weight (Kharitonenkov et al., 2005; Kharitonenkov et al., 2007; Coskun et al., 2008; Xu et al., 2009). Recent mechanistic studies have demonstrated that FGF21 is regulated by peroxisome proliferator-activated receptor (PPAR)-α in the liver and PPARγ in adipose tissues (Badman et al., 2007; Muise et al., 2008). FGF21 exerts its functions through activation of a unique dual receptor system including tyrosine kinase, FGF receptors (FGFRs), and a co-receptor, β-klotho (Ogawa et al., 2007). The functions of FGF21 are also regulated by PPARγ coactivator-1α (PGC1α) (Estall et al., 2009), a key regulator of energy homeostasis, and by activation of the AMP-activated protein kinase-sirtuin 1 pathway (Chau et al., 2010).
Previous studies have suggested that the liver is the major tissue for FGF21 expression and that the adipose tissue is the major target of FGF21 function (Hotta et al., 2009). FGF21 plays an important role in regulating energy homeostasis during fasting and is required for activation of hepatic lipid oxidation and triglyceride (TG) clearance. It has also been demonstrated that FGF21 is abundantly expressed in other tissues including the intestine (Fon Tacer et al., 2010), indicating a possible role of FGF21 in intestine. Interestingly, its unique co-receptor, β-Klotho, is highly expressed in colon tissues (Fon Tacer et al., 2010). However, the role of FGF21 and how it is regulated in the intestine are not known.
The intestine absorbs and transports the nutrients from the diet. Dietary triglyceride (TG) is hydrolyzed into free fatty acids (FFA) in the intestine lumen, which they, in turn, are taken up by enterocytes from their apical side, transported to the endoplasmic reticulum (ER), re-synthesized into TG, and assembled into chylomicrons and transported into blood stream. Therefore, this intestine-mediated TG metabolism process is critical for whole body energy homeostasis (Green and Glickman, 1981; Shepherd, 1994). Intestinal epithelial cells are positioned between an anaerobic lumen and a highly metabolic lamina propria (Taylor and Colgan, 2007). Hypoxia exists under physiological conditions and becomes severe under pathological conditions when more oxygen is demanded due to metabolism of nutrients and a decreased oxygen supply. Prolonged hypoxia has many cytotoxic effects including oxidative stress (LeGrand and Aw, 1996). Adaptation to hypoxia in the intestine is mainly provided by regulation of a transcription factor, hypoxia-inducible factor (HIF), which up-regulates many genes that mediate cellular energy homeostasis.
To elucidate the correlation of FGF21 regulation and hypoxia in TG metabolism in the intestine, the present study investigated the mechanisms of how hypoxia regulates intestinal FGF21 expression and the effects on TG metabolism with a chemical exposure that has a similar effect as oxygen deprivation, which is sometimes called “chemical hypoxia”. We demonstrate that chemical hypoxia induces a down-regulation of FGF21 in Caco-2 cells; this regulation is not associated with HIF stabilization by hypoxia, but depends on hypoxia-induced oxidative stress and mRNA stabilization.
Materials and Methods
Cell Culture and Treatments
Caco-2 cells were purchased from the American Type Culture Collection (Mannasas, VA, Cat#:HTB-37). Caco-2 cells were cultured in EMEM, supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% fetal bovine serum, at 37 °C in a humidified 5% CO2 environment. Culture media were changed every 2 days. Cells were subcultured after partial digestion with 0.25% trypsin-EDTA. Hypoxic conditions were introduced by incubating cells in a tightly sealed chamber maintained at 1% O2, 5% CO2, and balanced with N2 at 37°C. Caco-2 cells were also treated with cobalt chloride (CoCl2), and in some experiments with deferoxamine (DFO). Cells were treated with or without antioxidant n-acetylcysteine (NAC) or manganese [III] tetrakis (4-benzoic acid) porphyrin (MnTBAP) as indicated.
RNA Interference
SiRNAs targeting human HIF1α/2α and a negative mismatched control were designed and synthesized by Ambion (Austin, TX). After monolayer cultures reached 50% confluency, the cells were transfected with 100 nM HIF1α -targeted or negative mismatched siRNA using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instruction. Antibiotics were added to the medium 24 h after transfection, and cells were used for experimental procedures 48 h after transfection.
Nuclear Extract Preparation
In brief, cells were washed once on a dish with ice-cold phosphate-buffered saline (PBS). Ice-cold buffer (10 mM Tris-HCl, pH 7.8, 1.5 mM MgCl2, and 10 mM KCl) containing freshly added 0.4 mM phenylmethylsulfonyl fluoride (PMSF), 0.5 mM dithiothreitol (DTT), and 1% protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) was overlaid on cells in the dish and incubated for 10 min. The cells were then harvested by scraping with a rubber cell policeman and lysed by Dounce homogenization. Nuclei were pelleted by centrifugation of 10,000 g for 10 min and then resuspended in ice-cold buffer (20 mM Tris-HCl, pH 7.8, 420 mM KCl, 1.5 mM MgCl2, and 20% glycerol) containing freshly added 0.4 mM PMSF, 0.5 mM DTT, 1% protease inhibitor cocktail, and 1 mM Na3VO4, and incubated for 30 min on ice with occasional tapping. The extracts were clarified by centrifugation at 12,000 g for 15 min at 4°C, placed in aliquots, and stored at -80°C.
Oil red O staining
Caco-2 cells were seeded in 6-well plate and were cultured in serum-free media overnight. Twenty- four hours after treatment with CoCl2, cells were washed three times with ice-cold PBS and fixed with 4% paraformaldehyde for 1 h. After fixation, cells were washed three times and stained with Oil Red O solution (working solution, 0.5 g Oil Red O powder dissolved in 60% ethanol) for 40 min at room temperature. Cells were washed again with PBS to remove unbound staining. Cells were then observed under a light microscope.
Detection of superoxide formation
Superoxide, one of the major forms of reactive oxygen species (ROS), accumulation in Caco-2 cells was detected by dihydroethidium fluorescence microscopy. Nonfluorescent dihydroethidium is oxidized by superoxide to yield the red fluorescent product, ethidium, which binds to nucleic acids, staining the nucleus a bright fluorescent red. Caco-2 cells in chamber slides were incubated with 5 μM dihydroethidium (Molecular Probes, Eugene, OR) for 30 min at 37°C in the dark. The superoxide-catalyzed ethidium red fluorescence was examined under fluorescence microscopy.
Quantitative Real time RT-PCR
The mRNA expression was assessed by real-time PCR. Total RNA from cultured cells was isolated with Trizol according to manufacturer's protocol (Invitrogen, Carlsbad, CA) and reverse-transcribed using GenAmp RNA PCR kit (Applied Biosystems, Foster City, CA). The cDNA was amplified in 96-well reaction plates with a SYBR green PCR Master Mix (Applied Biosystems) on an ABI 7500 real-time PCR thermocycler. Primer sequences are listed in supplemental table 1. The ddCt-method (change in dCt [=Ct of the target gene minus Ct of the housekeeping gene]) was used for relative quantification. Fold changes in expression were calculated according to the transformation: fold increase =2(-ΔΔCt) . PCR efficiency was tested and ensured to be similar for both the target gene and β-actin control gene (Livak and Schmittgen, 2001).
