Abstract
Recent studies have demonstrated an important physiologic link between bone and fat. Bone and fat cells arise from the same mesenchymal precursor cell within bone marrow, capable of differentiation into adipocytes or osteoblasts. Increased BMI appears to protect against osteoporosis. However, recent studies have suggested detrimental effects of visceral fat on bone health. Increased visceral fat may also be associated with decreased growth hormone (GH) and insulin-like growth factor 1 (IGF-1) levels which are important for maintenance of bone homeostasis. The purpose of our study was to assess the relationship between vertebral bone marrow fat and trabecular bone mineral density (BMD), abdominal fat depots, GH and IGF-1 in premenopausal women with obesity. We studied 47 premenopausal women of various BMI (range: 18–41 kg/m2, mean 30 ± 7 kg/m2) who underwent vertebral bone marrow fat measurement with proton magnetic resonance spectroscopy (1H-MRS), body composition, and trabecular BMD measurement with computed tomography (CT), and GH and IGF-1 levels. Women with high visceral fat had higher bone marrow fat than women with low visceral fat. There was a positive correlation between bone marrow fat and visceral fat, independent of BMD. There was an inverse association between vertebral bone marrow fat and trabecular BMD. Vertebral bone marrow fat was also inversely associated with IGF-1, independent of visceral fat. Our study showed that vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 and BMD. This suggests that the detrimental effect of visceral fat on bone health may be mediated in part by IGF-1 as an important regulator of the fat and bone lineage.
INTRODUCTION
Obesity is a major public health problem and visceral adiposity is associated with increased metabolic and cardiovascular risk (1,2). However, it has been thought that obese women are at decreased risk for developing osteoporosis, and that increased body weight protects against bone loss (3,4). Despite these data, there is increasing evidence that visceral adiposity and the metabolic syndrome have potential detrimental effects on bone health, such as a higher incidence of osteoporotic fractures and impaired bone structure and strength (5,6). Obese women have been found to have lower rates of bone formation, as measured by type I collagen, suggesting that increased body fat suppresses new collagen formation (7). Recent attention has focused on the emerging role of bone marrow fat and its relationship to bone lineage and osteoporosis. The relationship between bone and fat formation within the bone marrow microenvironment is complex and remains an area of active investigation. Bone and fat cells share a common mesenchymal precursor stem cell within bone marrow, capable of differentiating into adipocytes or osteoblasts (8,9) under the influence of several hormones and transcription factors, such as GH, insulin-like growth factor 1 (IGF-1), leptin and peroxisomal proliferator-activated receptor-γ (9–11). We have shown increased bone marrow fat in subjects with anorexia nervosa despite a severe depletion of body fat using proton magnetic resonance spectroscopy (1H-MRS) as a noninvasive technique (12). Increased bone marrow fat has been found in subjects with morphologic evidence of bone weakness such as endplate depression and compression fractures (13,14). Given the association of increased visceral fat and impaired bone health, we hypothesized that women with high visceral fat would have increased bone marrow fat compared to those with low visceral fat. In addition, we wanted to evaluate the association of vertebral bone marrow fat with abdominal fat compartments using 1H-MRS and computed tomography (CT) as noninvasive techniques.
Growth hormone (GH) and IGF-1 are important regulators of bone homeostasis and important for the maintenance of bone mass (15). GH stimulates the proliferation of cells of the osteoblastic lineage through stimulation of IGF-1 and inhibits adipogenesis (16). It has been suggested that IGF-1 is necessary to maintain cortical and trabecular bone and, consistent with this, adult GH deficiency causes low bone turnover osteoporosis with a high risk of fractures (17). The GH-IGF-1 system also plays a significant role in modulating body composition, and studies have established that with increased visceral adiposity physiologic GH secretion is impaired (18–20). We hypothesize that vertebral bone marrow fat would be inversely associated with IGF-1 levels in premenopausal women with obesity.
METHODS AND PROCEDURES
The study was approved by Partners Healthcare institutional review board and complied with Health Insurance Portability and Accountability Act guidelines. Written informed consent was obtained from all subjects after the nature of the procedures had been fully explained.
