‘Ethical conduct’ literally means simply doing the right thing, but in reality it means more. It involves acting in the right spirit, out of an abiding respect and concern for one's fellow creatures.
Human research is research conducted with or about people, or their data or tissues, with the sole intention to do good.
Human research involves significant risks and it is possible for things to go wrong. Despite the best of intentions and care in planning and practice, sometimes things go awry. Now and then mishaps may arise because of technical errors or an ethical insensitivity, neglect or disregard.
On rare occasions, the practice of research has even involved deliberate and appalling violation of human beings. Earlier, in the 1900s, there were no regulations regarding the ethical use of human subjects in research. There were no guidelines or any code drawn out for conduct and no Institutional Review Board (IRB). Here is a brief account of why rules and regulations were established and the need for all established research institutes to have an IRB became a necessity.[1]
THE NUREMBERG CODE
A well-known chapter in the history of research with human subjects opened on December 9, 1946, when an American military tribunal opened criminal proceedings against 23 leading German physicians and administrators for their willing participation in war crimes and crimes against humanity. Among the charges were that German physicians conducted medical experiments on thousands of concentration camp prisoners without their consent. Most of the subjects of these experiments died or were permanently crippled as a result.
As a direct result of the trial, the Nuremberg Code was established in 1948, stating that ‘The voluntary consent of the human subject is absolutely essential,’ making it clear that subjects should give consent and that the benefits of the research must outweigh the risks.
Although it did not carry the force of law, the Nuremberg Code was the first international document, which advocated voluntary participation and informed consent.[2]
THE DECLARATION OF HELSINKI
In 1964, the World Medical Association established recommendations guiding medical doctors in biomedical research involving human subjects. The Declaration governs international research ethics and defines rules for ‘research combined with clinical care’ and ‘non-therapeutic research.’ The Declaration of Helsinki was revised in 1975, 1983, 1989, and 1996, and is the basis for Good Clinical Practices used today.
Issues addressed in the declaration of Helsinki include:
Research with humans should be based on the results from laboratory and animal experimentation
Research protocols should be reviewed by an independent committee prior to initiation
Informed consent from research participants is necessary
Research should be conducted by medically / scientifically qualified individuals
Risks should not exceed benefits
THE TUSKEGEE SYPHILIS STUDY (1932 – 1972)
One of the turning points in the development of a consensus for guidelines for ethical conduct in research was a project conducted by the US Public Health Service. Six hundred low-income, African-American males, 400 of whom were infected with syphilis, were monitored for 40 years. Free medical examinations were conducted; however, the subjects were not told about their disease. Even though a proven cure (penicillin) became available in the 1950s, the study continued until 1972, with participants being denied treatment. In some cases, when the subjects were diagnosed as having syphilis by other physicians, researchers intervened to prevent treatment. The study sparked off a wide-scale public outrage when it became publicly known, and the US government had to close it in 1973.
Due to the publicity from the Tuskegee Syphilis Study, a National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research was formed in the US, which was in charge of identifying the basic ethical principles that should underline the conduct of biomedical and behavioral research involving human subjects and to develop guidelines that should be followed, to assure that such research is conducted in accordance with those principles. The Commission drafted the Belmont Report, a foundational document for the ethics of human subjects’ research in the United States.[3]
THE BELMONT REPORT
The Belmont Report was published in 1979, with attempts to summarize the basic ethical principles identified by the Commission in the course of its deliberations. The Report is a statement of the basic ethical principles and guidelines that should assist in resolving the ethical problems that surround the conduct of research with human subjects. The three basic ethical principles and their corresponding applications according to the report are:
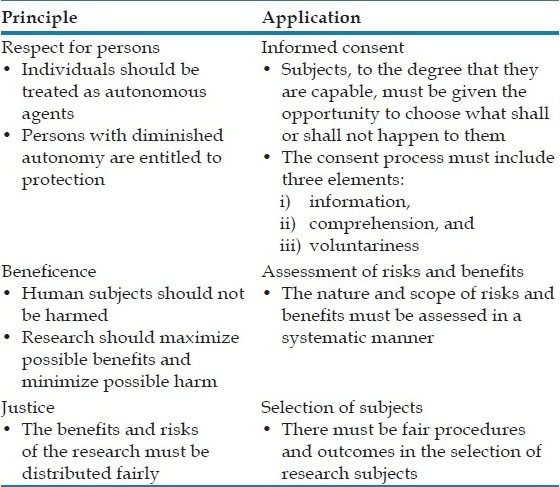
The Belmont Report established three basic ethical principles – respect for persons, beneficence, and justice – which are the cornerstones for the regulations involving human subjects.[3]
COMMON RULE
This is a set of regulations that have been adopted by many research agencies in the United States and elsewhere.
The main elements of the Common Rule include:[3]
requirements for assuring compliance by research institutions
requirements for researchers obtaining and documenting informed consent
requirements for Institutional Review Board (IRB) membership, function, operations, review of research, and record keeping
additional protection for certain vulnerable research subjects – pregnant women, prisoners, and children
Situation in India
As in the case of many other nations, India too has developed national guidelines for research involving human beings. In our country the guidelines, which are often cited and followed, are those issued by the Indian Council of Medical Research, New Delhi. The Indian Council of Medical research brought out the ‘Policy Statement on Ethical Considerations involved in Research on Human Subjects,’ in 1980, and revised these guidelines in 2000, as the ‘Ethical guidelines for Biomedical Research on Human Subjects’. Since then it has been revised and the latest version has been published in 2006.
In its general statement the document stresses on the fact that only such research should be undertaken whose purpose would be to advance the ‘betterment of all, especially the least advantaged’. The manner in which the research is conducted should not compromise the dignity and well-being of the subjects, and it should avoid the historical pitfalls of unethical research, To ensure sound scientific output ‘the research must be subjected to a strict regime of evaluation at all stages of the proposal’.[4]
All human beings are born free and equal in dignity and rights; it is the duty of each and every man related or unrelated to science to treat their fellow beings with love and respect.
REFERENCES
- 1.World Health Organization. Geneva: WHO Document Production Services; 2009. Research ethics committees: Basic concepts for capacity building. [Google Scholar]
- 2.Komesaroff P, Dodds S, McNeill P, Skene L. Canberra: Ausinfo; 2001. Human research ethics handbook - commentary on National statement on ethical conduct in research involving humans. [Google Scholar]
- 3.Las Vegas: University of Nevada; 2010. History of research ethics. Ref Type: Electronic Citation. [Google Scholar]
- 4.Ethical guidelines for biomedical research on human participants. New Delhi: Indian Council of Medical Research; 2010. Statement of general principles on ethical considerations involving human participants; pp. 2–8. [Google Scholar]