Western blot analysis
Cells were lysed on ice for 30 min in IPB (50 mM Tris·HCl, pH 7.4, 150 mM NaCl, 2 mM EDTA, 4 mM Na3VO4, 40 mM NaF, 1% Triton X-100, 1 mM PMSF, 1% protease inhibitor cocktail) and centrifuged at 14,000 g for 10 min. The supernatants were collected. An appropriate amount of protein in total cell lysates was resolved in a SDS-polyacrylamide gel and transferred onto a polyvinylidene difluoride membrane (Whatman, Sanford, ME). Membranes were blocked for 1 h in Tris-buffered saline/Tween 20 (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, and 0.05% Tween 20) containing 5% nonfat dry milk and incubated overnight at 4°C with the following primary antibodies diluted in blocking buffer: HIF1α (mouse anti-human mAb, BD Biosciences, San Jose, CA); β-actin (mouse anti-human mAb), PPARα (rabbit anti-human pAb) and PPARγ (mouse anti-human mAb, Santa Cruz Biotechnology, Santa Cruz, CA); and FGF21 (rabbit anti-human pAb, Abcam, San Francisco, CA). After washing with Tris-buffered saline/Tween 20, the membranes were incubated with a horseradish peroxidase-linked anti-mouse or anti-rabbit IgG antibody for 1 h at room temperature. Proteins were visualized using an enhanced chemiluminescence system (GE Healthcare, Piscataway, NJ) and quantified by densitometry analysis.
Triglyceride measurements
Caco-2 cells were seeded in 6-well plates and were cultured in serum-free media overnight. Twenty-four hours after treatment, cells were washed twice with cold PBS and collected. Total TG content in the cells was determined using a TG assay kit (the assay is linear when the TG concentration is up to 885 mg/dl; Wako Chemical, Osaka, Japan). The protein concentration was determined with a BioRad DC Protein Assay kit (Bio-Rad Laboratories, Hercules, CA, USA). TG values were normalized by protein concentration.
FGF21 ELISA assay
Caco-2 cells were plated in 6-well plates one day before treatment. After treatment, the media were collected and concentrated at 10,000 g using Centricon (Millipore, Bedford, MA), with a cut-off of 3 kDa. FGF21 protein levels were analyzed using an ELISA kit according to the manufacturer's instructions (the appropriate range of this assay is 31.25 pg/ml to 2000 pg/ml human FGF21, Millipore, Bedford, MA). All assays were performed in triplicate.
Statistical analysis
All experiments were performed at least three times. Data are expressed as means ± SEM. ANOVA and Newman-Keuls multiple-comparison test were used to determine statistical significance. Differences between groups were considered significant at P<0.05.
Results
Hypoxia down-regulates FGF21 expression
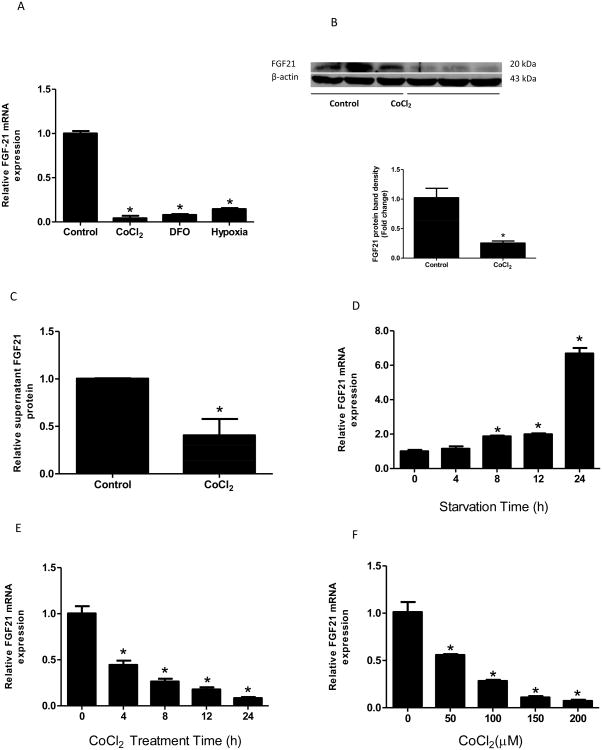
To determine whether hypoxia plays a role in FGF21 regulation in the intestine, we treated Caco-2 cells with well-known chemical hypoxia mimics, CoCl2 or DFO, or exposed cells to 1% O2 in the presence and absence of serum for 24 hours. The levels of FGF21 expression were analyzed by real-time RT-PCR, ELISA and Western blot. Real-time PCR analysis showed that 1% oxygen treatment resulted in a ten-fold reduction in FGF21 mRNA levels. Similarly, CoCl2 treatment dramatically reduced FGF21 mRNA expression by twenty folds. DFO, which depletes cellular Fe2+ and inactivates HIF prolyl hydroxylases, also decreased FGF21 mRNA expression significantly (Fig 1A). Western blot (Fig 1B) and ELISA (Fig 1C) analyses showed a significant decrease in cellular and secreted FGF21 protein levels in CoCl2-treated cells. Serum presence or absence had no effect on the changes of FGF21 mRNA levels under hypoxia treatment (data not shown). Because of the similar effects of CoCl2, DFO and 1% oxygen on FGF21 expression, we used CoCl2 as a hypoxia inducer in the following experiments.
Figure 1. Hypoxia down-regulates FGF21 expression.
Caco-2 cells were exposed to 1% O2 or treated with chemical hypoxia mimics, CoCl2 or DFO, in the absence of serum for 24 hours. FGF21 mRNA (A) and cellular FGF21 protein (B) expression and secreted protein in the medium (C) in Caco-2 cells were measured by real-time PCR, immunoblotting or ELISA, respectively. Caco-2 cells were incubated in serum-free medium for 0, 4, 8, 12, and 24 hours in the absence (D) or presence (E) of CoCl2 (200 μM), and mRNA levels were measured. The cells were exposed to CoCl2 in various concentrations (0, 50, 100, 150, 200 μM) for 24 hours in the absence of serum, and mRNA expression (F) were measured. *P<0.05 vs controls.
Next, we sought to study the mechanism by which CoCl2 regulates FGF21 mRNA expression. Caco-2 cells were exposed to 200 μM CoCl2 for 0, 4, 8, 12, and 24 hours, respectively, or treated with various concentrations of CoCl2 (0, 50, 100, 150, 200 μM) for 24 hours. As shown in Figure 1D, serum starvation increased FGF21 mRNA levels in a time-dependent manner, which is in agreement with the results in animal studies (Inagaki et al., 2007). In contrast, CoCl2 incubation dramatically decreased FGF21 mRNA expression in a time-dependent manner (Fig 1E). The suppression of FGF21 mRNA expression by CoCl2 was also dose-dependent (Fig 1F). In following experiments, a concentration of 200 μM of CoCl2 and a time period of 24 hours were used.
