Abstract
Objective
To analyze headache patterns prior to and following treatment of unruptured intracranial aneurysms and identify factors associated with different headache outcomes.
Methods
A prospective observational study of patients being treated for unruptured intracranial aneurysms. Headache patterns were established prior to aneurysm treatment and for 6 months following treatment. Factors associated with different headache outcomes were investigated.
Results
In all patients (n=44), 90-day headache frequency decreased from an average of 31 days prior to aneurysm treatment to 17 days following treatment (p<0.001). In patients with active pretreatment headaches (n=28), 90-day headache frequency decreased from 49 days to 26 days (p=0.002). Headache frequency was reduced in 68% of patients, while 9% of patients had new or worsened headaches following aneurysm treatment. Pretreatment migraine, more severe pretreatment headaches, higher pretreatment trait anxiety, and stent-assisted aneurysm coiling were associated with a lack of headache improvement.
Conclusions
The majority of patients with headaches at the time of aneurysm treatment had reductions in headache frequency during the 6 months following treatment. Potential risk factors for poor headache outcomes were identified but need to be studied further.
Keywords: Aneurysm clip, aneurysm coil, central sensitization, headache, intracranial aneurysm
Introduction
Intracranial aneurysms are common, present in approximately 3–6% of the population over the age of 30 (1). Headaches are a frequent symptom in patients with cerebral aneurysms, often leading to the aneurysm diagnosis. Headaches are the presenting symptom of unruptured intracranial aneurysms in approximately one-third of cases (2). Following treatment of intracranial aneurysms, there may be a change in headache patterns, with some patients improving, some worsening, and others having the onset of new headaches (3). To date, there are few data regarding headache patterns after treatment of unruptured intracranial aneurysms (3,4).
Although the nature of the association between headaches and unruptured intracranial aneurysms is not defined, when headache is a symptom leading to the diagnosis of an aneurysm, patients and their physicians may believe that the aneurysm is causing headache. Thus, patients often expect improvements in their headaches following aneurysm treatment. Our clinical experience and a previously conducted retrospective analysis led us to hypothesize that although many patients do improve after aneurysm treatment, others have no appreciable change in headache patterns, while others have worsening or new onset of headaches (5). In our specialty headache clinic, we have found that patients with new or worsening headaches following aneurysm treatment are often severely disabled from these headaches and are typically difficult to treat effectively. We hypothesized that these new and worsening headaches are incited by the aneurysm treatment, and that the development of central sensitization may contribute to the persistence of these headaches.
The aim of this study was to prospectively investigate headache patterns before and following aneurysm treatment in order to assess headache outcomes following the treatment. To improve the ability to predict headache outcomes after aneurysm treatment, we aimed to identify patient characteristics, pretreatment headache patterns, aneurysm characteristics, and aneurysm treatments associated with headache improvement. To test our hypothesis that central sensitization contributes to the persistence of new and worsening headaches following aneurysm treatment, patients were assessed for symptoms of cutaneous allodynia and their cutaneous pain thresholds were measured.
Methods
Following Washington University Institutional Review Board approval, adult patients with planned treatments for unruptured intracranial aneurysms (coiling, clipping, liquid embolic agent) at Washington University were offered enrollment into this prospective study conducted between 2007 and 2009. After completing the informed consent process, all enrolled patients were interviewed and completed baseline assessments prior to the aneurysm intervention. Collected pretreatment data included patient age and sex, medical history, medications used, aneurysm size and location, symptoms leading to aneurysm diagnosis, symptoms of depression via the Beck Depression Inventory (BDI), symptoms of anxiety via the State-Trait Anxiety Inventory (STAI), symptoms of allodynia via the Allodynia Symptom Checklist (ASC-12), and headache- related disability using the Migraine Disability Assessment Score (MIDAS) (6–9). When patients had a history of headaches, they provided information on headache quality, severity, frequency, and associated symptoms. Patients with a history of headaches were assigned a headache diagnosis according to the International Classification of Headache Disorders II (ICHD-II) criteria (10). Patients were then asked to repeat assessments at 1, 3 and 6 months following the aneurysm treatment. At each assessment patients provided information regarding their headache characteristics and medication use, and they completed the BDI, MIDAS, STAI and ASC-12. Headache frequency and severity were determined at each time point via MIDAS responses. All patients were asked to present to our institution for quantitative sensory testing (QST) prior to aneurysm treatment and 1, 3 and 6 months following aneurysm treatment. Patients who were not able to present to our institution for this testing were asked to participate in the rest of the study.