Subjects
The study group was comprised of 47 healthy premenopausal women who were recruited from the community through advertisements. Study participants ranged in BMI from 18.1 to 41.4 kg/m2, with a mean BMI of 29.5 ± 6.9 kg/m2. Inclusion criteria were female gender, age >18 years, and eumenorrhea. Exclusion criteria included hypothalamic or pituitary disorders, diabetes mellitus or other chronic illnesses, estrogen or glucocorticoid use and weight >280 pounds (due to the limitations of the CT and magnetic resonance imaging scanner).
To determine the effect of visceral fat on vertebral bone marrow fat, subjects were divided into two groups for comparison based on visceral adipose tissue (VAT), as determined by CT. The 24 women with the least VAT were grouped into a low VAT group and the 23 women with the most VAT were grouped into the high VAT group based on the VAT median as previously described (20,21).
Participants were admitted to the Clinical Research Center at the Massachusetts General Hospital (Boston, MA), where testing was performed. Each participant underwent 1H-MRS and CT as detailed below and fasting blood tests. A growth hormone-releasing hormone (GHRH)-arginine stimulation test was performed in a subset of patients (n = 25) as the GHRH became unavailable in the United States. Clinical characteristics and peak GH after GHRH-arginine stimulation have been previously published in 31 of the 47 subjects (22).
Endocrine testing
All subjects had blood drawn for fasting IGF-1. For the GHRH-arginine stimulation test, GHRH 1 mcg/kg plus arginine 0.5 g/kg (maximum 30 g) IV were administered and GH levels drawn at baseline and every 30 min for 2 h, the standard protocol used to diagnose GH deficiency in patients with hypopituitarism (23).
1H-MRS of bone marrow
All subjects underwent 1H-MRS of the 4th lumbar vertebral body to determine bone marrow lipid content using a 3.0T MR imaging system (Siemens Trio; Siemens Medical Systems, Erlangen, Germany) as previously described (12). Fitting of all 1H-MRS data was performed using LCModel (version 6.1-4A; Stephen Provencher, Oakville, Ontario, Canada) (24). Data were transferred from the scanner to a Linux workstation and metabolite quantification was performed using eddy current correction and water scaling. A customized fitting algorithm for bone marrow analysis provided estimates for all lipid signals combined (0.9, 1.3, and 2.3 p.p.m.). LCModel bone marrow lipid estimates were automatically scaled to unsuppressed water peak (4.7 p.p.m.) and expressed as lipid to water ratio (%).
CT-body composition
Each subject underwent cross-sectional CT scan of the abdomen at the level of L4. Assessment of total, visceral and subcutaneous abdominal fat compartments was performed by single slice CT. Scan parameters were standardized (144 table height, 80 kV, 70 mA, 2 s, 1-cm slice thickness, 48 field of view). Fat attenuation coefficients were set at −50 to −250 hounse field units as described by Borkan et al. (25). Total abdominal cross-sectional area was computed by outlining the outer contour of the abdomen. A second outline along the abdominal wall and back musculature (inner contour) was used to define the subcutaneous adipose tissue area, which is the area located between the outer and inner contour. VAT was defined as the area within the inner contour comprising all pixels with attenuation coefficients between −50 and −250 hounse field units. The total adipose tissue area was calculated as the sum of subcutaneous and visceral abdominal fat. Analyses were performed using Alice software (version 4.3.9; Parexel, Waltham, MA).
CT-bone mineral density
Trabecular bone mineral density (BMD) assessment of L4 was performed using quantitative CT on a LightSpeed CT scanner (General Electric, Milwaukee, WI) with the patient lying supine on a K2 HPO4 CT calibration phantom. Scan parameters were: 144-cm table height, 80 kV, 70 mA, 2 s, 1-cm slice thickness, 48 cm field of view. Images were analyzed utilizing an Impax workstation (AGFA Diagnostic Software, version 4; Agfa, Ridgefield Park, NJ). Trabecular BMD results are expressed in mg/cm3.
