Abstract
Background:
Strongyloidiasis is endemic in many tropical and subtropical countries and reports from northeast India are very few.
Aim:
A community-based study was carried out in Assam, India, to find out the occurrence of Strongyloides stercoralis.
Materials and Methods:
Stool samples were obtained from 198 randomly selected individuals from the community. Samples were processed using direct microscopy with formal-ether concentration methods.
Results and Conclusion:
Seventeen numbers of samples showed the presence of Strongyloides stercoralis (8.5%). A total of 105 (53%) individuals were positive for at least one intestinal parasite. The other intestinal parasites detected were Ascaris lumbricoides (33.3%), Trichuris trichiura (9.1%), hookworm (7.1%), Enterobius vermicularis (1.5%), and Giardia cyst (1.5%). Five out of 17 individuals positive for S.stercoralis had associated gastrointestinal, respiratory, and/or cutaneous symptoms.The present study although not exhaustive for true prevalence calls for attention in the backdrop of high malnutrition and pulmonary tuberculosis.
KEYWORDS: Strongyloidiasis, Strongyloides stercoralis, Assam, India, prevalence
INTRODUCTION
Strongyloidiasis, an important emerging infection that is endemic in many tropical and subtropical countries, and reported to have infected about 100 million people worldwide, is a neglected tropical disease.[1] Even though the fecal-oral route of transmission is described, humans mostly acquire the infection through penetration of filariform larvae through the skin. The larvae are transferred through the circulation to the lungs and finally reach their final location in the small intestine where the female adult worms and rhabditiform larvae live in the jejunal crypts and cause mild eosinophilia and chronic inflammation.[2] The entire life cycle of Strongyloides stercoralis can be completed in the soil as a free living cycle after being shed in stools as rhabditiform larvae, or can persist in the host by autoinfection. In patients with suppressed immunity, the infective larvae are more likely to penetrate the intestinal mucosa and invade the bloodstream and further disseminate to multiple organs such as the skin, lungs or even the central nervous system, and sometimes lead to sepsis or meningitis.[3]
There are many studies reporting the prevalence as well as serious complications of hyperinfection and dissemination as a result of Strongyloidiasis.[3,4] Reports from northeast India are very few and most of them are limited hospital-based data or case reports.[4] We report here a community-based survey carried out in Assam, India, to study the occurrence of S. stercoralis.
MATERIALS AND METHODS
The study was carried out in the Dibrugarh district of Assam, India, in 2009. The area is inhabited by the indigenous people of Assam, the Ahom and Sonowal Kachari group belonging to the Tibeto-Burman linguistic tribe. Most of them are farmers with a low socioeconomic status. Most of the houses are with ‘kuccha’ floor and the people work and walk barefoot. All houses have shallow tube wells as a drinking water source.
People were informed about the study and a copy of the village registry, with house numbers containing the basic demographic information was obtained from the local health worker (Accredited Social Health Assistant).There were 134 households with a total population of 688. A total of 45 households were randomly selected for the study (every third house). From these households which included 225 individuals, every member were invited for the study. All the families were contacted by the health worker and the aim and procedures of the study were explained, and a verbal informed consent was obtained from the head of the household. Samples were collected on the following day and were brought to the main laboratory within two hours.
The samples were processed immediately after bringing them to the laboratory using direct microscopy and with formal-ether concentration methods. The infected individuals were advised to attend the health center for treatment. Identification of S. stercoralis larva was based on morphology, namely the size of buccal cavity, genital primordium, and appearance of a tail.
Those individuals whose stool samples were positive for S. stercoralis larvae were revisited for further clinical probing.
RESULTS AND DISCUSSION
Out of the 225 individuals recruited, 198 (88%) submitted a stool specimen. The mean age was 25.32 years (3 months to 90 years) and 100 were males. A total of 105 (53%) individuals were positive for at least one intestinal parasite.
The intestinal parasites detected were Ascaris lumbricoides (33.3%), Trichuris trichiura (9.1%), hookworm (7.1%), Enterobius vermicularis (1.5%), and Giardia cyst (1.5%). S. stercoralis larvae were detected in 8.5% (17 / 198) by direct fecal smear preparation No increase in detection rate was observed following concentration.
The demographic and associated symptoms of the positive cases are presented in Table 1. The prevalence of S. stercoralis was similar in females and males (10.2 versus 7.0%, P = 0.58). S. stercoralis positive cases were restricted to 13 households only. In three families more than one member was involved (three in one family and two in two families). A detailed examination and past history for the last six months for all the positive cases are summarized in Table 1. Diarrhea, constipation, abdominal pain, cough, and skin rashes [Figure 1] were seen among the S. stercoralis positive cases. None of the individuals were suspected or investigated by the attending physician for helminthic infection, and as a result none received anti-helminthic treatment. This calls for attention by the physicians serving in these areas to consider strongyloidiasis in the differential diagnosis, while treating their patients. If not recognized it could have devastating consequences.