QST was performed to determine heat, cold and mechanical pain thresholds. Patients were given verbal instructions prior to each QST testing session using a standard written script. Each patient underwent testing at the right and left forehead. Thermal testing was performed using the Medoc Pathway platform with a 30mm × 30mm thermode. The thermode was applied to the skin and fastened with a Velcro strap. The method of limits was used for testing. The thermode started at a baseline temperature of 32°C. Depending upon the modality being tested (heat or cold), thermode temperature increased or decreased by 1°C/second. The patient pressed a button on the response unit when the pain threshold being tested (heat pain, cold pain) was reached. The heating or cooling process stopped immediately and the thermode returned to the baseline temperature. This test was performed three times for each modality in each body location. The mean of the three trials for each modality in each body location was the thermal pain threshold. ‘Thermal pain threshold’ was defined as the first instant that the stimulus felt painful. Following thermal testing, pressure pain threshold at the right and left forehead was tested using a set of 20 calibrated von Frey filaments (Bioseb, Vitrolles, France). In an ascending pattern of increasing tensile strength, each filament was applied three times until the patient reported pain on two of three applications. Each filament was held in place for 2 seconds. ‘Pressure pain threshold’ was defined as the lowest force that resulted in pain on two of three applications.
Statistical analyses were performed using SPSS version 18.0. Descriptive statistics were used to describe patient demographics, headache histories and aneurysm characteristics. Primary analyses used paired two-tailed t-tests or Wilcoxon signed-rank tests to determine change in mean headache frequency and severity pre- and post-aneurysm treatment (pretreatment vs. last follow-up). One-way ANOVA with Dunnett t-tests were used to compare 3-month and 6-month headache frequency to pretreatment headache frequency. For all analyses, p-values of <0.05 were considered significant. The proportions of patients with active pretreatment headaches who improved and who worsened were calculated. A patient was considered to have an active headache disorder at baseline if they had at least 3 headache days in the 90 days preceding aneurysm treatment. Improvement was defined as at least a 50% reduction in monthly headache frequency at the time of last follow-up. Worsening was defined as at least a 50% increase in monthly headache frequency at the time of last follow-up. ASC-12, MIDAS, BDI and STAI scores pretreatment vs. post-treatment (time of last follow-up) were compared using paired t-tests or Wilcoxon signed-rank tests as appropriate.
Characteristics of patients with headache improvement following treatment were compared to those with no improvement/worsening headaches/new headaches following treatment using unpaired two-tailed t-tests, independent samples Mann–Whitney U tests, or Pearson’s chi-square tests as appropriate. Univariate comparisons included age, sex, pretreatment migraine, pretreatment chronic migraine, pretreatment chronic daily headache of any subtype, pretreatment headache frequency, pretreatment headache severity, aneurysm location (posterior circulation vs. anterior circulation), type of aneurysm treatment (coiling, clipping, stent-assisted coiling), and pretreatment scores on MIDAS, BDI, STAI and ASC-12.
ASC-12 scores and pain thresholds pretreatment and post-treatment were compared among all patients who had QST as well as among those patients with headaches who had QST. Pain thresholds were averaged across right and left forehead. Group comparisons were made using two-tailed paired t-tests or Wilcoxon signed-rank tests as appropriate.
Results
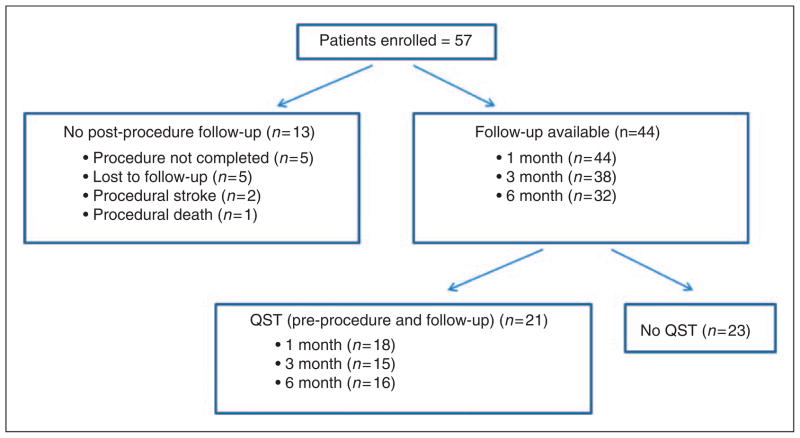
Patient enrollment is summarized in Figure 1; a total of 57 patients were enrolled into this study. Post-procedure data were not attainable from 13 of these patients. Thus, pretreatment and post-treatment data were available from 44 patients.