CoCl2 increases HIF1α protein levels
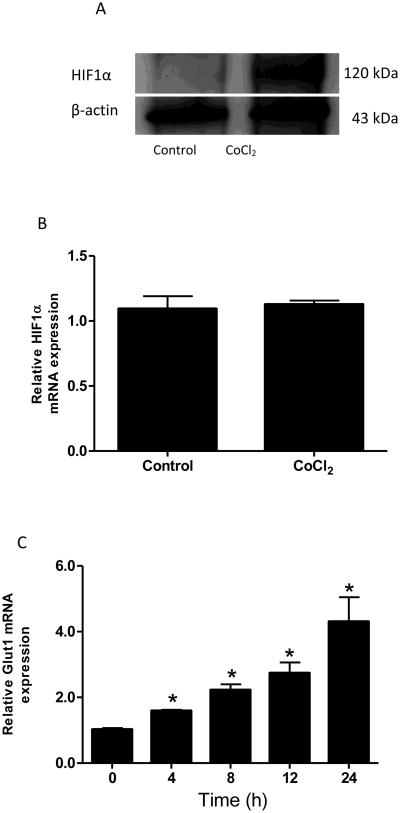
It is well-known that CoCl2 induces HIF1α protein accumulation in a variety of cell types. As anticipated, CoCl2 treatment increased nuclear HIF1α protein level (Fig 2A) in Caco-2 cells, but not the mRNA levels (Fig 2B), which agrees with other studies (Wu et al., 2005). Furthermore, we examined the expression of Glut1, a well-known HIF1α transcriptional target, in response to CoCl2 treatment. As expected, CoCl2 significantly increased Glut1 mRNA expression in Caco-2 cells in a time-dependent manner (Fig 2C).
Figure 2. CoCl2 increases HIF1α protein level.
Caco-2 cells were exposed to CoCl2 in the absence of serum for 24 hours. Nuclear protein (A) and mRNA (B) levels of HIF1α were determined. The cells were incubated for 0, 4, 8, 12, and 24 hours in the presence of CoCl2 (200 μM), and mRNA levels of GLUT1 were measured (C). *P<0.05 vs 0h.
CoCl2 down-regulates FGF21 expression independent of HIF1α/2α
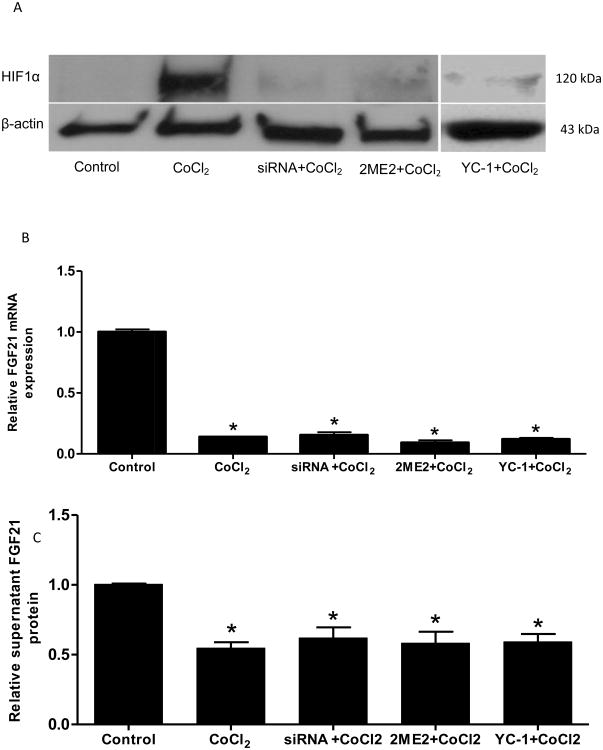
In order to further investigate whether CoCl2-induced FGF21 suppression is mediated by HIF1α, HIF1α gene was silenced by its specific siRNA or HIF1α protein accumulation was inhibited by specific inhibitors, 2-Methoxyestradiol (2ME2) . As shown in Figure 3A, CoCl2 induced a drastic HIF1α protein nuclear accumulation, and it was completely eliminated by siRNA or 2ME2 treatment. However, silencing of the HIF1α gene did not restore the CoCl2-suppressed FGF21 mRNA levels and protein secretion into the medium (Fig 3B). We also utilized two distinct HIF1α inhibitors to further determine the effect of decreased HIF1α expression on FGF21 expression. Caco-2 cells were treated with 2ME2 (100μM), a HIF inhibitor at the posttranscriptional level (Mabjeesh et al., 2003) or YC-1 (50μM), a HIF inhibitor at the post-translational level (Li et al., 2008), in the presence of CoCl2 for 24 hours. The treatment with both inhibitors diminished CoCl2-induced HIF1α protein accumulation (Fig 3A), but failed to restore CoCl2-suppressed FGF21 mRNA and secreted protein levels (Fig 3B &C). Besides HIF1α, Caco-2 cells also express HIF2α. In order to further investigate whether CoCl2-induced decrease in FGF21 expression is mediated by HIF2α, HIF2α gene was silenced by its specific siRNA. Silencing of the HIF2α gene did not restore the CoCl2-suppressed FGF21 mRNA levels (Supplemental Fig 1). Thus, these data strongly suggest that the negative effect of CoCl2 on FGF21 expression in Caco-2 cells is independent of HIF1α/2α.
Figure 3. CoCl2-mediated FGF21 down-regulation is independent of HIF1α/2 α.
Caco-2 cells were transfected with HIF1α siRNA for 48 hours or treated with HIF1α inhibitors, 2ME2 (100 μM) or YC-1(50 μM), for 12 hours, respectively, followed by CoCl2 treatment for 24 hours. Expression of HIF1α protein (A), FGF21 mRNA (B), and protein secreted in the medium (C) were measured. *P<0.05 vs controls.
Effect of CoCl2 on FGF21 is independent of PPARα and PPARγ in Caco-2 cells
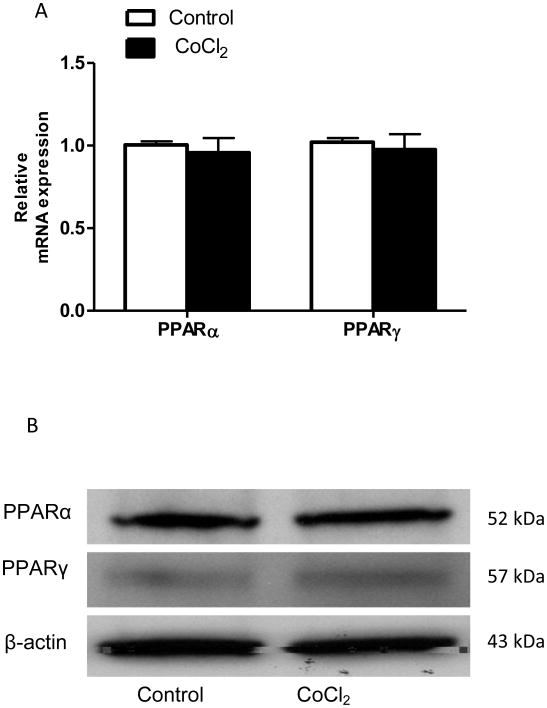
Numerous studies have demonstrated that PPARα and PPARγ are key transcriptional regulators of FGF21 in the liver and in the adipose tissue (Lundasen et al., 2007; Wang et al., 2008). To investigate whether the decrease in FGF21 expression due to the treatment of CoCl2 was a consequence of the alteration of upstream regulation of transcription, mRNA and protein levels of PPARα and PPARγ were examined. As shown in Fig 4A, CoCl2 treatment did not affect PPARα and PPARγ either at mRNA levels or at protein levels in Caco-2 cells (Fig 4C). These data suggest that the suppression of FGF21 expression is unlikely to be dependent on PPARα and PPARγ -mediated pathway(s).
Figure 4. CoCl2-mediated FGF21 down-regulation is independent of PPARα and γ.
Caco-2 cells were exposed to CoCl2 in the absence of serum for 24 hours. Cellular PPARα and PPARγ mRNA (A) and protein (B) expression were measured.
