Abstract
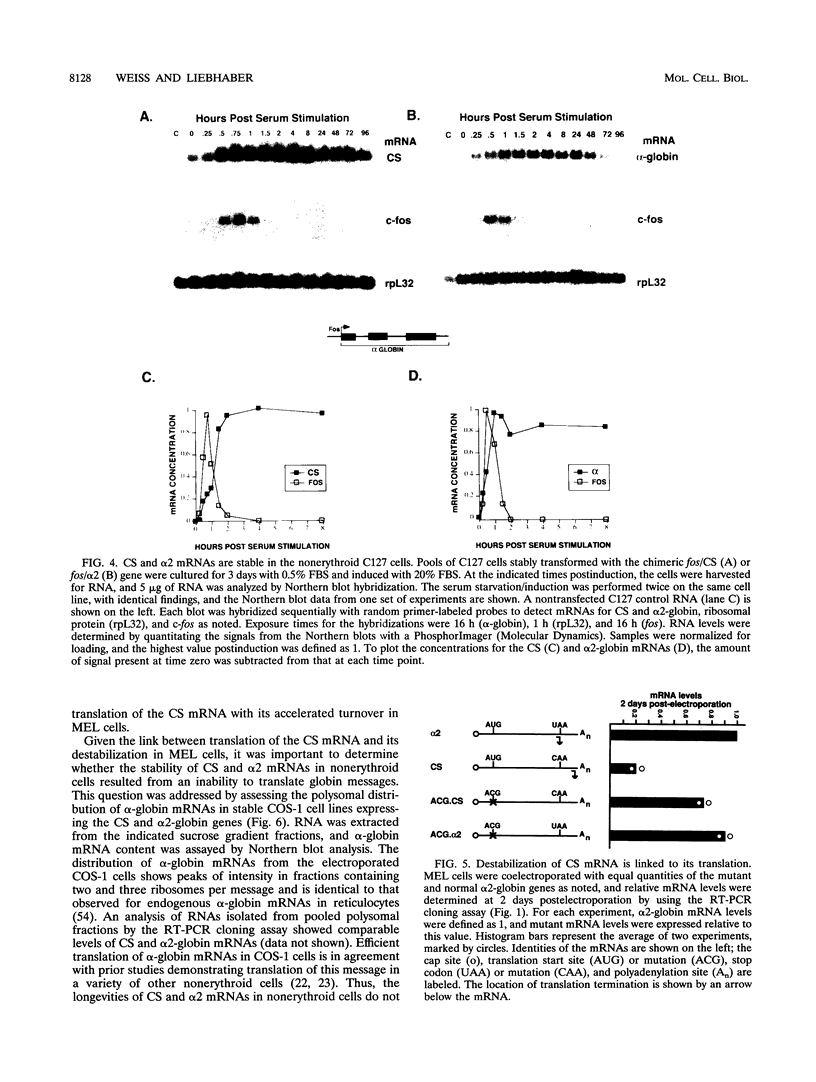
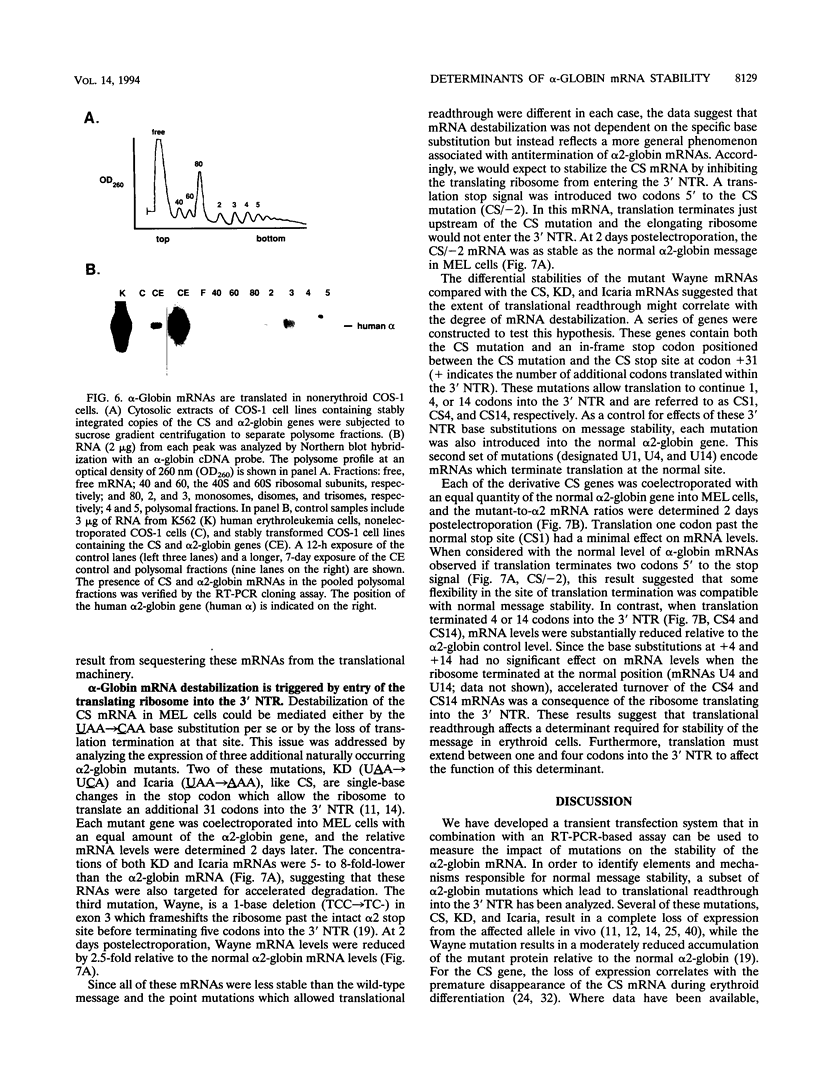
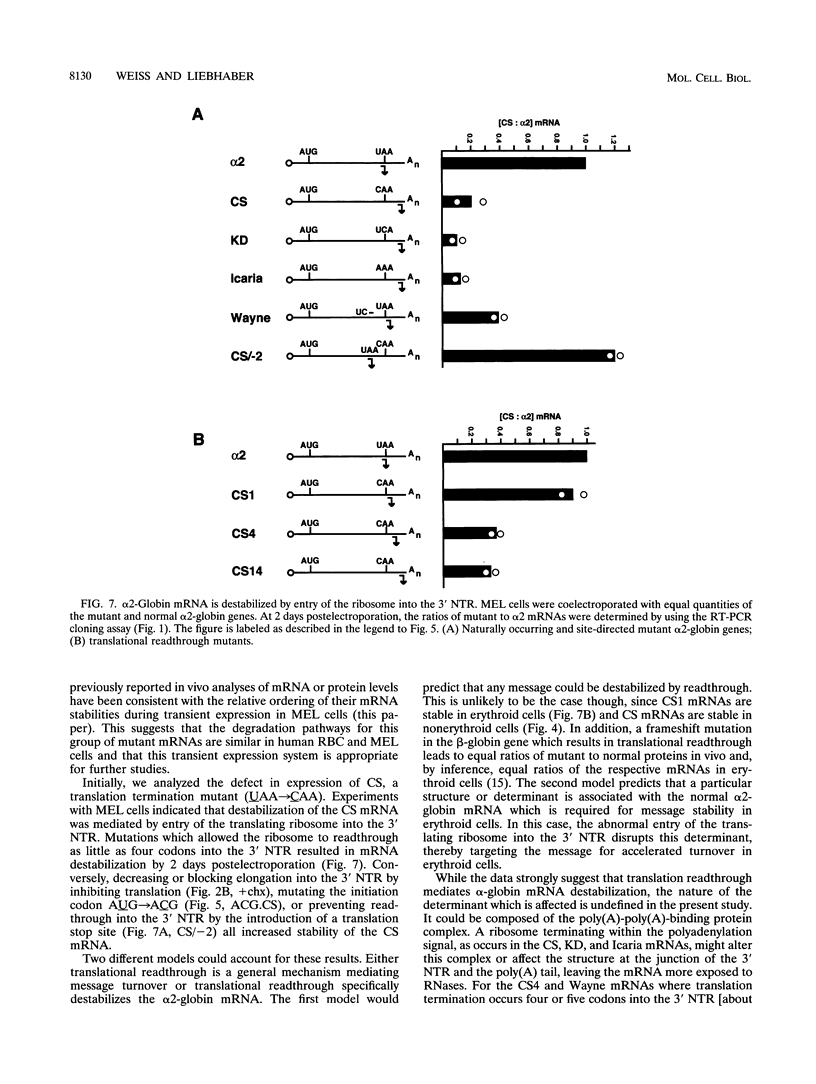
Although globin mRNAs are considered prototypes of highly stable messages, the mechanisms responsible for their longevity remain largely undefined. As an initial step in identifying potential cis-acting elements or structures which contribute to their stability, we analyzed the defect in expression of a naturally occurring alpha 2-globin mutant, alpha Constant Spring (CS). The CS mutation is a single-base change in the translation termination codon (UAA-->CAA) that allows the ribosome to read through into the 3' nontranslated region (NTR). The presence of CS mRNA in transcriptionally active erythroid precursors and its absence (relative to normal alpha-globin mRNA) in the more differentiated transcriptionally silent erythrocytes suggest that this mutation disrupts some feature of the alpha-globin mRNA required for its stability. Using a transient transfection system, we demonstrate that in murine erythroleukemia cells the CS mRNA is unstable compared with the normal alpha 2-globin mRNA. The analyses of several other naturally occurring and site-directed mutant alpha-globin genes in murine erythroleukemia cells indicate that entry of a translating ribosome into the 3' NTR targets the message for accelerated degradation in erythroid cells. In contrast, both the CS and alpha 2-globin mRNAs are stable in several nonerythroid cell lines. These results suggest that translational readthrough disrupts a determinant associated with the alpha 2-globin 3' NTR which is required for mRNA stability in erythroid cells.
Full text
PDF


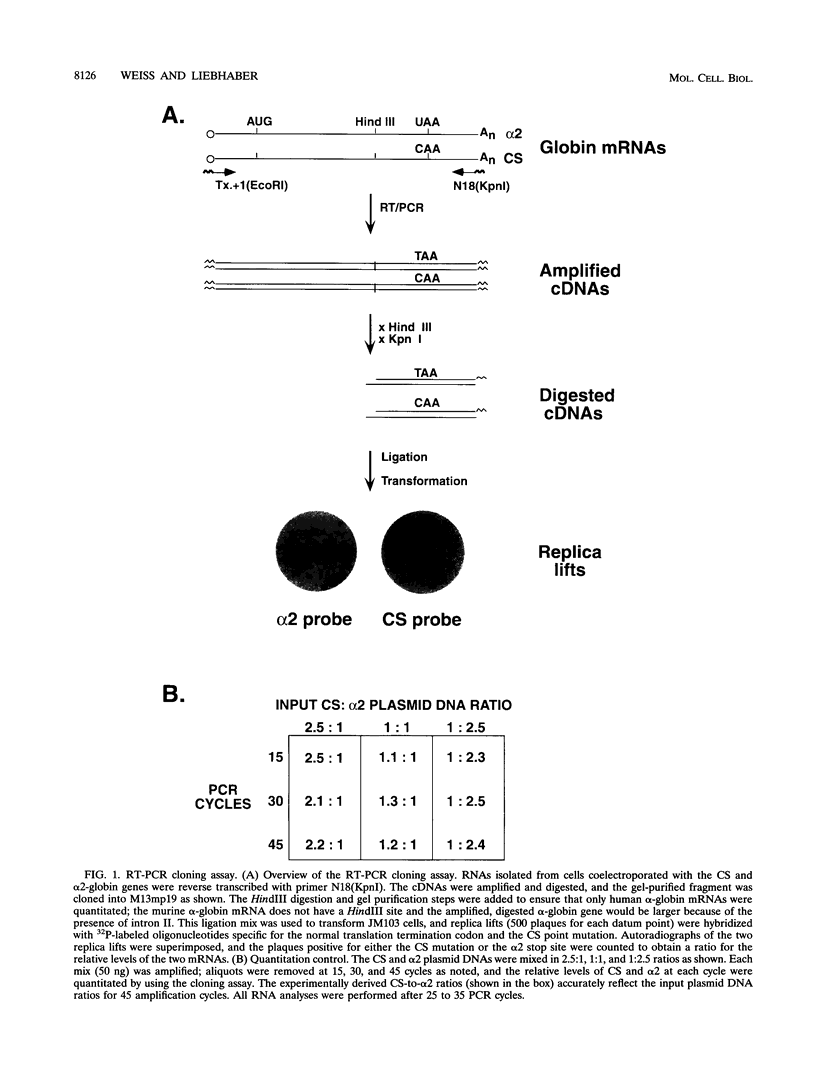
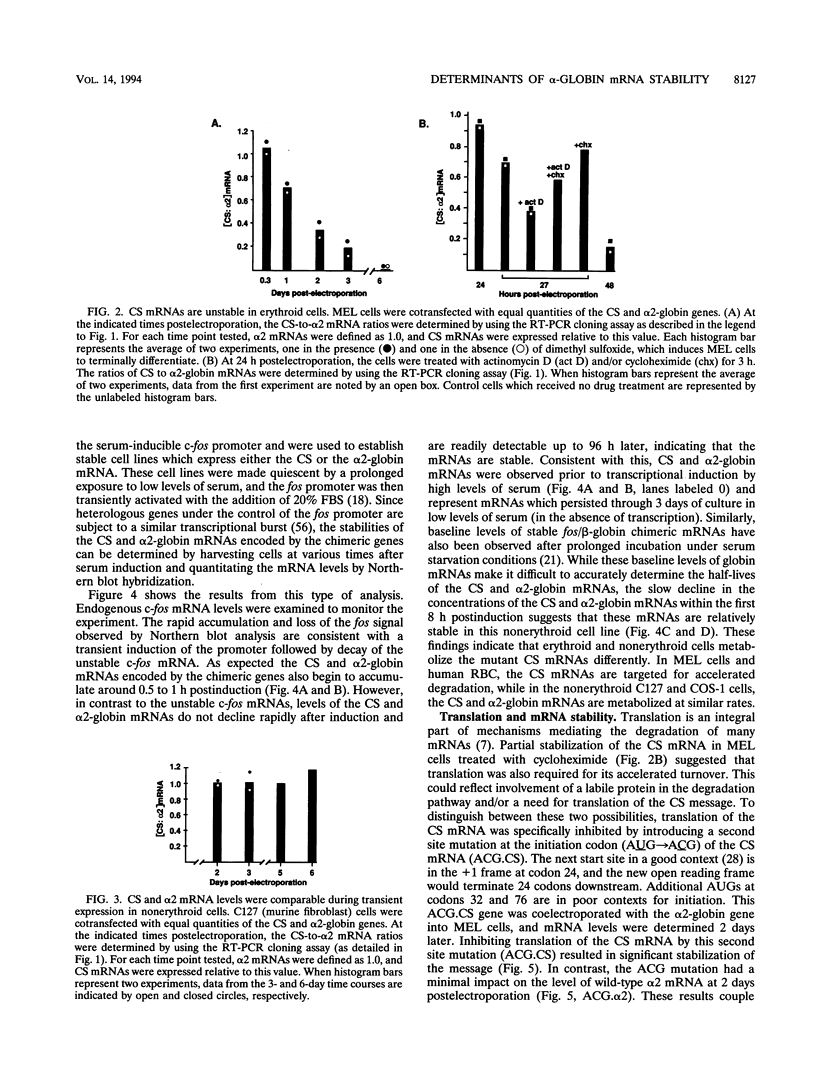


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahern S. M., Miyata T., Sadler J. E. Regulation of human tissue factor expression by mRNA turnover. J Biol Chem. 1993 Jan 25;268(3):2154–2159. [PubMed] [Google Scholar]