Figure 1.
Study enrollment.
Of the 44 patients with follow-up, 37 were female and 7 were male. Median age was 57 years (mean 58 years, range 36–86 years). Risk factors for intracranial aneurysms were present in 31 patients: 26 patients reported a diagnosis of hypertension, 16 hypercholesterolemia, and 7 had a disorder likely secondary to atherosclerosis (myocardial infarction in 4, coronary artery disease in 2, transient ischemic attack in 1) (11). Thirty-seven had a history of headaches that were either active or inactive at the time of enrollment. ICHD-II headache diagnoses included: 11 episodic tension-type headache, 8 chronic tension-type headache, 8 episodic migraine, 6 chronic migraine, 2 occipital neuralgia, 1 post-traumatic headache, and 1 primary stabbing headache. Twenty-eight had active headaches at the time of aneurysm treatment. This included 8 with chronic tension-type headache, 6 with chronic migraine, 5 with episodic tension-type headache, 5 with episodic migraine, 2 with occipital neuralgia, 1 with post-traumatic headache, and 1 with primary stabbing headache. In addition, 4 patients were classified as having “headache attributed to saccular aneurysm” according to ICHD-II criteria (10). Presenting symptoms that led to a diagnosis of an unruptured aneurysm included: headache (n=26), visual symptoms (n=15), asymptomatic (e.g. s/p head trauma, evaluation for parotid gland cysts, screening exam) (n=6), dizziness (n=5), auditory symptoms (e.g. tinnitus, hearing loss) (n=3), sensory loss (n=3), memory loss (n=3), weakness (n=2) and seizure (n=1). Many patients had more than one symptom leading to aneurysm diagnosis. Nine patients had headache as an isolated presenting symptom. Thirty-two patients had aneurysms located in the anterior circulation and 13 had aneurysms in the posterior circulation. One patient had aneurysms in both the anterior and posterior circulation. Thirty-eight patients underwent aneurysm coiling, 5 underwent clipping, and 3 had liquid embolic agent infusion (2 also had coils placed). Nine patients had stent-assisted coiling.
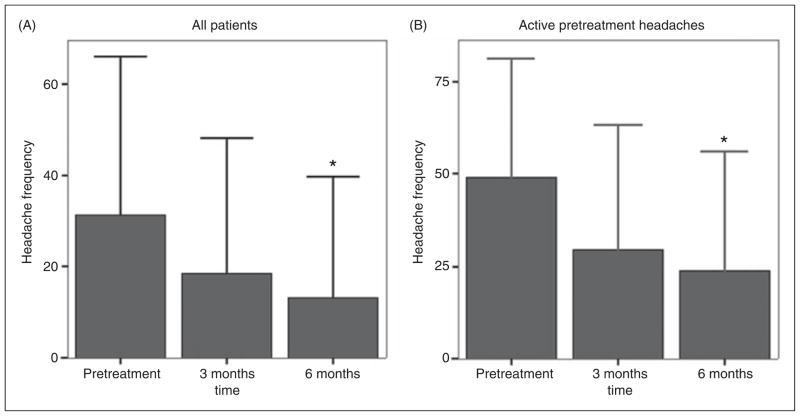
Among all patients, headache frequency declined from 31 days with headache per 90 days prior to aneurysm treatment to 17 days per 90 days at the time of last follow-up (p<0.001). There was a continued decline in 90-day headache frequency from pretreatment to 6 months following treatment: pretreatment 31 days, 3 months 19 days (3 months vs. pretreatment p=0.12), and 6 months 13 days (6 months vs. pretreatment p=0.02) (Figure 2). There was no change in headache severity, averaging 4/10 both pretreatment and post-treatment.