Down-regulation of FGF21 expression in Caco-2 cells is mediated by oxidative stress
Previous studies have shown that hypoxia induces ROS generation (Rathore et al., 2008; Desireddi et al., 2010). To evaluate the effects of CoCl2 on ROS formation, Caco-2 cells were treated with CoCl2 for 24 hours without serum, and superoxide formation was determined by fluorescent ethidium measurement. As shown in Fig 5A, CoCl2 significantly increased superoxide accumulation.
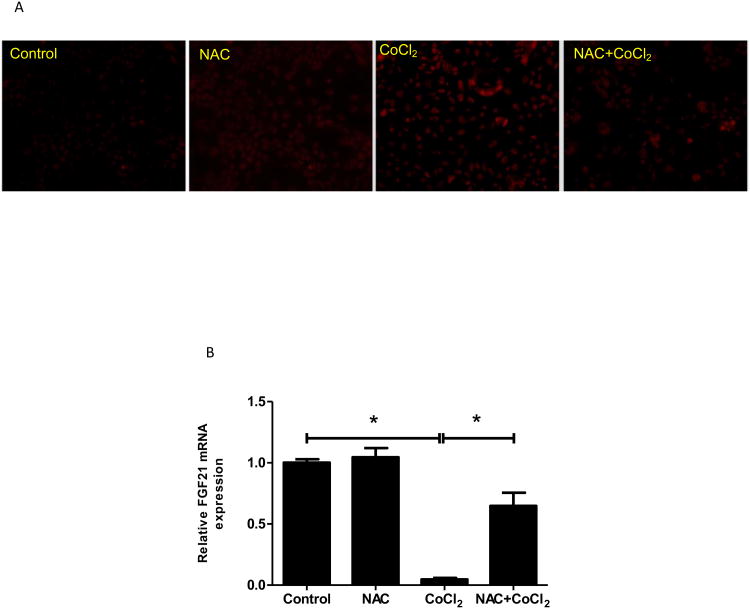
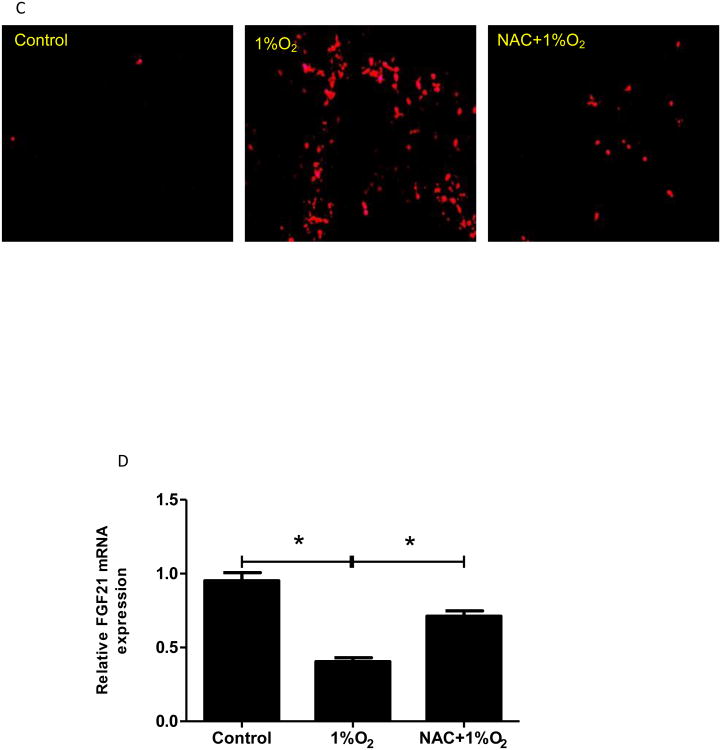
Figure 5. Down-regulation of FGF21 expression in Caco-2 cells is mediated by oxidative stress.
Caco-2 cells were exposed to CoCl2 or hypoxia with/without NAC in the absence of serum for 24 hours. (A&C) Superoxide accumulation in Caco-2 cells was examined by dihydroethidium fluorescence microscopy. (B&D) Expression of FGF21 mRNA was measured by real time RT-PCR.
To determine whether the decreased FGF21 expression by CoCl2 was due to the oxidative stress, a well-known antioxidant, NAC (3 mM), was added to Caco-2 cell culture along with CoCl2. As shown in Fig 5A, addition of NAC completely inhibited CoCl2-induced superoxide accumulation. Importantly, CoCl2-induced reduction in FGF21 expression was completely restored by NAC addition (Fig 5B). Besides being an antioxidant, NAC may also act as a cobalt chelator. To further analyze the specificity of the role of CoCl2-induced oxidative stress in the FGF21 expression, another ROS scavenger, MnTBAP, which does not show to be a cobalt chelator, was used to treat Caco-2 cells along with CoCl2. Addition of MnTBAP completely inhibited CoCl2-induced FGF21 down-regulation (Supplemental Fig 2). These results demonstrate that the effects of CoCl2 on FGF21 expression in Caco-2 cells are mediated by oxidative stress. To determine whether hypoxia also induces a similar effect on superoxide in Caco-2 cells and whether NAC ameliorates the effects of CoCl2 on FGF21 expression, Caco-2 cells were incubated under 1% O2 in the presence and absence of NAC. As shown in Fig 5C, addition of NAC completely inhibited hypoxia-induced superoxide formation. Importantly, hypoxia-reduced FGF21 expression was completely restored by NAC addition (Fig 5D). Taken altogether, the effects of CoCl2 and hypoxia on FGF21 expression in Caco-2 cells are mediated by oxidative stress.
CoCl2 decreases FGF21 mRNA stability in Caco-2 cells
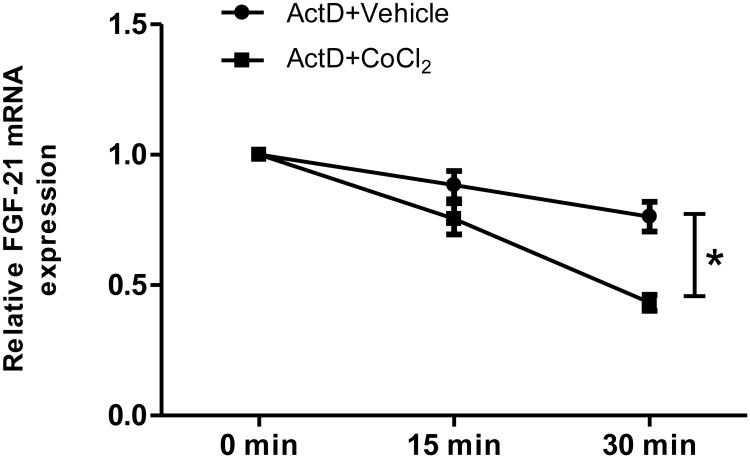
Previous studies have shown that ROS causes oxidative modification of major cellular macromolecules, lipids, DNA, and proteins (England et al., 2006; Niki, 2008; Guachalla and Rudolph, 2010) and decrease DNA stability (Savu et al., 2011). Because CoCl2 decreases FGF21 expression and this event is independent of PPARα and -γ-mediated transcription, we sought to determine if CoCl2 could alter FGF21 mRNA stability. Actinomycin D (ActD), a transcription inhibitor, was used to inhibit mRNA transcription. Consistent with our hypothesis, ActD addition inhibited the mRNA synthesis, and CoCl2 significantly decreased FGF21 mRNA stability compared with vehicle group in 30 min. (Fig 6). Thus, ROS-induced mRNA instability is involved in FGF21 down-regulation by CoCl2 treatment.
Figure 6. CoCl2 decreases FGF21 mRNA stability.