- Atwater J. A., Wisdom R., Verma I. M. Regulated mRNA stability. Annu Rev Genet. 1990;24:519–541. doi: 10.1146/annurev.ge.24.120190.002511. [DOI] [PubMed] [Google Scholar]
- Aviv H., Voloch Z., Bastos R., Levy S. Biosynthesis and stability of globin mRNA in cultured erythroleukemic Friend cells. Cell. 1976 Aug;8(4):495–503. doi: 10.1016/0092-8674(76)90217-8. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay R., Coutts M., Krowczynska A., Brawerman G. Nuclease activity associated with mammalian mRNA in its native state: possible basis for selectivity in mRNA decay. Mol Cell Biol. 1990 May;10(5):2060–2069. doi: 10.1128/mcb.10.5.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos R. N., Aviv H. Theoretical analysis of a model for globin messenger RNA accumulation during erythropoiesis. J Mol Biol. 1977 Feb 25;110(2):205–218. doi: 10.1016/s0022-2836(77)80069-7. [DOI] [PubMed] [Google Scholar]
- Bastos R. N., Volloch Z., Aviv H. Messenger RNA population analysis during erythroid differentiation: a kinetical approach. J Mol Biol. 1977 Feb 25;110(2):191–203. doi: 10.1016/s0022-2836(77)80068-5. [DOI] [PubMed] [Google Scholar]
- Bennett V. D., Adams S. L. Characterization of the translational control mechanism preventing synthesis of alpha 2(I) collagen in chicken vertebral chondroblasts. J Biol Chem. 1987 Oct 25;262(30):14806–14814. [PubMed] [Google Scholar]
- Bernstein P., Peltz S. W., Ross J. The poly(A)-poly(A)-binding protein complex is a major determinant of mRNA stability in vitro. Mol Cell Biol. 1989 Feb;9(2):659–670. doi: 10.1128/mcb.9.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey J. L., Hentze M. W., Koeller D. M., Caughman S. W., Rouault T. A., Klausner R. D., Harford J. B. Iron-responsive elements: regulatory RNA sequences that control mRNA levels and translation. Science. 1988 May 13;240(4854):924–928. doi: 10.1126/science.2452485. [DOI] [PubMed] [Google Scholar]