Table 1.
Clinical symptoms in persons infested with Strongyloides stercoralis
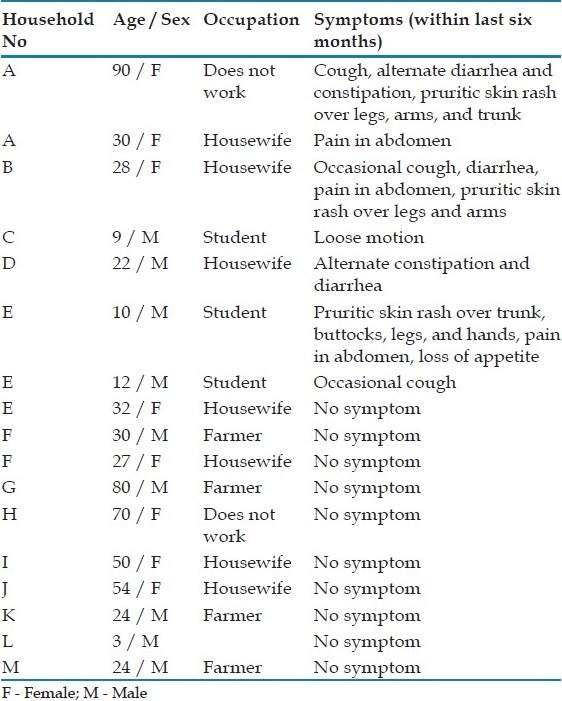
Figure 1.
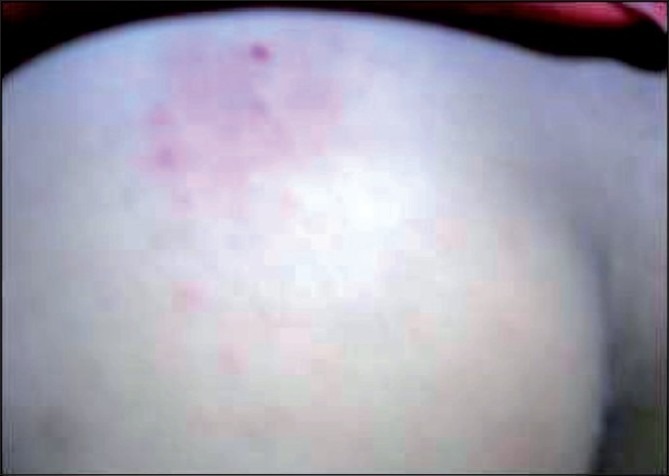
Skin rash over buttocks in an individual with fecal sample positive for Strongyloides stercoralis. The individual had similar rash over her trunk, legs, hands and neck
Familial aggregation of infection with S. stercoralis was seen in three families. None of the persons in the present study had hyperinfection or disseminated infection. The risk factors for disseminated strongyloidiasis include, immunosuppressive therapy, transplant, malignant disease, HIV infection, malnutrition, diabetes mellitus, and so on.[3] This part of the country is reported to have more prevalence of malnutrition and pulmonary tuberculosis.[5] Therefore, more attention should be paid by the attending physician, especially in a rural setup, as untreated infection could cause serious problems in the community.
The present study, although not exhaustive for true prevalence (8.5%), shows minimum community prevalence and calls for more attention of the physician, in the backdrop of high malnutrition and pulmonary tuberculosis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diement D, et al. Soil-transmitted helminthic infection: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–32. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 2.Onah DN, Nawa Y. Mucosal immunity against parasitic gastrointestinal nematodes. Korean J Parasitol. 2000;38:209–36. doi: 10.3347/kjp.2000.38.4.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keiser PB, Nutman TB. Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev. 2004;17:208–217. doi: 10.1128/CMR.17.1.208-217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agrawal V, Agarwal T, Ghoshal UC. Intestinal strongyloidiasis: a diagnosis frequently missed in tropics. Trans R Soc Trop Med Hyg. 2009;103:242–6. doi: 10.1016/j.trstmh.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Medhi GK, Hazarika NC, Shah B, Mahanta J. Study of health problems and nutritional status of tea garden population of Assam. Indian J Med Sci. 2006;60:496–505. [PubMed] [Google Scholar]