Figure 2.
Headache frequency pre- and post-aneurysm treatment.
A. Headache frequency pre- and post-aneurysm treatment in all patients. Examination of the whole sample (n=44) reveals a significant reduction in headache frequency (headache days/90 days) following aneurysm treatment. Six-month headache frequency was significantly lower than pretreatment frequency (13 days/90 days vs. 31 days/90 days, p=0.02). Values are group means. Error bars indicate + 1 SD. *indicates p=0.02.
B. Headache frequency pre- and post-aneurysm treatment in patients with active pretreatment headaches. Examination of patients with active headache disorders prior to aneurysm treatment (n=28) reveals a significant reduction in headache frequency (headache days/90 days) following aneurysm treatment. Six-month headache frequency was significantly lower than pretreatment frequency (24 days/90 days vs. 49 days/90 days, p=0.03). Values are group means. Error bars indicate + 1 SD. *indicates p=0.03.
Among patients with active headaches prior to aneurysm treatment, mean headache frequency declined from 49 days per 90 days prior to treatment to 26 headache days per 90 days at the time of last follow-up after aneurysm treatment (p<0.01). There was a continued decline in 90-day headache frequency within the 6 months of follow-up: pretreatment 49 days, 3 months 30 days (3 months vs. pretreatment p=0.08), and 6 months 24 days (6 months vs. pretreatment p=0.03) (Figure 2). Headache frequency was substantially reduced (≥50% reduction) in 19 of 28 patients (68%), unchanged in 6 (21%), and substantially increased (≥50% increase) in 3 (11%). There was no change in headache severity, averaging 5/10 both pretreatment and post-treatment.
All 4 patients with headaches meeting diagnostic criteria for headache attributed to saccular aneurysm (new acute headaches which resolved within 72 hours) also had recurrent headaches which were likely attributable to primary headache disorders (1 episodic migraine, 1 chronic migraine, 1 episodic tension-type headache, 1 chronic tension-type headache). Each of these patients reported that their acute-onset headache was substantially different from their usual recurrent headaches. Thus, we presume that these patients had primary headache disorders and then had a superimposed acute-onset headache which was attributable to their unruptured aneurysm. At the time of last follow-up after aneurysm treatment, 2 of these patients had significant improvement in headache frequency (1 patient with episodic migraine and 1 with episodic tension-type headache), while 2 had no significant change in headache frequency (1 patient with chronic migraine and 1 with chronic tension-type headache).
Headache frequency, headache severity, ASC-12 scores, MIDAS scores, BDI scores, and anxiety scores pre- and post-aneurysm treatment are shown in Table 1. Among all patients, there were significant reductions in headache frequency, symptoms of allodynia, depression scores, and state anxiety. Among patients with active headache disorders at the time of aneurysm treatment there were significant reductions in headache frequency, state anxiety and trait anxiety.
Table 1.
Outcomes following aneurysm treatment. At the time of last follow-up, among all patients there were significant reductions in headache frequency, symptoms of cutaneous allodynia (ASC-12), symptoms of depression (BDI), and state anxiety. Among patients with headaches prior to aneurysm treatment there were significant reductions in headache frequency, state anxiety and trait anxiety
| All patients (n=44)
|
Patients with active pretreatment headaches (n=28)
|
|||||
|---|---|---|---|---|---|---|
| Pretreatment | Last follow-up | p | Pretreatment | Last follow-up | p | |
| Headache days/90 days | 31 (35) | 17 (29) | <0.001 | 49 (33) | 26 (33) | 0.002 |
| Headache severity | 4 (3.0) | 4 (4) | 0.52 | 5 (2) | 5 (3) | 0.82 |
| ASC-12 | 3 (4) | 1 (2) | 0.04 | 2 (2) | 2 (2) | 0.21 |
| MIDAS | 23 (39) | 15 (34) | 0.07 | 37 (43) | 23 (40) | 0.06 |
| BDI | 9 (10) | 8 (9) | 0.01 | 12 (11) | 10 (11) | 0.06 |
| State anxiety | 50 (6) | 48 (4) | 0.04 | 50 (6) | 47 (5) | 0.03 |
| Trait anxiety | 47 (5) | 46 (4) | 0.13 | 48 (5) | 46 (4) | 0.04 |
Values are means (SD).