Caco-2 cells were pretreated for 30 min with a transcription inhibitor, actinomycin D (5 μg/ml), before the addition of 200 μM CoCl2 or vehicle control for 15 min and 30 min. Expression of FGF21mRNA was measured by real-time RT-PCR. *Significantly different, p<0.05.
CoCl2 induces TG accumulation in Caco-2 cells
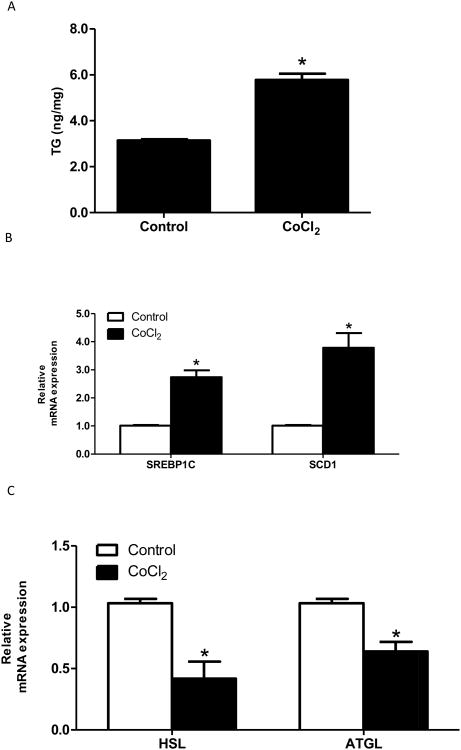
Both hypoxia and FGF21 regulation have been shown to play an important role in lipid metabolism (Rankin et al., 2009; Murata et al., 2011). In the present study, the role of hypoxia in lipid metabolism was investigated. Intestinal Caco-2 cells were cultured in serum-free media overnight and treated with CoCl2 for 24 hours. A significant increase in TG content was observed in Caco-2 cells when treated with CoCl2 (Fig 7A). To investigate the mechanism of how hypoxia regulates lipid accumulation, the expression of sterol regulatory element binding protein 1c (SREBP1c), an essential transcription factor promoting expression of lipogenesis-related genes, acetyl-CoA carboxylase (ACC), fatty acid synthase (FAS), stearoyl-CoA desaturase (SCD1), and lipolysis genes hormone-sensitive lipase (HSL) and adipose triglyceride lipase (ATGL), were measured. SREBP1c and SCD1 mRNA levels were significantly increased (Fig 7B); whereas HSL and ATGL, the two predominant lipases, were significantly decreased (Fig 7C).
Figure 7. CoCl2 induces TG accumulation.
Caco-2 cells were incubated in serum-free medium overnight and treated with CoCl2 (200 μM) for 24 hours. Cellular TG levels were measured (A). mRNA levels of lipogenesis genes (B) and lipolysis genes (C) were measured by real-time PCR. *Significantly different vs controls, p<0.05.
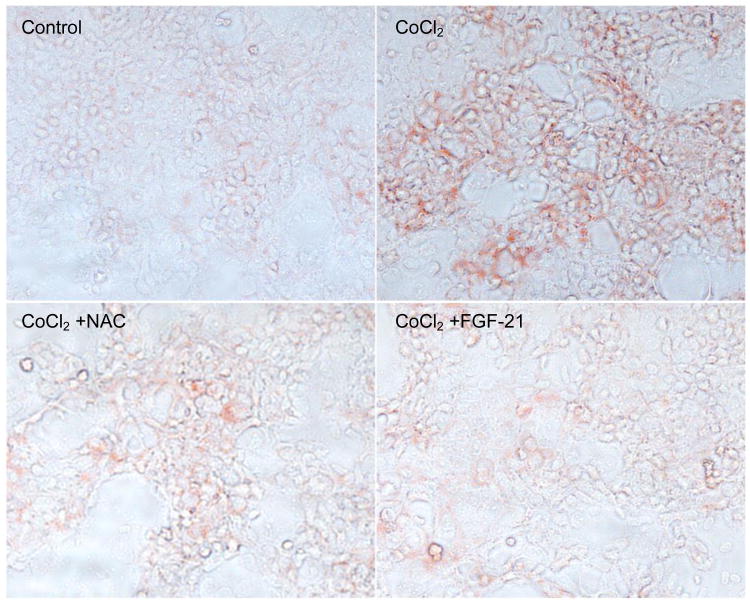
We then determined whether the addition of an antioxidant or recombinant FGF21 could lower the CoCl2-induced fat accumulation by Oil Red O staining. Similar to TG measurement, CoCl2 induced a marked increase in Oil Red O staining. As expected, NAC addition significantly decreased CoCl2-induced fat accumulation. Similarly, CoCl2-increased intracellular lipid accumulation was dramatically attenuated by FGF21 (1μg/ml) treatment (Fig 8).
Figure 8. Effects of anti-oxidant and FGF21 on CoCl2-induced lipid accumulation.
Caco-2 cells were incubated in serum-free medium overnight and treated with CoCl2 (200 μM), CoCl2 with NAC (3 mM) or CoCl2 with recombinant human FGF21 (1μg/ml) for 24 hours, and intracellular lipid accumulation was estimated by Oil red O staining.
Discussion
FGF21 is expressed mainly in the liver and adipose tissue, but is also present abundantly in the intestine (Fon Tacer et al., 2010). Our results demonstrated considerable expression of FGF21 in human intestinal cells. Although the biology of FGF21 in the intestine has not been investigated, lipid metabolism in the intestine profoundly affects gut function including immune-response, nutrient absorption and gut barrier function. In addition, the intestine is one of the unique organs that are exposed to physiologically hypoxic conditions. Although hypoxia has been attributed to lipid metabolic dysfunction in several tissues, and FGF21 has emerged as an important metabolic regulator of lipid metabolism, it is unknown whether FGF21 plays a role in hypoxia-induced lipid accumulation and how FGF21 is regulated by hypoxia. In the present study, we have demonstrated for the first time that physical hypoxia and hypoxia induced by chemical mimics significantly decrease FGF21 gene expression and protein production in Caco-2 cells, and this reduction is involved in hypoxia-induced lipid accumulation.
Previous studies showed that lipid dysregulation is associated with hypoxic states in several organs (Rankin et al., 2009; Yin et al., 2009). In a hypoxic environment, cells switch from aerobic to anaerobic metabolism to generate more ATP in an oxygen independent manner (Taylor, 2008). As a result, glucose uptake and glycolysis increase, and mitochondrial oxidative phosphorylation decreases. HIF plays a central role in this switch because it regulates glucose transporter 1 and several critical glycolytic enzymes, and inhibits mitochondrial oxidative phosphorylation by blocking pyruvate entry (Semenza, 2007). While the role of HIF in glucose metabolism under hypoxic conditions is well-studied, the regulation of lipid metabolism in response to low oxygen in still not clearly elucidated. In this study, we demonstrated that hypoxia and the chemical mimics decreased cellular FGF21 in an intestinal cell line and increased triglyceride accumulation. The reduction of FGF21 may be a novel mechanism by which hypoxia causes fat accumulation.