Statistical analysis
JMP Statistical Database Software (version 5.0.1; SAS Institute, Cary, NC) was used for statistical analyses. Variables were tested for normality of distribution using the Wilk–Shapiro test. Variables that were not normally distributed were log transformed. Groups were compared using the Student’s t-test. Linear regression analysis was performed. Multivariate standard least squares regression modeling was performed to control for age, BMI, BMD, and visceral adiposity. Forward stepwise regression modeling was also performed. P ≤ 0.05 was used to denote significance.
RESULTS
Clinical characteristics of study subjects
The age of study participants ranged from 19 to 45 years, with a mean of 32.8 ± 7.1 years. Study participants ranged in BMI from 18.1 to 41.4 kg/m2, with a mean of 29.5 ± 6.9 kg/m2. Clinical characteristics of the low VAT and high VAT groups are shown in Table 1. Subjects with high VAT had significantly higher mean BMI, abdominal fat, and vertebral bone marrow fat than the low VAT group. They also had significantly lower mean IGF-1 and peak-stimulated GH levels. There was no significant difference in trabecular BMD as determined by quantitative CT between the two groups.
Table 1.
Clinical characteristics of subjects with low- and high-visceral adipose tissue (low VAT, high VAT)
| Low VAT (n = 24) |
High VAT (n = 23) |
P | |
|---|---|---|---|
| Age (years) | 30.1 ± 6.1 | 35.7 ± 7.0 | 0.005 |
| BMI (kg/m2) | 24.8 ± 5.1 | 34.4 ± 4.9 | <0.0001 |
| Weight (kg) | 68.5 ± 14.3 | 92.1 ± 14.7 | <0.0001 |
| IGF-1 (ng/ml) | 147.3 ± 51.9 | 113.5 ± 47.3 | 0.02 |
| GH-stim peak (ng/ml) | 28.0 ± 10.5 | 15.4 ± 9.8 | 0.006 |
| Bone marrow fat/water (%) |
52.5 ± 21.2 | 66.5 ± 26.0 | 0.05 |
| Abdomen VAT (cm2) | 34.7 ± 10.8 | 129.4 ± 52.5 | <0.0001 |
| Abdomen SAT (cm2) | 251.5 ± 131.7 | 476.9 ± 147.1 | <0.0001 |
| Abdomen TAT (cm2) | 286.2 ± 135.7 | 606.3 ± 163.2 | <0.0001 |
| Trabecular BMD (mg/cm3) |
161.4 ± 31.2 | 158.2 ± 22.7 | NS |
Data presented as mean ± s.d.
BMD, bone mineral density; GH, growth hormone; IGF-1, insulin-like growth factor 1; SAT, subcutaneous adipose tissue; TAT, total adipose tissue; VAT, visceral adipose tissue.
Association of vertebral bone marrow fat with BMD, visceral fat, and IGF-1
There was a positive correlation between vertebral bone marrow fat and VAT (r = 0.34, P = 0.02) (Figure 1).
Figure 1.
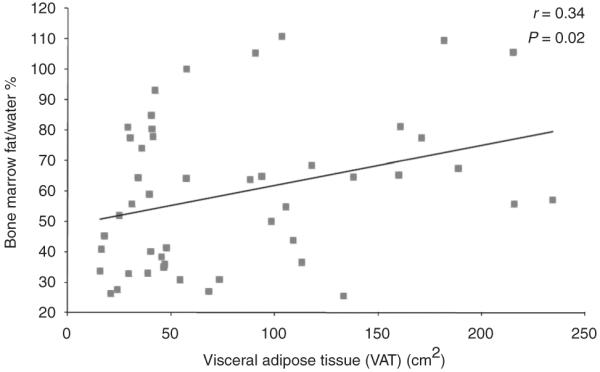
Regression analysis of vertebral bone marrow fat on visceral adipose tissue. There is a positive correlation between bone marrow fat and visceral adipose tissue, which remained significant after controlling for BMD (P = 0.05). BMD, bone mineral density; VAT, visceral adipose tissue.