- Clegg J. B., Weatherall D. J., Contopolou-Griva I., Caroutsos K., Poungouras P., Tsevrenis H. Haemoglobin Icaria, a new chain-termination mutant with causes alpha thalassaemia. Nature. 1974 Sep 20;251(5472):245–247. doi: 10.1038/251245a0. [DOI] [PubMed] [Google Scholar]
- Clegg J. B., Weatherall D. J., Milner P. F. Haemoglobin Constant Spring--a chain termination mutant? Nature. 1971 Dec 10;234(5328):337–340. doi: 10.1038/234337a0. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- De Jong W. W., Meera Khan P., Bernini L. F. Hemoglobin Koya Dora: high frequency of a chain termination mutant. Am J Hum Genet. 1975 Jan;27(1):81–90. [PMC free article] [PubMed] [Google Scholar]
- Flatz G., Kinderlerer J. L., Kilmartin J. V., Lehmann H. Haemoglobin Tak: a variant with additional residues at the end of the beta-chains. Lancet. 1971 Apr 10;1(7702):732–733. doi: 10.1016/s0140-6736(71)91994-5. [DOI] [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Hanash S. M., Winter W. P., Rucknagel D. L. Synthesis of haemoglobin Wayne in erythroid cells. Nature. 1977 Oct 20;269(5630):717–719. doi: 10.1038/269717a0. [DOI] [PubMed] [Google Scholar]
- Hargrove J. L., Schmidt F. H. The role of mRNA and protein stability in gene expression. FASEB J. 1989 Oct;3(12):2360–2370. doi: 10.1096/fasebj.3.12.2676679. [DOI] [PubMed] [Google Scholar]
- Herrick D. J., Ross J. The half-life of c-myc mRNA in growing and serum-stimulated cells: influence of the coding and 3' untranslated regions and role of ribosome translocation. Mol Cell Biol. 1994 Mar;14(3):2119–2128. doi: 10.1128/mcb.14.3.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huez G., Bruck C., Cleuter Y. Translational stability of native and deadenylylated rabbit globin mRNA injected into HeLa cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):908–911. doi: 10.1073/pnas.78.2.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huez G., Marbaix G., Hubert E., Cleuter Y., Leclercq M., Chantrenne H., Devos R., Soreq H., Nudel U., Littauer U. Z. Readenylation of polyadenylate-free globin messenger RNA restores its stability in vivo. Eur J Biochem. 1975 Nov 15;59(2):589–592. doi: 10.1111/j.1432-1033.1975.tb02486.x. [DOI] [PubMed] [Google Scholar]