FGF21 plays an important role in lipid metabolism. Recent studies indicate that, in rodents, FGF21 regulates lipid oxidation in the liver (Badman et al., 2007; Badman et al., 2009). Adenoviral knockdown of hepatic FGF21 in mice caused fatty liver, suggesting that FGF21 is required for hepatic TG clearance (Badman et al., 2007). In addition, serum TG levels were markedly reduced in both ob/ob and db/db mice by the administration of FGF21 (Kharitonenkov et al., 2005). While the role of FGF21 in rodents has been extensively studied, little is known about the role and regulation of FGF21 in humans. Increased serum FGF21 level has been found in several pathological conditions, including obesity, insulin-resistance, cardiovascular disease, and nonalcoholic liver disease (Chavez et al., 2009; Li et al., 2010; Lin et al., 2010). Several studies using human cell lines, such as HepG2, demonstrated that overexpression of FGF21 suppressed TG accumulation by inhibiting SREBP1c (Wright et al., 2011; Zhang et al., 2011), and knock-down of FGF21 increased TG accumulation (Wright et al., 2011). Our results show that along with the reduction of FGF21 expression, hypoxia causes cellular TG accumulation in human Caco-2 cells, further suggesting a strong link between FGF21 and lipid metabolism under hypoxia-induced pathological conditions.
HIF expression and accumulation is an early cellular response to low oxygen concentration. CoCl2 and DFO have been widely used in the treatment of anemia and diseases with iron imbalance, respectively. They are also used as hypoxia mimics in cell culture and it is known that they activate hypoxic signaling by stabilizing the HIFα. Indeed, CoCl2 administration caused a dramatic expression of HIF1α protein, but not mRNA, in Caco-2 cells. Previous studies have shown that FGF2, a member of the FGF family, is upregulated by hypoxia, and HIF1α is a transcription factor of FGF2 (Conte et al., 2008) However, the hypoxia responsive element has not been found in the promoter region of FGF21, indicating that regulation of FGF21 by hypoxia-induced HIF1 is an indirect event. Numerous studies have demonstrated that FGF21 expression is regulated by PPARα or PPAR-γ, depending on cell types (Lundasen et al., 2007; Muise et al., 2008). In lean rodents, FGF21 expression is strongly induced in the liver by fasting through a mechanism that involves PPARα (Lundasen et al., 2007), but in adipose tissue, FGF21 is induced via a PPARγ pathway (Muise et al., 2008). Our studies demonstrated that the regulation of FGF21 under hypoxic conditions was unlikely via PPARα and/or PPARγ pathway in Caco-2 cells. Indeed, the regulation of PPARα and PPARγ by hypoxia is cell type-dependent. Saiaja showed that hypoxia could rapidly down-regulate PPARα in epithelial cells in vitro and in vivo (Narravula and Colgan, 2001), while PPARγ is a target gene of HIF1α in cardiomyocytes, and HIF1α could activate PPARγ in response to pathologic stress (Krishnan et al., 2009).
Although HIF1 regulates many genes involved in the adaptation to hypoxic stress, intracellular ROS paradoxically increase under hypoxic conditions. Previous studies have demonstrated that hypoxia increases ROS in intestine epithelial cells and intestinal tissues (Kuhn et al., 2002). Consistent with these findings, we found that CoCl2 significantly increases superoxide anions in Caco-2 cells evaluated by DHE staining. Anti-oxidant addition decreased DHE staining, further confirming our finding. ROS-derived oxidative stress has profound effects on cellular components including modification of proteins and alteration of gene expression in various cell types (Long et al., 2004). While much evidence shows that ROS have a capability to increase mRNA expression (Salles et al., 2005), the present study demonstrates that CoCl2 treatment causes an increase in ROS, which results in the down-regulation of FGF21 gene expression in Caco-2 cells. Importantly, CoCl2-induced TG accumulation in Caco-2 cells could be attenuated by administration of an anti-oxidant or recombinant FGF21. These findings suggest that ROS-mediated FGF21 regulation has an important impact on cellular lipid metabolism.
How is FGF21 mRNA expression regulated in response to CoCl2 in Caco-2 cells? Transcription factor-mediated regulation is critical for mRNA synthesis. However, in the present study, the described major transcription factors of FGF21, PPARα and PPARγ, had no change in response to CoCl2, suggesting that CoCl2-decreased FGF21 mRNA expression is likely independent of PPARα and PPARγ. Previous studies demonstrated that mRNA stability plays an important role in mRNA turnover and gene expression (Hargrove and Schmidt, 1989). We hypothesized that the decreased FGF21 mRNA levels may be caused by accelerated degradation in response to the treatment of CoCl2 in Caco-2 cells. As anticipated, FGF21 mRNA degradation was accelerated by CoCl2 treatment evaluated by inhibition of the transcription by ActD. This finding suggests that the CoCl2-induced decrease in FGF21 mRNA levels is mediated by the mRNA stability, rather than decreased synthesis.
While CoCl2 and DFO mimic hypoxic conditions, exposure to both of them can lead to cellular toxicity. Indeed, treatment with CoCl2 and DFO decreased cell viability by about 30% in Caco-2 cells (data not shown). Previous genomic studies have shown that both CoCl2 and hypoxia regulate a similar group of genes on a global gene expression level (Vengellur et al., 2003), and CoCl2 cytotoxicity is dependent on HIF1α-mediated increase in cell death-promoting genes, such as BNiP3 and NIX, in mouse embryonic fibroblasts (Vengellur and LaPres, 2004). It is therefore unlikely that CoCl2-induced cytotoxicity has a major impact on FGF21 expression since the independency of FGF21 mRNA expression on HIF signaling by CoCl2 exposure in Caco-2 cells demonstrated in current study. In contrast, the hypoxic signaling pathway can activate cell survival genes involved in angiogenesis and glycolysis (Rey et al., 2011) and inhibit apoptotic pathways in certain cell types (Flamant et al., 2010). Thus, CoCl2-induced change in cell viability is complex. It remains to be tested whether CoCl2-induced cytotoxicity in Caco-2 cells is HIF signaling dependent; but it is well-known that CoCl2 exposure produces ROS in variety of cell types, which may account for the decreased cell viability (Patel et al., 2012). The effects of CoCl2-induced cell death and retardation of cell proliferation on FGF21 expression needs additional investigation.
It should be noted that our results are not in line with a previous study in which FGF21 expression was up-regulated upon nutrition deprivation, hypoxia, or double-deprivation stress in murine melanoma cells (Osawa et al., 2009). In line with this study, we did show that starvation increased FGF21 mRNA expression in Caco-2 cells. In contrast, we did not observe an increase of FGF21 expression by hypoxia. Instead, hypoxia causes a signifcant reduction in FGF21 expression in o-2 cells. This discrepancy suggests that the regulation of FGF21 expression is cell type specific, and it is also possibly caused by different treatment regimes. Indeed, important differences in the biology of FGF21 between mice and humans have been described (Ryden, 2009). Murine cells may act differently from human cells for FGF21 regulation by hypoxia In addition, the murine melanoma cells were treated with 20 cycles of hypoxia and reoxygenation (Osawa et al., 2009), while, in present study, Caco-2 cells were exposed to hypoxic condition for only one time or challenged with one dose of CoCl2. Another member of FGF family, FGF2, has been studied for association with HIF1α in response to hypoxia (Li et al., 2002). FGF2 and HIF1α regulate each other in a mutually inhibitory way when they are in small amounts, and are mutually stimulative when they exist abundantly (Conte et al., 2008). Additional studies are required for further understanding of the mechanism of FGF21 regulation in response to hypoxia.
In summary, our results reveal a novel scenario in which hypoxia down-regulates FGF21 expression via oxidative stress-mediated regulation of mRNA stability in Caco-2 cells. Decreased FGF21 expression is correlated with the compromised cellular capacity to maintain lipid homeostasis leading to triglyceride accumulation. Our data indicate that modulation of FGF21 might have a potential in maintaining intestinal energy homeostasis to improve intestinal diseases.
