Abstract
Background:
Survey on the prevalence of various intestinal parasitic infestations in different geographic regions is a prerequisite to obtain an accurate understanding of the burden and cause of intestinal parasitic infestations in a particular area. The aim of the present study was to determine the intestinal parasitic infestation among children in a semi-urban area.
Materials and Methods:
A total of 335 stool samples were collected, processed, and microscopically examined for intestinal parasites.
Results:
One hundred twenty-eight (38%) stool samples showed presence of ova/cysts. Multiple parasites were seen in 42 (32.8%) samples. Among the protozoans, Entamoeba histolytica (55.3%) was the most common followed by Giardia lamblia (40.4%). Ascaris lumbricoides and Hymenolepis nana (24.2%) were the most common helminths detected.
Conclusions:
In most of the cases, intestinal parasitic infestation spreads due to low standards of personal hygiene, poor sanitation, non-usage of toilets and an illiterate population, thus suggesting regular surveys to help in devising optimum methods of control.
KEYWORDS: Diarrhea, intestinal parasites, semi-urban population
INTRODUCTION
Rapid industrialization and a shift of the population from rural to urban areas have caused deterioration in the environmental quality. It is not unusual to find areas of poverty and poor sanitation within Indian cities. Helminthic infestations and parasitic infections put a severe strain on the nutrition of children in particular who live in these pockets.[1] As a result of this morbidity, they are at an increased risk for detrimental effects like poor cognitive performance and physical growth.[2] Therefore, children living in rural areas of developing countries are seriously affected.[3] Poor sanitation, scarcity of potable drinking water, and low standards of personal hygiene contribute to rapid spread of these infections.[4] Estimation of the global magnitude of morbidity and mortality due to parasitic infestations is a major concern.[5] The frequency of parasitic infestation varies with age and sex of the general population. Children aged below 10 years frequently complain of problems related to parasitic infestations than older children.[6,7]
It then becomes important to know the disease burden of parasitic infestations in the communities. Limited data regarding this is available in literature. Thus, it was rightfully considered worthwhile to undertake a study in one of the semi-urban population sample to examine the prevalence of different types of helminthic and parasitic infestations among children living in those conditions.
MATERIALS AND METHODS
This study was undertaken in the Department of Microbiology, Santosh Medical College and Hospital, Ghaziabad from June 2008 to December 2009. A total of 335 stool samples were collected from children aged below the age of 14 years. House visits were made and the samples were collected along with the requisite data. The children were selected randomly on the basis of age and weight. Demographic data from every child was recorded that included the age, sex, residence, education/occupation of mother, number of children in family, housing condition, type of toilet, hand washing practice after defecation and clinical symptoms.
Stool specimens (~1 gm) were collected in wide mouth containers without any preservative and transported to the laboratory within an hour. The stool samples were subjected to gross and microscopic examination. Naked eye examination was done for intestinal worms and segments of Taenia species. The microscopic examination was accomplished by normal saline preparation and Lugol's Iodine preparation directly from the stool.[8] The negative samples were examined by formal ether concentration technique.[9] Modified Ziehl-Neelsen stain was used for the identification of coccidian parasites.[10] Children who had taken antiparasitic drug during the last three months and had a history of loose stool during the last one month were excluded from the study.
RESULTS
During the study, 235 houses were visited from where 335 samples were collected, which included one sample each from 177 houses and two stool samples each from 79 houses i.e., 158 samples. On gross examination, no stool sample showed any worm or segment of helminthic worms.
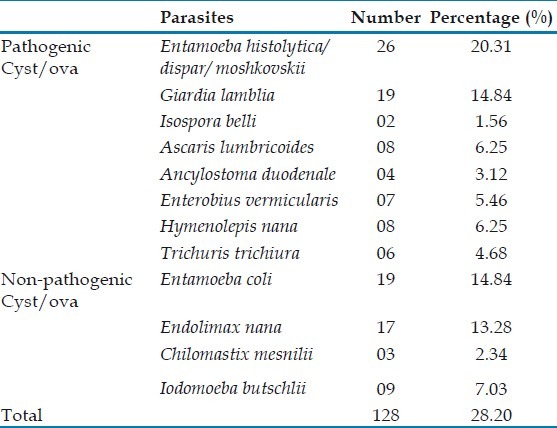
Microscopic examination showed that of the 335 samples screened, parasites were detected from 128 (38%) samples [Table 1]. Of these, the number of pathogenic ova and cysts found were 62.5% and nonpathogenic intestinal parasites found were 37.5%. Protozoans formed 58.7% of the total parasitic infestation while the helminthic infestation was 41.2%. The most common pathogenic intestinal parasite was Entamoeba histolytica (20.3%) followed by Giardia lamblia (14.8%) and Ascaris lumbricoides and Hymenolepis nana (6.2%). Among the protozoans, E. histolytica (55.3%) was the most common followed by G. lamblia (40.4%). A. lumbricoides and H. nana (24.2%) were the most common helminths detected.