- Hunt D. M., Higgs D. R., Winichagoon P., Clegg J. B., Weatherall D. J. Haemoglobin Constant Spring has an unstable alpha chain messenger RNA. Br J Haematol. 1982 Jul;51(3):405–413. doi: 10.1111/j.1365-2141.1982.tb02796.x. [DOI] [PubMed] [Google Scholar]
- Kan Y. W., Todd D., Dozy A. M. Haemoglobin Constant Spring synthesis in red cell precursors. Br J Haematol. 1974 Sep;28(1):103–107. doi: 10.1111/j.1365-2141.1974.tb06643.x. [DOI] [PubMed] [Google Scholar]
- Kazazian H. H., Jr The thalassemia syndromes: molecular basis and prenatal diagnosis in 1990. Semin Hematol. 1990 Jul;27(3):209–228. [PubMed] [Google Scholar]
- Klausner R. D., Rouault T. A., Harford J. B. Regulating the fate of mRNA: the control of cellular iron metabolism. Cell. 1993 Jan 15;72(1):19–28. doi: 10.1016/0092-8674(93)90046-s. [DOI] [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krowczynska A., Brawerman G. Structural features in the 3'-terminal region of polyribosome-bound rabbit globin messenger RNAs. J Biol Chem. 1986 Jan 5;261(1):397–402. [PubMed] [Google Scholar]
- Krowczynska A., Yenofsky R., Brawerman G. Regulation of messenger RNA stability in mouse erythroleukemia cells. J Mol Biol. 1985 Jan 20;181(2):231–239. doi: 10.1016/0022-2836(85)90087-7. [DOI] [PubMed] [Google Scholar]
- Langstaff J. M., Arnstein H. R. Messenger RNA turnover during bone marrow erythroid cell differentiation. Biochim Biophys Acta. 1985 Jul 24;825(3):316–325. doi: 10.1016/0167-4781(85)90019-3. [DOI] [PubMed] [Google Scholar]
- Liebhaber S. A., Cash F. E., Shakin S. H. Translationally associated helix-destabilizing activity in rabbit reticulocyte lysate. J Biol Chem. 1984 Dec 25;259(24):15597–15602. [PubMed] [Google Scholar]
