Abstract
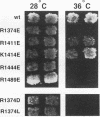
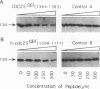
Previously we found that negatively charged residues at positions 62, 63, and 69 of H-Ras are involved in binding to the CDC25 guanine nucleotide exchange factor (GEF). Using site-directed mutagenesis, we have changed conserved, positively charged residues of CDC25GEF to glutamic acid. We find the nonfunctional CDC25R1374E mutant and the nonfunctional H-RasE63K mutant cooperate in suppression of the loss of CDC25 function in Saccharomyces cerevisiae. Also, peptides corresponding to residues 1364 to 1383 of CDC25GEF inhibit interaction between GEFs and H-Ras. We propose that residues 1374 of CDC25GEF and 63 of H-Ras form an ion pair and that when this ion pair is reversed, functional interaction can still occur.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Becker D. M., Guarente L. High-efficiency transformation of yeast by electroporation. Methods Enzymol. 1991;194:182–187. doi: 10.1016/0076-6879(91)94015-5. [DOI] [PubMed] [Google Scholar]
- Broek D., Toda T., Michaeli T., Levin L., Birchmeier C., Zoller M., Powers S., Wigler M. The S. cerevisiae CDC25 gene product regulates the RAS/adenylate cyclase pathway. Cell. 1987 Mar 13;48(5):789–799. doi: 10.1016/0092-8674(87)90076-6. [DOI] [PubMed] [Google Scholar]
- Buday L., Downward J. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell. 1993 May 7;73(3):611–620. doi: 10.1016/0092-8674(93)90146-h. [DOI] [PubMed] [Google Scholar]
- Chevray P. M., Nathans D. Protein interaction cloning in yeast: identification of mammalian proteins that react with the leucine zipper of Jun. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5789–5793. doi: 10.1073/pnas.89.13.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien C. T., Bartel P. L., Sternglanz R., Fields S. The two-hybrid system: a method to identify and clone genes for proteins that interact with a protein of interest. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9578–9582. doi: 10.1073/pnas.88.21.9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerby L. M., Escobar W. A., Fink A. L., Mitchinson C., Wells J. A. The role of lysine-234 in beta-lactamase catalysis probed by site-directed mutagenesis. Biochemistry. 1990 Jun 19;29(24):5797–5806. doi: 10.1021/bi00476a022. [DOI] [PubMed] [Google Scholar]
- Feig L. A. The many roads that lead to Ras. Science. 1993 May 7;260(5109):767–768. doi: 10.1126/science.8484117. [DOI] [PubMed] [Google Scholar]
- Hwang J. K., Warshel A. Why ion pair reversal by protein engineering is unlikely to succeed. Nature. 1988 Jul 21;334(6179):270–272. doi: 10.1038/334270a0. [DOI] [PubMed] [Google Scholar]
- Lai C. C., Boguski M., Broek D., Powers S. Influence of guanine nucleotides on complex formation between Ras and CDC25 proteins. Mol Cell Biol. 1993 Mar;13(3):1345–1352. doi: 10.1128/mcb.13.3.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B. X., Wei W., Broek D. The catalytic domain of the mouse sos1 gene product activates Ras proteins in vivo and in vitro. Oncogene. 1993 Nov;8(11):3081–3084. [PubMed] [Google Scholar]
- Lowy D. R., Willumsen B. M. Function and regulation of ras. Annu Rev Biochem. 1993;62:851–891. doi: 10.1146/annurev.bi.62.070193.004223. [DOI] [PubMed] [Google Scholar]
- Martegani E., Vanoni M., Zippel R., Coccetti P., Brambilla R., Ferrari C., Sturani E., Alberghina L. Cloning by functional complementation of a mouse cDNA encoding a homologue of CDC25, a Saccharomyces cerevisiae RAS activator. EMBO J. 1992 Jun;11(6):2151–2157. doi: 10.1002/j.1460-2075.1992.tb05274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosteller R. D., Han J., Broek D. Identification of residues of the H-ras protein critical for functional interaction with guanine nucleotide exchange factors. Mol Cell Biol. 1994 Feb;14(2):1104–1112. doi: 10.1128/mcb.14.2.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munder T., Fürst P. The Saccharomyces cerevisiae CDC25 gene product binds specifically to catalytically inactive ras proteins in vivo. Mol Cell Biol. 1992 May;12(5):2091–2099. doi: 10.1128/mcb.12.5.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl R. H., Gietz R. D. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989 Dec;16(5-6):339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- Shou C., Farnsworth C. L., Neel B. G., Feig L. A. Molecular cloning of cDNAs encoding a guanine-nucleotide-releasing factor for Ras p21. Nature. 1992 Jul 23;358(6384):351–354. doi: 10.1038/358351a0. [DOI] [PubMed] [Google Scholar]
- Wells J. A., Powers D. B., Bott R. R., Graycar T. P., Estell D. A. Designing substrate specificity by protein engineering of electrostatic interactions. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1219–1223. doi: 10.1073/pnas.84.5.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D., Riggs M., Field J., Vojtek A., Broek D., Wigler M. The adenylyl cyclase gene from Schizosaccharomyces pombe. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7989–7993. doi: 10.1073/pnas.86.20.7989. [DOI] [PMC free article] [PubMed] [Google Scholar]