Table 1.
Parasitic distribution in stool specimen (n = 128)
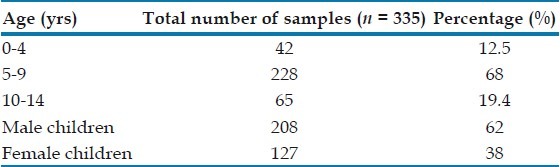
Among the study population, 208 were male and 127 were female children [Table 2]. The demographic correlation with the intestinal parasites isolated showed that intestinal parasites isolated were higher in the male children (63.2%). The age group of children ranged up to 14 years, out of which maximum number of stool samples (68%) belonged to children between 5 and 9 years. About 53.9% of parasites were isolated from children who had mothers who were uneducated and in 72% children who were not using soap for hand washing after toilet tested positive for intestinal parasites.
Table 2.
Parasitic infestation in relation to age and sex
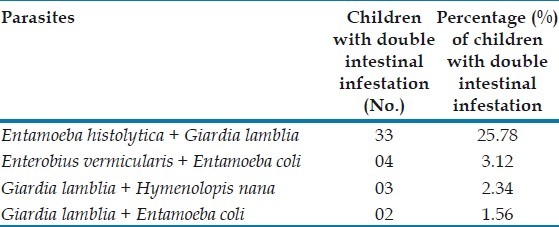
Existence of two different parasites in the same sample was observed commonly with E. histolytica and G. lamblia (25.7%) followed by Enterobius vermicularis and Entamoeba coli (3.1%), G. lamblia and H. nana (2.3%) [Table 3].
Table 3.
Distribution of double parasitic infection (n = 128)
DISCUSSION
In this study, 38% of the healthy children showed positive result for intestinal parasites, which is nearly comparable to the study of Mahajan et al. (26.8%).[11] The isolation of protozoal cysts was higher than that of the helminthic ova. Our study showed that the most common intestinal parasite observed was E. histolytica (20.3%). Prakash, Tandon, and Shrivastava have also reported 35.6% and 18.4% positivity for the same.[12,13] The prevalence of E histolytica has been observed as a common finding in tropical and subtropical countries and is responsible for diarrhea and amoebic liver abscess in several studies.[14] These intestinal parasites are commonly transmitted by infected drinking water and food. In India, the water supply poses a big problem due to faecal contamination of the same.
The most common helminthic infestation in our study was A. lumbricoides (6.2%) which was low compared to similar studies by Shrivastava where 22.2% of stool samples demonstrated A. lumbricoides.[13] In our study, 32.8% showed double parasitic infections. In a similar study, 46.7% had prevalence of one or more parasites as reported by Wani et al.[15] However, in their study, the prevalence of A. lumbricoides was highest (28.4%) followed by G. lamblia (7.2%), Trichuris trichiura (4.9%) and Taenia saginata (3.7%).
The presence of cysts of Isospora belli (1.50%) was an unusual finding in our study, as it is usually associated with AIDS patients and is responsible for chronic diarrhea. Dalvi et al, have reported I. belli as the most common pathogen among HIV associated diarrhea.[16]
A higher percentage of infestation was seen among children of age group 5-9 years, most of them belonging to the lower strata of society who are deprived of healthy living conditions. Celiksoz et al, in their study have also observed that poor economic conditions, low personal hygiene, lack of clean drinking water, and improper waste disposal are important contributory factors for intestinal parasitism in slums.[4] In this study, a higher percentage of children of uneducated mothers were found positive for intestinal parasites (69%). Okayay et al, observed that mother's education was important for bringing up of children in rural and economically weaker sections of the society and educated mothers can train their children well and maintain a better health than the uneducated mothers.[17] None of the children used toilet paper though the usage of toilet papers significantly reduces the incidence of parasitic infections.[18]
This study was limited to single sample observation. Perhaps a larger sample size and longitudinal study with more parameters might be necessary for continuation of this surveillance study to obtain an accurate understanding of the burden and cause of parasitic infestation in this area.
CONCLUSION
In most of the cases, intestinal parasitic infestation spreads due to low standards of personal hygiene, poor sanitation, non-usage of toilets (and toilet papers), and an illiterate population. An integrated approach of drug treatment and focussed participatory hygiene education is required to control parasite load among rural children in India. These measures would mitigate the severity of frequent outbreak of parasitic infestation. However, it also highlights the fact that in spite of the easy availability of antihelminthic and antiprotozoal drugs, these conditions are not yet eradicated. Hence, regular surveys serve as a very useful purpose and should be an ongoing process.