- Liebhaber S. A., Kan Y. W. Differentiation of the mRNA transcripts originating from the alpha 1- and alpha 2-globin loci in normals and alpha-thalassemics. J Clin Invest. 1981 Aug;68(2):439–446. doi: 10.1172/JCI110273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Small B. Different lifetimes of reticulocyte messenger RNA. Cell. 1976 Jan;7(1):59–65. doi: 10.1016/0092-8674(76)90255-5. [DOI] [PubMed] [Google Scholar]
- Lumelsky N. L., Forget B. G. Negative regulation of globin gene expression during megakaryocytic differentiation of a human erythroleukemic cell line. Mol Cell Biol. 1991 Jul;11(7):3528–3536. doi: 10.1128/mcb.11.7.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquat L. E., Kinniburgh A. J. A beta zero-thalassemic beta-globin RNA that is labile in bone marrow cells is relatively stable in HeLa cells. Nucleic Acids Res. 1985 Apr 25;13(8):2855–2867. doi: 10.1093/nar/13.8.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod D. L., Shreeve M. M., Axelrad A. A. Improved plasma culture system for production of erythrocytic colonies in vitro: quantitative assay method for CFU-E. Blood. 1974 Oct;44(4):517–534. [PubMed] [Google Scholar]
- Meyuhas O., Perry R. P. Construction and identification of cDNA clones for mouse ribosomal proteins: application for the study of r-protein gene expression. Gene. 1980 Jul;10(2):113–129. doi: 10.1016/0378-1119(80)90129-8. [DOI] [PubMed] [Google Scholar]
- Milner P. F., Clegg J. B., Weatherall D. J. Haemoglobin-H disease due to a unique haemoglobin variant with an elongated alpha-chain. Lancet. 1971 Apr 10;1(7702):729–732. doi: 10.1016/s0140-6736(71)91992-1. [DOI] [PubMed] [Google Scholar]
- Müllner E. W., Kühn L. C. A stem-loop in the 3' untranslated region mediates iron-dependent regulation of transferrin receptor mRNA stability in the cytoplasm. Cell. 1988 Jun 3;53(5):815–825. doi: 10.1016/0092-8674(88)90098-0. [DOI] [PubMed] [Google Scholar]
- Nielsen D. A., Shapiro D. J. Insights into hormonal control of messenger RNA stability. Mol Endocrinol. 1990 Jul;4(7):953–957. doi: 10.1210/mend-4-7-953. [DOI] [PubMed] [Google Scholar]