Supplementary Material
Highlights.
Hypoxia down-regulates FGF-21 expression in Caco-2 cells.
FGF-21 down-regulation is HIF-α independent.
FGF-21 down-regulation is modulated by oxidative stress-mediated mRNA stability.
FGF-21 is involved in hypoxia-induced triglyceride accumulation in Caco-2 cells.
Acknowledgments
We thank Dr. Moosa Mohammadi (New York University) for providing the recombinant human FGF21, and Marion McClain for manuscript proof reading. The work presented in this study was supported by NIH grants (P01AA017103, P30AA019360, R01AA015970, R01AA018016, R01AA018869, R37AA010762, RC2AA019385 and R01DK071765), the VA, ADA grant (1-12-BS-47) and NSFC grants (30971515 and 81170203).
Footnotes
Conflict of Interest Statement: The authors have no conflict of interest to be claimed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Badman MK, Koester A, Flier JS, Kharitonenkov A, Maratos-Flier E. Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology. 2009;150:4931–4940. doi: 10.1210/en.2009-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell metabolism. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Chau MD, Gao J, Yang Q, Wu Z, Gromada J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1alpha pathway. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12553–12558. doi: 10.1073/pnas.1006962107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez AO, Molina-Carrion M, Abdul-Ghani MA, Folli F, Defronzo RA, Tripathy D. Circulating fibroblast growth factor-21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes care. 2009;32:1542–1546. doi: 10.2337/dc09-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte C, Riant E, Toutain C, Pujol F, Arnal JF, Lenfant F, Prats AC. FGF2 translationally induced by hypoxia is involved in negative and positive feedback loops with HIF-1alpha. PloS one. 2008;3:e3078. doi: 10.1371/journal.pone.0003078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149:6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- Desireddi JR, Farrow KN, Marks JD, Waypa GB, Schumacker PT. Hypoxia increases ROS signaling and cytosolic Ca(2+) in pulmonary artery smooth muscle cells of mouse lungs slices. Antioxid Redox Signal. 2010;12:595–602. doi: 10.1089/ars.2009.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England K, Driscoll CO, Cotter TG. ROS and protein oxidation in early stages of cytotoxic drug induced apoptosis. Free Radic Res. 2006;40:1124–1137. doi: 10.1080/10715760600838209. [DOI] [PubMed] [Google Scholar]
- Estall JL, Ruas JL, Choi CS, Laznik D, Badman M, Maratos-Flier E, Shulman GI, Spiegelman BM. PGC-1alpha negatively regulates hepatic FGF21 expression by modulating the heme/Rev-Erb(alpha) axis. Proceedings of the NAtional Academy of Sciences of the United States of America. 2009;106:22510–22515. doi: 10.1073/pnas.0912533106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamant L, Notte A, Ninane N, Raes M, Michiels C. Anti-apoptotic role of HIF-1 and AP-1 in paclitaxel exposed breast cancer cells under hypoxia. Molecular cancer. 2010;9:191. doi: 10.1186/1476-4598-9-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fon Tacer K, Bookout AL, Ding X, Kurosu H, John GB, Wang L, Goetz R, Mohammadi M, Kuro-o M, Mangelsdorf DJ, Kliewer SA. Research resource: Comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol Endocrinol. 2010;24:2050–2064. doi: 10.1210/me.2010-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PH, Glickman RM. Intestinal lipoprotein metabolism. J Lipid Res. 1981;22:1153–1173. [PubMed] [Google Scholar]
- Guachalla LM, Rudolph KL. ROS induced DNA damage and checkpoint responses: influences on aging? Cell Cycle. 2010;9:4058–4060. doi: 10.4161/cc.9.20.13577. [DOI] [PubMed] [Google Scholar]
- Hargrove JL, Schmidt FH. The role of mRNA and protein stability in gene expression. FASEB J. 1989;3:2360–2370. doi: 10.1096/fasebj.3.12.2676679. [DOI] [PubMed] [Google Scholar]
- Hotta Y, Nakamura H, Konishi M, Murata Y, Takagi H, Matsumura S, Inoue K, Fushiki T, Itoh N. Fibroblast growth factor 21 regulates lipolysis in white adipose tissue but is not required for ketogenesis and triglyceride clearance in liver. Endocrinology. 2009;150:4625–4633. doi: 10.1210/en.2009-0119. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell metabolism. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB. FGF-21 as a novel metabolic regulator. The Journal of clinical investigation. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, Etgen GJ. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148:774–781. doi: 10.1210/en.2006-1168. [DOI] [PubMed] [Google Scholar]
- Krishnan J, Suter M, Windak R, Krebs T, Felley A, Montessuit C, Tokarska-Schlattner M, Aasum E, Bogdanova A, Perriard E, Perriard JC, Larsen T, Pedrazzini T, Krek W. Activation of a HIF1alpha-PPARgamma axis underlies the integration of glycolytic and lipid anabolic pathways in pathologic cardiac hypertrophy. Cell metabolism. 2009;9:512–524. doi: 10.1016/j.cmet.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Kuhn MA, Xia G, Mehta VB, Glenn S, Michalsky MP, Besner GE. Heparin-binding EGF-like growth factor (HB-EGF) decreases oxygen free radical production in vitro and in vivo. Antioxid Redox Signal. 2002;4:639–646. doi: 10.1089/15230860260220148. [DOI] [PubMed] [Google Scholar]
- LeGrand TS, Aw TY. Chronic hypoxia and glutathione-dependent detoxication in rat small intestine. Am J Physiol. 1996;270:G725–729. doi: 10.1152/ajpgi.1996.270.4.G725. [DOI] [PubMed] [Google Scholar]
- Li H, Fang Q, Gao F, Fan J, Zhou J, Wang X, Zhang H, Pan X, Bao Y, Xiang K, Xu A, Jia W. Fibroblast growth factor 21 levels are increased in nonalcoholic fatty liver disease patients and are correlated with hepatic triglyceride. Journal of hepatology. 2010;53:934–940. doi: 10.1016/j.jhep.2010.05.018. [DOI] [PubMed] [Google Scholar]
- Li J, Shworak NW, Simons M. Increased responsiveness of hypoxic endothelial cells to FGF2 is mediated by HIF-1alpha-dependent regulation of enzymes involved in synthesis of heparin sulfate FGF2-binding sites. J Cell Sci. 2002;115:1951–1959. doi: 10.1242/jcs.115.9.1951. [DOI] [PubMed] [Google Scholar]
- Li SH, Shin DH, Chun YS, Lee MK, Kim MS, Park JW. A novel mode of action of YC-1 in HIF inhibition: stimulation of FIH-dependent p300 dissociation from HIF-1{alpha} Mol Cancer Ther. 2008;7:3729–3738. doi: 10.1158/1535-7163.MCT-08-0074. [DOI] [PubMed] [Google Scholar]
- Lin Z, Wu Z, Yin X, Liu Y, Yan X, Lin S, Xiao J, Wang X, Feng W, Li X. Serum levels of FGF-21 are increased in coronary heart disease patients and are independently associated with adverse lipid profile. PloS one. 2010;5:e15534. doi: 10.1371/journal.pone.0015534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Long H, Shi T, Borm PJ, Maatta J, Husgafvel-Pursiainen K, Savolainen K, Krombach F. ROS-mediated TNF-alpha and MIP-2 gene expression in alveolar macrophages exposed to pine dust. Part Fibre Toxicol. 2004;1:3. doi: 10.1186/1743-8977-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundasen T, Hunt MC, Nilsson LM, Sanyal S, Angelin B, Alexson SE, Rudling M. PPARalpha is a key regulator of hepatic FGF21. Biochemical and biophysical research communications. 2007;360:437–440. doi: 10.1016/j.bbrc.2007.06.068. [DOI] [PubMed] [Google Scholar]