There was an inverse association between vertebral bone marrow fat and trabecular BMD as determined by quantitative CT (r = −0.39, P = 0.007) (Figure 2). There was an inverse association between vertebral bone marrow fat and IGF-1 (r = −0.39, P = 0.007) (Figure 3). There were no significant correlations between vertebral bone marrow fat and age, BMI, weight, subcutaneous and total abdominal adipose tissue (P = 0.1–0.8). There was no significant correlation between vertebral bone marrow fat and peak GH following stimulation with GHRH-arginine (r = −0.19, P = 0.4).
Figure 2.
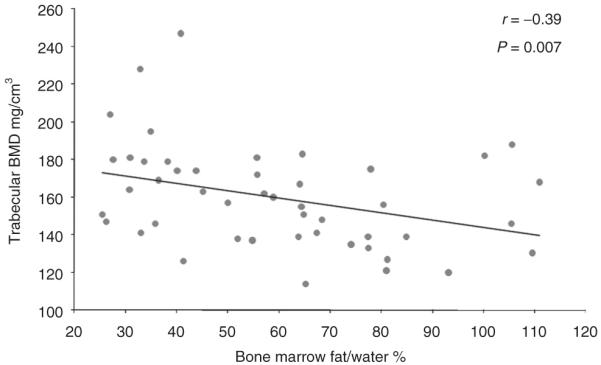
Regression analysis of BMD on vertebral bone marrow fat. There is an inverse association between bone marrow fat and trabecular BMD, which remained significant after controlling for visceral adipose tissue (P = 0.03). BMD, bone mineral density.
Figure 3.
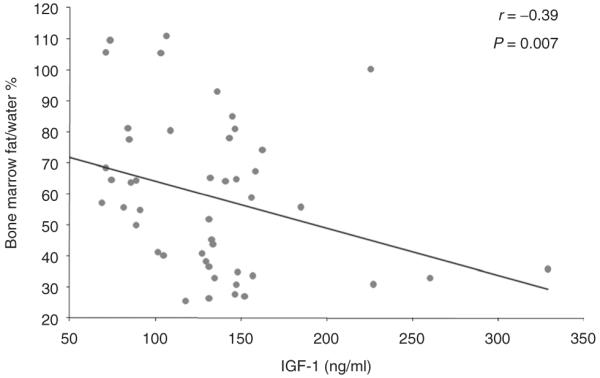
Regression analysis of vertebral bone marrow fat on IGF-1. There is an inverse association between vertebral bone marrow fat and IGF-1, which remained significant after controlling for age and BMI (P = 0.05). IGF-1, insulin-like growth factor 1.
Because VAT is associated with BMD, we controlled for VAT and BMD using multivariate analysis. After controlling for BMD, the correlation between vertebral bone marrow fat and VAT remained significant (P = 0.05). After controlling for VAT, the correlation between vertebral bone marrow fat and BMD remained significant (P = 0.03).
As IGF-1 decreases with increasing age and BMI, we controlled for age and BMI using multivariate analysis. After controlling for age and BMI the correlation between vertebral bone marrow fat and IGF-1 remained significant (P = 0.05).
When vertebral bone marrow fat was entered as a dependent variable and IGF-1, visceral fat, and trabecular BMD as independent variables in a forward stepwise regression model, IGF-1 and BMD were significant predictors of vertebral bone marrow fat. IGF-1 explained 25% (r2 = 0.25, P = 0.02) and trabecular BMD explained 14% (r2 = 0.14, P = 0.007) of the variability of vertebral bone marrow fat.
When trabecular BMD was entered as a dependent variable and vertebral bone marrow fat, IGF-1, and BMI as independent variables in a forward stepwise regression model, vertebral bone marrow fat was a significant predictor of trabecular BMD and explained 15% of the variability of trabecular BMD (r2 = 0.15, P = 0.007).
DISCUSSION
Our study demonstrated that premenopausal women with increased visceral fat had increased vertebral bone marrow fat compared to women with low visceral fat despite normal BMD. Moreover, our data suggest that increased vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in this population.
Despite being a risk factor for cardiovascular and metabolic disease, obesity has been thought to protect against osteoporosis (3,4) due to stresses from mechanical loading and from metabolic effects of hormones secreted by adipocytes (26). Recent studies have suggested that increased visceral fat has negative effects on bone health and correlates inversely with BMD (5,6).
