Abstract
Lamin A/C gene-related cardiomyopathy is associated with progressive heart failure and malignant arrhythmias. Current guidelines advise the use of implantable defibrillators to prevent arrhythmogenic sudden cardiac death only in situations where there is evidence of severe left ventricular dysfunction. We describe a case of a woman with genetically confirmed Lamin C deficiency with preserved left ventricular function in whom an implantable defibrillator was inserted and within a month of implantation was used to terminate symptomatic ventricular tachycardia.
Keywords: Lamin A and C deficiency, Dilated Cardiomyopathy, Sudden Cardiac Death
Implications for Practice
Mutations in the Lamin A/C gene can affect the heart, skeletal muscles, adipose tissues, bones and nervous tissues with most cases resulting in dilated cardiomyopathy, conduction defects, dysrrhythmias and premature death.
Malignant ventricular arrhythmias may occur independently of left ventricular dysfunction and can predate the diagnosis of dilated cardiomyopathy.
The early implantation of cardiac defibrillators where there are resting ECG abnormalities may reduce mortality due to sudden cardiac death.
Background
Lamin A/C (LMNA) gene mutations are rare but highly penetrant diseases that result in a variety of clinical phenotypes, the majority of which have major cardiac involvement. These include high rates of dilated cardiomyopathy (DCM) and life-threatening arrhythmias with as many as 46% of patients dying suddenly.1 Current guidelines for the use of implantable cardiac defibrillators (IDC) as primary prevention recognise this increased risk but only in a cohort of patients with established ventricular systolic dysfunction (ejection fraction <35%).2,3
We report a case of a woman with documented Lamin C gene mutation but with preserved systolic function.
Case details
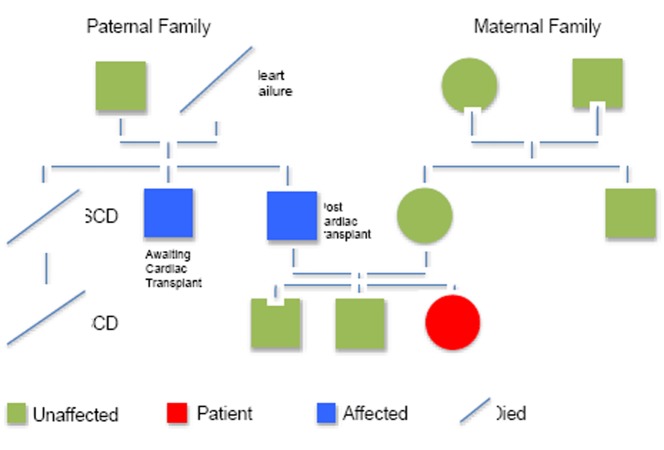
A 32-year-old female was referred for pacemaker insertion after a two-year history of recurrent palpitations thought to be secondary to atrial fibrillation. She had previously been fit and well. There was no history of syncope. There was an extensive cardiac family history (Figure 1). Her father undergone cardiac transplantation for DCM and had proven Lamin C deficiency. A paternal uncle and his son died suddenly at the age of 45 and 38 respectively. A further paternal uncle has been diagnosed with DCM and is awaiting cardiac transplantation. Her paternal grandmother had also died aged 55 years with decompensated heart failure.
Figure 1. Family pedigree.
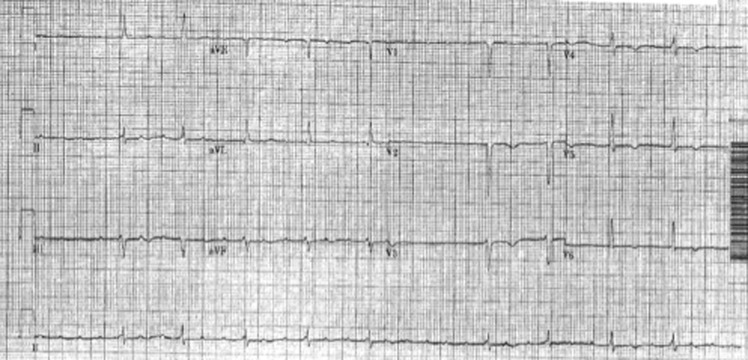
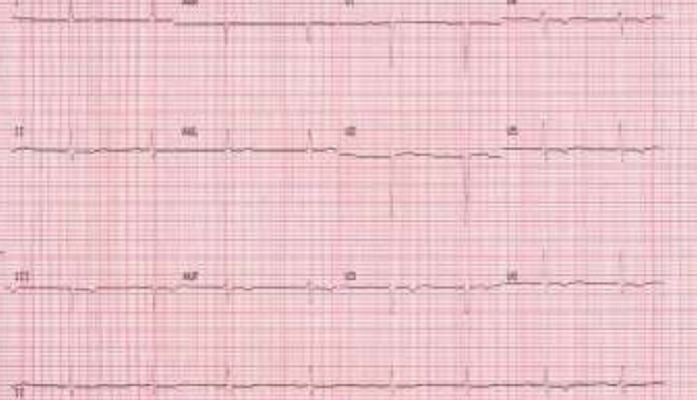
During palpitations she complained of an irregular heart beat, malaise, shortness of breath and fatigue. An ECG revealed an atrial tachycardia with variable atrioventricular block (Figure 2a). During her initial presentation, treatment with 25mg of oral metoprolol resulted in profound bradycardia with heart rates as low as 20 beat per minute. Spontaneous conversion to sinus rhythm occurred the day after admission. The ECG showed PR prolongation at 240ms as well as abnormal left axis deviation. There were also T wave repolarisation abnormalities in the praecordial leads (Figure 2b). The QT interval was normal (QTc of 410ms).