Exploratory analyses were performed to identify potential predictors for the absence of headache improvement following aneurysm treatment (Table 2). For these analyses, patients with pretreatment headaches (n=28) and the patient with new headaches following treatment (n=1) were included. The following were associated with a lack of headache improvement: (i) having migraine prior to treatment; (ii) having more severe headaches prior to treatment; (iii) stent-assisted coiling; (iv) higher pretreatment trait anxiety.
Table 2.
Factors associated with headache outcomes. Comparison of characteristics between patients with headache improvement following aneurysm treatment to patients with no change (n = 6), worsening (n = 3) or new headaches (n = 1) revealed factors associated with headache outcomes. Patients with pretreatment migraine, more severe headaches, and with higher pretreatment anxiety were less likely to improve following aneurysm treatment. Furthermore, stent-assisted coiling of the aneurysm was associated with a lack of headache improvement
| Headaches improved (n = 19) | Headaches did not improve/new headaches (n = 10) | p | |
|---|---|---|---|
| Age in years (SD) | 57 (11) | 53 (9) | 0.32 |
| Female (%) | 14 (74%) | 10 (100%) | 0.08 |
| Chronic migraine (%) | 2 (10.5%) | 4 (40%) | 0.06 |
| CDH (%) | 8 (42%) | 6 (60%) | 0.36 |
| Migraine (%) | 5 (26%) | 7 (70%) | 0.02 |
| Headache frequency (SD) | 44 (32) | 53 (37) | 0.50 |
| Headache severity (SD) | 4 (2) | 7 (2) | <0.01 |
| Aneurysm size mm (SD) | 7 (6) | 6 (3) | 0.85 |
| Posterior circulation (%) | 4 (21%) | 4 (40%) | 0.28 |
| Coiled (%) | 16 (84.2%) | 8 (80%) | 0.48 |
| Stent-assisted coiling (%) | 1 (5.3%) | 4 (40%) | 0.02 |
| Clipped (%) | 2 (10.5%) | 2 (20%) | 0.48 |
| MIDAS (SD) | 24 (30) | 56 (56) | 0.11 |
| BDI (SD) | 10 (10) | 17 (12) | 0.11 |
| Trait anxiety (SD) | 46 (4) | 51 (5) | 0.01 |
| Stait anxiety (SD) | 49 (5) | 50 (7) | 0.69 |
| ASC-12 (SD) | 2 (2) | 3 (2) | 0.23 |
Twenty-one patients completed QST, including 16 with active headaches at the time of aneurysm treatment. Eleven patients who had headache improvement following aneurysm treatment and 6 patients without headache improvement or new headaches had QST. Pain thresholds and ASC-12 scores in each of these groups are illustrated in Table 3. There were no significant changes in any of these parameters in our study population.
Table 3.
Pain thresholds and allodynia symptoms. Among patients who had quantitative sensory testing (QST), there were no significant changes in allodynia symptoms (ASC-12) or heat, cold or pressure pain thresholds following aneurysm treatment
| Patients with QST (n = 21)
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All patients (n = 21)
|
Patients with active pretreatment headaches (n = 16)
|
Headaches improved (n = 11)
|
Headaches did not improve/new headaches (n = 6)
|
|||||||||
| Pretreatment | Last follow-up | p | Pretreatment | Last follow-up | p | Pretreatment | Last follow-up | p | Pretreatment | Last follow-up | p | |
| ASC-12 | 2 (2) | 1 (2) | 0.13 | 2 (2) | 2 (2) | 0.28 | 2 | 1 | 0.12 | 2 (2) | 2 (2) | 1 |
| Heat pain, °C | 42.0 (4.4) | 43.2 (3.9) | 0.18 | 42.2 (4.0) | 43.5 (3.9) | 0.26 | 41.9 (4.6) | 43.8 (4.3) | 0.21 | 41.5 (4.3) | 42.0 (3.5) | 0.75 |
| Cold pain, °C | 22.6 (6.8) | 21.1 (7.2) | 0.34 | 23.4 (6.0) | 21.1 (6.6) | 0.26 | 24.1 (5.5) | 20.5 (7.6) | 0.15 | 23.2 (7.5) | 23.7 (4.5) | 0.84 |
| Pressure pain, g | 61.2 (85.0) | 81.6 (89.9) | 0.34 | 56.1 (72.5) | 86.2 (78.4) | 0.23 | 67.4 (82.6) | 97.5 (87.9) | 0.24 | 26.5 (36.8) | 51.5 (51.4) | 0.25 |
Values are means (SD).