Gilsanz et al. (5) demonstrated the beneficial effects of subcutaneous and detrimental effects of visceral fat on bone structure and strength as determined by CT of the femur. In our study, visceral fat correlated with vertebral bone marrow fat, but there was no association between bone marrow fat and BMI, subcutaneous or total abdominal adipose tissue. This suggests distinct metabolic roles for visceral and subcutaneous fat.
Our observed correlation between visceral fat and bone marrow adiposity may provide clues as to the underlying mechanism for the negative effects of visceral fat on BMD. Our data are consistent with the data from a study by Shen et al. (27) showing a positive correlation between visceral fat and bone marrow fat of the pelvis and extremities determined by magnetic resonance imaging in a cohort of healthy women ranging from 18 to 88 years. As in our study, there was no significant correlation between subcutaneous and total fat and marrow fat. Shen et al. were unable to reliably quantify bone marrow fat in the spine using standard magnetic resonance imaging technique (27). We were able to quantify bone marrow fat in the spine using 1H-MRS. The importance of this is that vertebral bone marrow fat has been shown to be a predictor of bone fragility at this skeletal site (13). In a prior study, we have shown increased bone marrow fat in the spine, femoral metaphysis and diaphysis in women with anorexia nervosa compared to normal weight controls. However, we did not find correlations between abdominal fat depots and vertebral bone marrow fat in this population (12). This may be due to the markedly decreased abdominal fat depots in anorexia nervosa.
It has been suggested that vertebral bone marrow fat determined by 1H-MRS, in combination with BMD, is of significance in evaluating skeletal integrity, more valuable than either parameter alone. Increased bone marrow fat affects biomechanical strength of bone, as yellow marrow is a weaker biomechanical support medium than red bone marrow (13,14). Therefore, we used 1H-MRS to quantify bone marrow fat in the spine. Fatty infiltration of bone marrow has been shown to be a characteristic feature of older individuals and is inversely related to BMD and skeletal integrity (28,29).
In our study, vertebral bone marrow fat was inversely associated with BMD and was a significant predictor of BMD. In addition, we saw a significant difference in bone marrow fat in premenopausal women with low and high visceral fat whereas there was no difference in BMD, suggesting a distinct role of bone marrow fat in skeletal integrity. A limitation of our study was that subjects with high VAT were older than subjects with low VAT. Therefore, we adjusted for age when comparing the two groups and the differences remained significant.
Our study is the first to examine the relationship between IGF-1 and bone marrow fat in humans. Osteoblasts and adipocytes are derived from a common precursor mesenchymal stem cell capable of differentiating into bone and fat. Several hormones and transcription factors are known to regulate bone and fat mass (9–11). GH and IGF-1 are important regulators of bone homeostasis and GH deficiency leads to decreased bone growth and osteopenia (17,30). GH and IGF-1 are also known to regulate fat mass (30). Adipocytes produce IGF-1 in response to GH and are a source of circulating IGF-1 (11). IGF-1 is an important differentiation factor for osteoblasts and studies in mice with low-serum IGF-1 have shown reduced bone size and trabecular density as well as increased bone marrow fat with reduced expression of several transcription factors necessary for osteoblasts differentiation (31). As GH secretion decreases with obesity (18,20), we investigated the relationship between bone marrow fat and the GH/IGF-1 axis in women covering the spectrum from normal weight to obesity. We found a significant inverse correlation between vertebral bone marrow fat and IGF-1 which persisted after controlling for age and BMI. However, we did not find a significant correlation between peak GH and vertebral bone marrow fat. This may have been due to the lack of peak-stimulated GH data in all patients, as GHRH became unavailable in the United States during the study, or may reflect differential effects of GH and IGF-1. Our data supports the role of IGF-1 as an important regulator of the fat and bone lineage.