- Nienhuis A. W., Benz E. J., Jr Regulation of hemoglobin synthesis during the development of the red cell (first of three parts). N Engl J Med. 1977 Dec 15;297(24):1318–1328. doi: 10.1056/NEJM197712152972404. [DOI] [PubMed] [Google Scholar]
- Osley M. A. The regulation of histone synthesis in the cell cycle. Annu Rev Biochem. 1991;60:827–861. doi: 10.1146/annurev.bi.60.070191.004143. [DOI] [PubMed] [Google Scholar]
- Owen D., Kühn L. C. Noncoding 3' sequences of the transferrin receptor gene are required for mRNA regulation by iron. EMBO J. 1987 May;6(5):1287–1293. doi: 10.1002/j.1460-2075.1987.tb02366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey N. B., Marzluff W. F. The stem-loop structure at the 3' end of histone mRNA is necessary and sufficient for regulation of histone mRNA stability. Mol Cell Biol. 1987 Dec;7(12):4557–4559. doi: 10.1128/mcb.7.12.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltz S. W., Jacobson A. mRNA stability: in trans-it. Curr Opin Cell Biol. 1992 Dec;4(6):979–983. doi: 10.1016/0955-0674(92)90129-z. [DOI] [PubMed] [Google Scholar]
- Ross J. Messenger RNA turnover in eukaryotic cells. Mol Biol Med. 1988 Feb;5(1):1–14. [PubMed] [Google Scholar]
- Ross J., Pizarro A. Human beta and delta globin messenger RNAs turn over at different rates. J Mol Biol. 1983 Jul 5;167(3):607–617. doi: 10.1016/s0022-2836(83)80101-6. [DOI] [PubMed] [Google Scholar]
- Ross J., Sullivan T. D. Half-lives of beta and gamma globin messenger RNAs and of protein synthetic capacity in cultured human reticulocytes. Blood. 1985 Nov;66(5):1149–1154. [PubMed] [Google Scholar]
- Sachs A. B. Messenger RNA degradation in eukaryotes. Cell. 1993 Aug 13;74(3):413–421. doi: 10.1016/0092-8674(93)80043-e. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakin S. H., Liebhaber S. A. Destabilization of messenger RNA/complementary DNA duplexes by the elongating 80 S ribosome. J Biol Chem. 1986 Dec 5;261(34):16018–16025. [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Treisman R. Transient accumulation of c-fos RNA following serum stimulation requires a conserved 5' element and c-fos 3' sequences. Cell. 1985 Oct;42(3):889–902. doi: 10.1016/0092-8674(85)90285-5. [DOI] [PubMed] [Google Scholar]