Discussion
Headache is one of the most common symptoms leading to the diagnosis of an unruptured intracranial aneurysm (2). According to the ICHD-II criteria, headache attributed to unruptured saccular aneurysm requires the presence of a new acute headache (including thunderclap headache) and/or painful third nerve palsy, evidence of causation by the unruptured aneurysm, and resolution of headache within 72 hours (10). When patients present in this fashion, there is a high suspicion for a direct relationship between the presenting symptoms and the unruptured aneurysm. However, when a patient presents with recurrent headaches with characteristics of migraine or tension-type headache, the relationship between these recurrent headaches and the unruptured aneurysm is usually unclear. Nonetheless, patients often presume a direct relationship and thus expect headache improvement following aneurysm treatment. Although some patients do improve, others will have worsening of headaches, new headaches, or no change in pretreatment headache patterns. Determining the typical headache course following aneurysm treatment and identifying predictors of headache outcomes will allow clinicians to give their patients realistic expectations. Furthermore, identification of modifiable risk factors for headache outcomes might allow for small modifications in treatment procedures that could increase the likelihood for favorable headache outcomes.
This prospective analysis demonstrates that the majority of patients with pretreatment headaches have headache improvement (reduced headache frequency) following aneurysm intervention. In this study, 68% of patients with active pretreatment headaches had substantial improvement following aneurysm treatment, 21% had no change in their headache frequency, and 11% had a substantial increase in headache frequency. Only one patient transformed from inactive headaches to an active headache pattern following aneurysm treatment. These results are limited by our sample size and the potential for recall bias related to completion of MIDAS questionnaires.
The explanation for headache improvement following aneurysm treatment is not clear. Although speculative at best, it is possible that in some patients headaches have a direct relationship to presence of an unruptured intracranial aneurysm. This association may seem relatively clear in the patient presenting with a thunderclap headache (often called ‘sentinel headache’ or ‘warning headache’ when an unruptured aneurysm is found) or painful third nerve palsy in whom an unruptured aneurysm is identified. The intracranial arteries, especially the proximal portions of the major vessels, are innervated by sensory nerves that could potentially be activated by aberrant blood flow and/or structural changes of the vessel wall (12–15). However, caution must be exercised when considering a causal relationship between unruptured aneurysms and recurrent headaches that resemble primary headache disorders. There are numerous other factors, such as anxiety and depression about harboring an unruptured aneurysm and the pending aneurysm treatment, which may transiently increase headache frequency after aneurysm diagnosis but prior to treatment. Patient expectations for headache improvement following aneurysm treatment could also contribute to improvements via a placebo effect. Furthermore, medications used at the time of treatment could have positive effects on headache patterns. Although changes in daily medications following aneurysm treatment could have had some effect on headache patterns in our patient population, we believe that this effect was minimal. Antihypertensive and antiplatelet medications were used commonly in our population. Prior to aneurysm treatment, 28 patients took daily antihypertensives and 35 used antiplatelets. The antihypertensive regimen was changed in 12 of our patients during their study involvement. This included 7 patients who had new antihypertensives started or had their dosage increased. Four of these patients had active headaches prior to aneurysm treatment. Three of these four patients had headache improvement (benazepril dosage increased in 1 patient; metoprolol stopped and lisinopril added in another patient; lisinopril added in the third patient) while one of these patients had headache worsening (metoprolol added). Twenty-four patients took clopidogrel surrounding the time of aneurysm treatment, typically for a duration ranging from 2 weeks to 2 months. Since the last follow-up occurred well after discontinuation of clopidogrel in the majority of our subjects, we anticipate that periprocedural use of clopidogrel had minimal effects on our study results. Although this study placed no restrictions on the use of headache prophylactic therapy, only one patient was started on a prophylactic medication (topiramate) other than an antihypertensive during the time of follow-up. This patient had no change in her headache frequency during study participation.