In conclusion, we have demonstrated an association between vertebral bone marrow fat and visceral fat in obese premenopausal women. We have also shown increased bone marrow fat in women with increased visceral fat, consistent with recent studies suggesting a detrimental effect of visceral fat on bone health. We have also demonstrated an inverse correlation between vertebral bone marrow fat and IGF-1, supporting the role of IGF-1 as an important regulator of the fat and bone lineage.
ACKNOWLEDGMENT
This work was supported in part by National Institutes of Health Grants RO1 HL-077674, UL1 RR025758, and K23 RR-23090.
Footnotes
DISCLOSURE The authors declared no conflict of interest.
REFERENCES
- 1.Rexrode KM, Carey VJ, Hennekens CH, et al. Abdominal adiposity and coronary heart disease in women. JAMA. 1998;280:1843–1848. doi: 10.1001/jama.280.21.1843. [DOI] [PubMed] [Google Scholar]
- 2.Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation. 2008;117:1658–1667. doi: 10.1161/CIRCULATIONAHA.107.739714. [DOI] [PubMed] [Google Scholar]
- 3.Albala C, Yáñez M, Devoto E, et al. Obesity as a protective factor for postmenopausal osteoporosis. Int J Obes Relat Metab Disord. 1996;20:1027–1032. [PubMed] [Google Scholar]
- 4.Reid IR, Plank LD, Evans MC. Fat mass is an important determinant of whole body bone density in premenopausal women but not in men. J Clin Endocrinol Metab. 1992;75:779–782. doi: 10.1210/jcem.75.3.1517366. [DOI] [PubMed] [Google Scholar]
- 5.Gilsanz V, Chalfant J, Mo AO, et al. Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab. 2009;94:3387–3393. doi: 10.1210/jc.2008-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Muhlen D, Safii S, Jassal SK, Svartberg J, Barrett-Connor E. Associations between the metabolic syndrome and bone health in older men and women: the Rancho Bernardo Study. Osteoporos Int. 2007;18:1337–1344. doi: 10.1007/s00198-007-0385-1. [DOI] [PubMed] [Google Scholar]
- 7.Papakitsou EF, Margioris AN, Dretakis KE, et al. Body mass index (BMI) and parameters of bone formation and resorption in postmenopausal women. Maturitas. 2004;47:185–193. doi: 10.1016/S0378-5122(03)00282-2. [DOI] [PubMed] [Google Scholar]
- 8.Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol. 2006;2:35–43. doi: 10.1038/ncprheum0070. [DOI] [PubMed] [Google Scholar]
- 9.Takada I, Suzawa M, Matsumoto K, Kato S. Suppression of PPAR transactivation switches cell fate of bone marrow stem cells from adipocytes into osteoblasts. Ann N Y Acad Sci. 2007;1116:182–195. doi: 10.1196/annals.1402.034. [DOI] [PubMed] [Google Scholar]
- 10.Rosen CJ. Bone remodeling, energy metabolism, and the molecular clock. Cell Metab. 2008;7:7–10. doi: 10.1016/j.cmet.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Vikman K, Isgaard J, Edén S. Growth hormone regulation of insulin-like growth factor-I mRNA in rat adipose tissue and isolated rat adipocytes. J Endocrinol. 1991;131:139–145. doi: 10.1677/joe.0.1310139. [DOI] [PubMed] [Google Scholar]
- 12.Bredella MA, Fazeli PK, Miller KK, et al. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. 2009;94:2129–2136. doi: 10.1210/jc.2008-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schellinger D, Lin CS, Hatipoglu HG, Fertikh D. Potential value of vertebral proton MR spectroscopy in determining bone weakness. AJNR Am J Neuroradiol. 2001;22:1620–1627. [PMC free article] [PubMed] [Google Scholar]
- 14.Schellinger D, Lin CS, Lim J, et al. Bone marrow fat and bone mineral density on proton MR spectroscopy and dual-energy X-ray absorptiometry: their ratio as a new indicator of bone weakening. AJR Am J Roentgenol. 2004;183:1761–1765. doi: 10.2214/ajr.183.6.01831761. [DOI] [PubMed] [Google Scholar]