Living situations are changing fast, and environmental deterioration will further complicate the health situation. There is a great need to monitor human settlements around the big cities; otherwise they will remain a permanent source of health hazard for all diseases including intestinal parasitism. This could thus have a severe economic impact on the Indian healthcare system.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Amar CF, Dear PH, Pedraza-Diaz S, Looker N, Linnane E, Mclauchlin J. Sensitive PCR restriction fragment length polymosphism assay for detection and genotyping of Giardia duodenalis in human feces. J Clin Microbiol. 2002;40:446–52. doi: 10.1128/JCM.40.2.446-452.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nokes C, Grantham SM, Swayer AW, Cooper ES, Bundy DA. Parasitic helminthic infection and cognitive function in schoolchildren. Proceed Roy Soc London B. 1992;247:77–81. doi: 10.1098/rspb.1992.0011. [DOI] [PubMed] [Google Scholar]
- 3.Hagne R, Ali IM, Sack RB, Farr BM, Ramakrishnan G, Petri WA., Jr Amoebiasis and mucosal IgA antibody against the Entamoeba histolytica adherence lectin in Bangladeshi children. J Infect Dis. 2001;183:1787–93. doi: 10.1086/320740. [DOI] [PubMed] [Google Scholar]
- 4.Celiksoz A, Güler N, Guler G, Oztop AY, Degeril S. Prevalence of intestinal parasites in three socioeconomically different regions of Sivas, Turkey. J Health Popul Nutr. 2005;23:184–91. [PubMed] [Google Scholar]
- 5.Phiri K, Whitty CJ, Graham SM, Ssembatya–Lule G. Urban/rural differences in prevalence and risk factor for intestinal helminth infection in southern Malawi. Ann Trop Med Parasitol. 2000;94:381–7. doi: 10.1080/00034983.2000.11813553. [DOI] [PubMed] [Google Scholar]
- 6.Easton A. Intestinal worm impair child health in the Philippines. BMJ. 1999;318:214. doi: 10.1136/bmj.318.7178.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oberhelman RA, Guerroro ES, Fernandes ML, Siho M, Mercado D, Comiskey N, et al. Corelations between intestinal parasitosis, physical growth and psychomotor development among infant and children from rural Nicaragua. Am J Trop Med Hyg. 1998;58:470–5. doi: 10.4269/ajtmh.1998.58.470. [DOI] [PubMed] [Google Scholar]
- 8.Proctor EM. Laboratory diagnosis of amoebiasis. Clin Lab Med. 1991;11:829–59. [PubMed] [Google Scholar]
- 9.Petri WA, Jr, Singh U. Diagnosis and management of amoebiasis. Clin Infect Dis. 1999;29:1117–25. doi: 10.1086/313493. [DOI] [PubMed] [Google Scholar]
- 10.Ash IR, Orihel TC. Parasites: A guide to laboratory procedures and identification. 3rd ed. Chicago: American Society of Clincial Pathologists; 1987. pp. 51–2. [Google Scholar]
- 11.Mahajan M, Mathur M, Talwar V, Revathi G. Prevalence of intestinal parasitic infections in East Delhi. Indian J Community Med. 1993;111:177–9. [Google Scholar]
- 12.Prakash O, Tandon BN. Intestinal parasites with special reference to Entamoeba histolytica complex as revealed by routine concentration and cultural examination of stool samples from patient with gastro-intestinal symptoms. Indian J Med Res. 1966;54:10–4. [Google Scholar]
- 13.Shrivastava JB. A survey of intestinal parasites in human population in Bombay with special reference to Entamoeba histolytica. Indian J Med Res. 1977;21:29–33. [PubMed] [Google Scholar]
- 14.Blessmann J, Van Linh P, Nu PA, Thi HD, Muller-Myhsok B, Buss H, et al. Epidemiology of amoebiasis in a region of high incidence of amoebic liver abscess in central Vietnam. Am J Trop Med Hyg. 2002;66:578–83. doi: 10.4269/ajtmh.2002.66.578. [DOI] [PubMed] [Google Scholar]
- 15.Wani SA, Ahmad F, Zargar SA, Ahmad Z, Ahmad P, Tak H. Prevalence of intestinal parasites and associated risk factors among schoolchildren in Srinagar city, Kashmir, India. J Parasitol. 2007;93:1541–3. doi: 10.1645/GE-1255.1. [DOI] [PubMed] [Google Scholar]
- 16.Dalvi S, Mehta P, Koticha A, Gita N. Microsporidia as an emerging cause of parasitic diarrhoea in HIV seropositive indiviuals in Mumbai. J Bombay Hosp J. 2006;48:592–7. [Google Scholar]
- 17.Okyay P, Erting S, Gultekin B, Onen O, Beser E. Intestinal parasites prevalence and related factor in school children. BMC Public Health. 2004;4:64. doi: 10.1186/1471-2458-4-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gamboa MI, Basualdo JA, Córdoba MA, Pezzani BC, Minvielle MC, Lahitte HB. Distribution of intestinal parasites in relation to environmental and sociocultural parameter in LaPlata, Argentina. J Helminthol. 2003;77:15–20. doi: 10.1079/JOH2002142. [DOI] [PubMed] [Google Scholar]