We anticipated a higher rate of worsening and new headaches following aneurysm treatment, likely due to a biased view that developed while working in a specialty headache center. Nonetheless, this relatively low incidence of new and worsening headaches following aneurysm treatment should not deter from considering this a significant problem. Our experience suggests that patients with new and worsening headaches after aneurysm treatment frequently suffer with severe chronic pain that is often only partially responsive to conventional therapies.
Univariate exploratory analyses identified several possible predictors for absence of headache improvement following aneurysm treatment. Factors associated with a lack of headache improvement included having pretreatment migraine, having more severe pretreatment headaches, having higher pretreatment trait anxiety, and stent-assisted coiling of the aneurysm. These associations should be further assessed in future studies which are powered for multivariate analyses. We anticipate that some of these potential predictors are not independent. For example, having more severe pretreatment headaches may be a marker for having migraine.
Stent-assisted coiling was associated with a lack of headache improvement following aneurysm treatment. Stent-assisted coiling is typically employed to treat wide neck and fusiform aneurysms (16). Stent-assisted coiling is associated with a decreased frequency of angiographic recurrence but with the cost of increased morbidity and mortality (16). Stent-assisted coiling could be associated with poorer headache outcomes via numerous possible mechanisms to include underlying differences in aneurysm characteristics, activation of vascular sensory afferents by the stent, and remodeling of the aneurysm neck and parent vessel (17).
Among patients with headaches, QST did not reveal any significant changes in pain thresholds after aneurysm treatment. However, there were non-significant increases in pain thresholds to heat, cold and pressure in patients with headache improvement following aneurysm treatment. A similar pattern was not seen in our small group of patients in whom headaches did not improve. Thus, future studies will continue to explore a potential role for sensitization in headaches related to aneurysms and their treatment.
To our knowledge, this study is the first prospective analysis of headache patterns following treatment of unruptured intracranial aneurysms. Two retrospective studies both found that the majority of patients with pretreatment headaches improved following aneurysm treatment (3,4). Qureshi and colleagues received questionnaire responses from 47 patients who had undergone endovascular treatment of an unruptured intracranial aneurysm during a 9.5-year period (3). Thirty-two reported the presence of headaches prior to treatment, 19 of whom reported headache improvement following treatment. Two patients had worsening headaches and 5 patients without pretreatment headaches reported onset of new headaches following aneurysm treatment. Kong and colleagues retrospectively analyzed 81 patients who had undergone surgical clipping or endovascular treatment of unruptured intracranial aneurysms over a 5-year period (4). Forty-nine had recurrent headaches prior to aneurysm treatment. Following treatment, 44/49 had headache improvement, 4 had no change, and 1 patient had headache worsening. Although investigators aimed to identify predictors of headache outcomes, none were found. Possible predictors investigated included aneurysm clipping vs. coiling, age, gender, aneurysm size, aneurysm location, associated symptoms, and hypertension history. Our prospective study demonstrating headache improvement following aneurysm treatment is in agreement with these two retrospective studies. Furthermore, we have identified factors associated with a lack of headache improvement, factors that may help physicians and their patients predict headache outcomes following aneurysm treatment.
In conclusion, in this study approximately 2/3 of patients with pretreatment headaches had substantial reductions in headache frequency following treatment of unruptured aneurysms. Less than 10% of all treated patients (those with and without pretreatment headaches) had worsening or new headaches following aneurysm treatment. Based on available data to date, patients with headaches and planned treatments for unruptured intracranial aneurysms can be informed that the majority of patients have at least short-term (up to 6 months) reductions in headache frequency following treatment and that about 10% have worsening or new headaches. Possible predictors for the absence of headache improvement include having migraine, having more severe headaches prior to treatment, having higher trait anxiety, and stent-assisted coiling. These potential risk factors need to be further explored in follow-up studies.
Acknowledgments
We would like to thank the physicians and nurses who assisted with identifying patients for this study: Ralph Dacey MD, Michael Chicoine MD, Greg Zipfel MD, DeWitte Cross MD, Christopher Moran MD, Randall Edgell MD, Bridget Filiput RN, Dawn Weinstock RN and Brenda Hall RN.
Funding
These studies were supported by NIH grants KL2RR024994 and UL1RR024992 to TJS, NIH grant K24DA00417 to EDK, and NIH grant R01NS048602 to RWG.