- 15.Ohlsson C, Bengtsson BA, Isaksson OG, Andreassen TT, Slootweg MC. Growth hormone and bone. Endocr Rev. 1998;19:55–79. doi: 10.1210/edrv.19.1.0324. [DOI] [PubMed] [Google Scholar]
- 16.Gevers EF, Loveridge N, Robinson IC. Bone marrow adipocytes: a neglected target tissue for growth hormone. Endocrinology. 2002;143:4065–4073. doi: 10.1210/en.2002-220428. [DOI] [PubMed] [Google Scholar]
- 17.Giustina A, Mazziotti G, Canalis E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev. 2008;29:535–559. doi: 10.1210/er.2007-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwu CM, Kwok CF, Lai TY, et al. Growth hormone (GH) replacement reduces total body fat and normalizes insulin sensitivity in GH-deficient adults: a report of one-year clinical experience. J Clin Endocrinol Metab. 1997;82:3285–3292. doi: 10.1210/jcem.82.10.4311. [DOI] [PubMed] [Google Scholar]
- 19.Makimura H, Stanley T, Mun D, You SM, Grinspoon S. The effects of central adiposity on growth hormone (GH) response to GH-releasing hormone-arginine stimulation testing in men. J Clin Endocrinol Metab. 2008;93:4254–4260. doi: 10.1210/jc.2008-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller KK, Biller BM, Lipman JG, et al. Truncal adiposity, relative growth hormone deficiency, and cardiovascular risk. J Clin Endocrinol Metab. 2005;90:768–774. doi: 10.1210/jc.2004-0894. [DOI] [PubMed] [Google Scholar]
- 21.Pijl H, Langendonk JG, Burggraaf J, et al. Altered neuroregulation of GH secretion in viscerally obese premenopausal women. J Clin Endocrinol Metab. 2001;86:5509–5515. doi: 10.1210/jcem.86.11.8061. [DOI] [PubMed] [Google Scholar]
- 22.Bredella MA, Torriani M, Thomas BJ, et al. Peak growth hormone-releasing hormone-arginine-stimulated growth hormone is inversely associated with intramyocellular and intrahepatic lipid content in premenopausal women with obesity. J Clin Endocrinol Metab. 2009;94:3995–4002. doi: 10.1210/jc.2009-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biller BM, Samuels MH, Zagar A, et al. Sensitivity and specificity of six tests for the diagnosis of adult GH deficiency. J Clin Endocrinol Metab. 2002;87:2067–2079. doi: 10.1210/jcem.87.5.8509. [DOI] [PubMed] [Google Scholar]
- 24.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 25.Borkan GA, Gerzof SG, Robbins AH, et al. Assessment of abdominal fat content by computed tomography. Am J Clin Nutr. 1982;36:172–177. doi: 10.1093/ajcn/36.1.172. [DOI] [PubMed] [Google Scholar]
- 26.Klein KO, Larmore KA, de Lancey E, et al. Effect of obesity on estradiol level, and its relationship to leptin, bone maturation, and bone mineral density in children. J Clin Endocrinol Metab. 1998;83:3469–3475. doi: 10.1210/jcem.83.10.5204. [DOI] [PubMed] [Google Scholar]
- 27.Shen W, Chen J, Punyanitya M, et al. MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos Int. 2007;18:641–647. doi: 10.1007/s00198-006-0285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Justesen J, Stenderup K, Ebbesen EN, et al. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2:165–171. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- 29.Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-γ2 transcription factor and TGF-β/BMP signaling pathways. Aging Cell. 2004;3:379–389. doi: 10.1111/j.1474-9728.2004.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mukherjee A, Murray RD, Shalet SM. Impact of growth hormone status on body composition and the skeleton. Horm Res. 2004;62(Suppl 3):35–41. doi: 10.1159/000080497. [DOI] [PubMed] [Google Scholar]
- 31.Rosen CJ, Ackert-Bicknell CL, Adamo ML, et al. Congenic mice with low serum IGF-I have increased body fat, reduced bone mineral density, and an altered osteoblast differentiation program. Bone. 2004;35:1046–1058. doi: 10.1016/j.bone.2004.07.008. [DOI] [PubMed] [Google Scholar]