- Vakalopoulou E., Schaack J., Shenk T. A 32-kilodalton protein binds to AU-rich domains in the 3' untranslated regions of rapidly degraded mRNAs. Mol Cell Biol. 1991 Jun;11(6):3355–3364. doi: 10.1128/mcb.11.6.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volloch V., Housman D. Stability of globin mRNA in terminally differentiating murine erythroleukemia cells. Cell. 1981 Feb;23(2):509–514. doi: 10.1016/0092-8674(81)90146-x. [DOI] [PubMed] [Google Scholar]
- Weiss I., Cash F. E., Coleman M. B., Pressley A., Adams J. G., Sanguansermsri T., Liebhaber S. A., Steinberg M. H. Molecular basis for alpha-thalassemia associated with the structural mutant hemoglobin Suan-Dok (alpha 2 109leu----arg) Blood. 1990 Dec 15;76(12):2630–2636. [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Hudson S., Tournay O., Bittenbender S., Shane S. S., Lange B., Tsujimoto Y., Caton A. J., Rovera G. Detection of minimal disease in hematopoietic malignancies of the B-cell lineage by using third-complementarity-determining region (CDR-III)-specific probes. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5123–5127. doi: 10.1073/pnas.86.13.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen T. J., Machlin P. S., Cleveland D. W. Autoregulated instability of beta-tubulin mRNAs by recognition of the nascent amino terminus of beta-tubulin. Nature. 1988 Aug 18;334(6183):580–585. doi: 10.1038/334580a0. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis using M13-derived vectors: an efficient and general procedure for the production of point mutations in any fragment of DNA. Nucleic Acids Res. 1982 Oct 25;10(20):6487–6500. doi: 10.1093/nar/10.20.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]