Footnotes
Reprints and permissions: sagepub.co.uk/journalsPermissions.nav
References
- 1.Wardlaw JM, White PM. The detection and management of unruptured intracranial aneurysms. Brain. 2000;123(Pt 2):205–221. doi: 10.1093/brain/123.2.205. [DOI] [PubMed] [Google Scholar]
- 2.Unruptured intracranial aneurysms – risk of rupture and risks of surgical intervention. International Study of Unruptured Intracranial Aneurysms Investigators. N Engl J Med. 1998;339:1725–1733. doi: 10.1056/NEJM199812103392401. [DOI] [PubMed] [Google Scholar]
- 3.Qureshi AI, Suri MF, Kim SH, et al. Effect of endovascular treatment on headaches in patients with unruptured intracranial aneurysms. Headache. 2003;43:1090–1096. doi: 10.1046/j.1526-4610.2003.03211.x. [DOI] [PubMed] [Google Scholar]
- 4.Kong DS, Hong SC, Jung YJ, et al. Improvement of chronic headache after treatment of unruptured intracranial aneurysms. Headache. 2007;47:693–697. doi: 10.1111/j.1526-4610.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 5.Schwedt TJ, Samples S, Rasmussen P, Stillman M. New headache after endovascular or microsurgical treatment of intracranial aneurysm. Neurology. 2005;64(Suppl 1):A401. [Google Scholar]
- 6.Arnau RC, Meagher MW, Norris MP, et al. Psychometric evaluation of the Beck Depression Inventory-II with primary care medical patients. Health Psychol. 2001;20:112–119. doi: 10.1037//0278-6133.20.2.112. [DOI] [PubMed] [Google Scholar]
- 7.Stewart WF, Lipton RB, Kolodner KB, et al. Validity of the Migraine Disability Assessment (MIDAS) score in comparison to a diary-based measure in a population sample of migraine sufferers. Pain. 2000;88:41–52. doi: 10.1016/S0304-3959(00)00305-5. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy BL, Schwab JJ, Morris RL, et al. Assessment of state and trait anxiety in subjects with anxiety and depressive disorders. Psychiatr Q. 2001;72:263–276. doi: 10.1023/a:1010305200087. [DOI] [PubMed] [Google Scholar]
- 9.Lipton RB, Bigal ME, Ashina S, et al. Cutaneous allodynia in the migraine population. Ann Neurol. 2008;63:148–158. doi: 10.1002/ana.21211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders. Cephalalgia. (2) 2004;24(Suppl 1):1–151. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 11.Inagawa T. Risk factors for the formation and rupture of intracranial saccular aneurysms in Shimane, Japan. World Neurosurg. 2010;73:155–164. doi: 10.1016/j.surneu.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 12.O’Connor TP, van der Kooy D. Pattern of intracranial and extracranial projections of trigeminal ganglion cells. J Neurosci. 1986;6:2200–2207. doi: 10.1523/JNEUROSCI.06-08-02200.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keller JT, Beduk A, Saunders MC. Origin of fibers innervating the basilar artery of the cat. Neurosci Lett. 1985;58:263–268. doi: 10.1016/0304-3940(85)90175-2. [DOI] [PubMed] [Google Scholar]
- 14.Saito K, Moskowitz MA. Contributions from the upper cervical dorsal roots and trigeminal ganglia to the feline circle of Willis. Stroke. 1989;20:524–526. doi: 10.1161/01.str.20.4.524. [DOI] [PubMed] [Google Scholar]
- 15.Mayberg M, Langer RS, Zervas NT, et al. Perivascular meningeal projections from cat trigeminal ganglia: possible pathway for vascular headaches in man. Science. 1981;213:228–230. doi: 10.1126/science.6166046. [DOI] [PubMed] [Google Scholar]
- 16.Piotin M, Blanc R, Spelle L, et al. Stent-assisted coiling of intracranial aneurysms: clinical and angiographic results in 216 consecutive aneurysms. Stroke. 41:110–115. doi: 10.1161/STROKEAHA.109.558114. [DOI] [PubMed] [Google Scholar]
- 17.Yahia AM, Gordon V, Whapham J, et al. Complications of Neuroform stent in endovascular treatment of intracranial aneurysms. Neurocrit Care. 2008;8:19–30. doi: 10.1007/s12028-007-9001-7. [DOI] [PubMed] [Google Scholar]