- Mabjeesh NJ, Escuin D, LaVallee TM, Pribluda VS, Swartz GM, Johnson MS, Willard MT, Zhong H, Simons JW, Giannakakou P. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3:363–375. doi: 10.1016/s1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- Muise ES, Azzolina B, Kuo DW, El-Sherbeini M, Tan Y, Yuan X, Mu J, Thompson JR, Berger JP, Wong KK. Adipose fibroblast growth factor 21 is up-regulated by peroxisome proliferator-activated receptor gamma and altered metabolic states. Molecular pharmacology. 2008;74:403–412. doi: 10.1124/mol.108.044826. [DOI] [PubMed] [Google Scholar]
- Murata Y, Konishi M, Itoh N. FGF21 as an Endocrine Regulator in Lipid Metabolism: From Molecular Evolution to Physiology and Pathophysiology. Journal of nutrition and metabolism. 2011;2011:981315. doi: 10.1155/2011/981315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narravula S, Colgan SP. Hypoxia-inducible factor 1-mediated inhibition of peroxisome proliferator-activated receptor alpha expression during hypoxia. J Immunol. 2001;166:7543–7548. doi: 10.4049/jimmunol.166.12.7543. [DOI] [PubMed] [Google Scholar]
- Niki E. Lipid peroxidation products as oxidative stress biomarkers. Biofactors. 2008;34:171–180. doi: 10.1002/biof.5520340208. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Kurosu H, Yamamoto M, Nandi A, Rosenblatt KP, Goetz R, Eliseenkova AV, Mohammadi M, Kuro-o M. BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7432–7437. doi: 10.1073/pnas.0701600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa T, Muramatsu M, Watanabe M, Shibuya M. Hypoxia and low-nutrition double stress induces aggressiveness in a murine model of melanoma. Cancer science. 2009;100:844–851. doi: 10.1111/j.1349-7006.2009.01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel E, Lynch C, Ruff V, Reynolds M. Co-exposure to nickel and cobalt chloride enhances cytotoxicity and oxidative stress in human lung epithelial cells. Toxicology and applied pharmacology. 2012;258:367–375. doi: 10.1016/j.taap.2011.11.019. [DOI] [PubMed] [Google Scholar]
- Rankin EB, Rha J, Selak MA, Unger TL, Keith B, Liu Q, Haase VH. Hypoxia-inducible factor 2 regulates hepatic lipid metabolism. Molecular and cellular biology. 2009;29:4527–4538. doi: 10.1128/MCB.00200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathore R, Zheng YM, Niu CF, Liu QH, Korde A, Ho YS, Wang YX. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCepsilon signaling axis in pulmonary artery smooth muscle cells. Free radical biology & medicine. 2008;45:1223–1231. doi: 10.1016/j.freeradbiomed.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey S, Luo W, Shimoda LA, Semenza GL. Metabolic reprogramming by HIF-1 promotes the survival of bone marrow-derived angiogenic cells in ischemic tissue. Blood. 2011;117:4988–4998. doi: 10.1182/blood-2010-11-321190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryden M. Fibroblast growth factor 21: an overview from a clinical perspective. Cell Mol Life Sci. 2009;66:2067–2073. doi: 10.1007/s00018-009-0003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salles N, Szanto I, Herrmann F, Armenian B, Stumm M, Stauffer E, Michel JP, Krause KH. Expression of mRNA for ROS-generating NADPH oxidases in the aging stomach. Exp Gerontol. 2005;40:353–357. doi: 10.1016/j.exger.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Savu O, Sunkari VG, Botusan IR, Grunler J, Nikoshkov A, Catrina SB. Stability of mitochondrial DNA against reactive oxygen species (ROS) generated in diabetes. Diabetes Metab Res Rev. 2011;27:470–479. doi: 10.1002/dmrr.1203. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE. 2007;2007:cm8. doi: 10.1126/stke.4072007cm8. [DOI] [PubMed] [Google Scholar]
- Shepherd J. Lipoprotein metabolism. An overview. Drugs. 1994;47(2):1–10. doi: 10.2165/00003495-199400472-00003. [DOI] [PubMed] [Google Scholar]
- Taylor CT. Mitochondria and cellular oxygen sensing in the HIF pathway. Biochem J. 2008;409:19–26. doi: 10.1042/BJ20071249. [DOI] [PubMed] [Google Scholar]
- Taylor CT, Colgan SP. Hypoxia and gastrointestinal disease. J Mol Med (Berl) 2007;85:1295–1300. doi: 10.1007/s00109-007-0277-z. [DOI] [PubMed] [Google Scholar]
- Vengellur A, LaPres JJ. The role of hypoxia inducible factor 1alpha in cobalt chloride induced cell death in mouse embryonic fibroblasts. Toxicological sciences: an official journal of the Society of Toxicology. 2004;82:638–646. doi: 10.1093/toxsci/kfh278. [DOI] [PubMed] [Google Scholar]
- Vengellur A, Woods BG, Ryan HE, Johnson RS, LaPres JJ. Gene expression profiling of the hypoxia signaling pathway in hypoxia-inducible factor 1alpha null mouse embryonic fibroblasts. Gene expression. 2003;11:181–197. doi: 10.3727/000000003108749062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Qiang L, Farmer SR. Identification of a domain within peroxisome proliferator-activated receptor gamma regulating expression of a group of genes containing fibroblast growth factor 21 that are selectively repressed by SIRT1 in adipocytes. Molecular and cellular biology. 2008;28:188–200. doi: 10.1128/MCB.00992-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright LE, Brandon AE, Hoy AJ, Forsberg GB, Lelliott CJ, Reznick J, Lofgren L, Oscarsson J, Stromstedt M, Cooney GJ, Turner N. Amelioration of lipid-induced insulin resistance in rat skeletal muscle by overexpression of Pgc-1beta involves reductions in long-chain acyl-CoA levels and oxidative stress. Diabetologia. 2011;54:1417–1426. doi: 10.1007/s00125-011-2068-x. [DOI] [PubMed] [Google Scholar]
- Wu Q, Yang SH, Wang RY, Ye SN, Xia T, Ma DZ. Effect of silencing HIF-1alpha by RNA interference on expression of vascular endothelial growth factor in osteosarcoma cell line SaOS-2 under hypoxia. Ai zheng = Aizheng = Chinese journal of cancer. 2005;24:531–535. [PubMed] [Google Scholar]
- Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, Chen JL, Jung DY, Zhang Z, Ko HJ, Kim JK, Veniant MM. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58:250–259. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Gao Z, He Q, Zhou D, Guo Z, Ye J. Role of hypoxia in obesity-induced disorders of glucose and lipid metabolism in adipose tissue. Am J Physiol Endocrinol Metab. 2009;296:E333–342. doi: 10.1152/ajpendo.90760.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lei T, Huang JF, Wang SB, Zhou LL, Yang ZQ, Chen XD. The link between fibroblast growth factor 21 and sterol regulatory element binding protein 1c during lipogenesis in hepatocytes. Molecular and cellular endocrinology. 2011;342:41–47. doi: 10.1016/j.mce.2011.05.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.