Figure 2a. ECG documenting Atrial Tachycardia with variable ventricular rate.
Figure 2b. ECG in Sinus rhythm with PR prolongation (240ms), left axis deviation and praecordial T wave inversion.
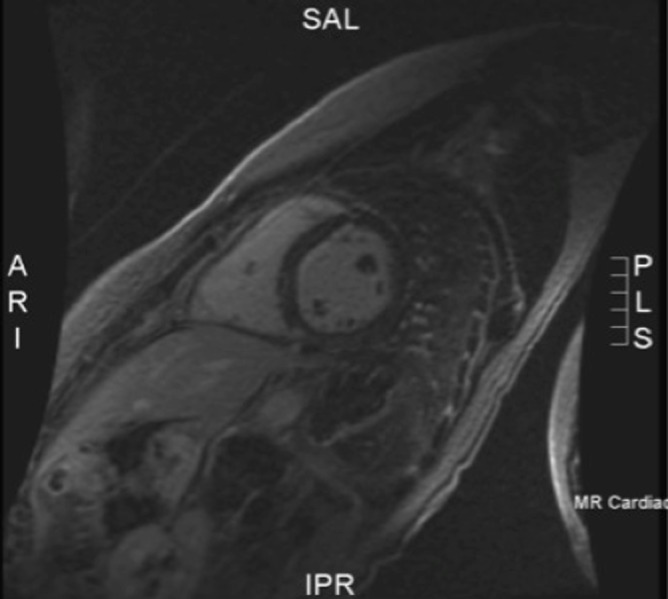
Trans-thoracic echocardiography and signal-averaged ECG were normal. A treadmill exercise stress test was also normal with normal QT interval shortening. Holter monitoring did not document ventricular arrhythmias. Cardiac MR (1.5 Tesla Siemens machine) revealed mild septal mid wall enhancement on post gadolinium delayed imaging but with preserved left ventricular size and systolic function (Figure 3). The ejection fraction was 51%. There were no radiographic features of arrhythmogenic right ventricular dysplasia (ARVD). Blood genetic testing confirmed Lamin C deficiency with a mutation in genotype C (1711 C>A).
Figure 3. Cardiac MR short axis view 20 minutes post gadolinium showing mild septal mid-wall enhancement mild.
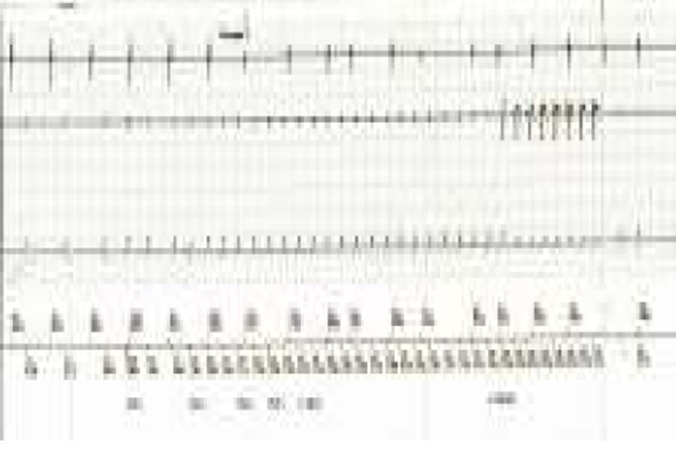
After detailed consultation with the patient and family, it was decided to proceed with an implantation of a dual chamber cardiac defibrillator. One month after the procedure, she had a pre-syncopal episode with collapse but no frank syncope. Interrogation of her device revealed one episode of ventricular tachycardia with a cycle length of 240ms successfully terminated with anti-tachycardia pacing during charging (Figure 4). Over the following six-month period, she had 37% atrial and 41% ventricular pacing for bradycardia. There were also 71 episodes of non-sustained atrial tachycardia.
Figure 4. EGM post ICD interrogation documenting sudden onset ventricular tachycardia with average cycle length of 240ms. Successful anti-tachycardia pacing shown at the end of strip.
Discussion
Lamin A/C gene-related DCM is characterised by progressive heart failure, conduction disease, absent or variable skeletal muscle involvement and a high incidence of sudden cardiac death. The mutation is thought to be responsible for 6%–8% of DCM.4,5 Becane et al1 first reported the high mortality and morbidity associated with the LMNA gene mutation in a large French kindred, with as many as 50% dying suddenly. Left ventricular dysfunction rapidly progressed to heart failure and cardiac transplantation. Furthermore, LMNA mutation carriers also had a poorer prognosis than noncarrier DCM with an event free survival at the age of 45 years of 31% versus 75% for non-carriers.
Pasotti et al6 published the longest follow up of patients with the LMNA mutation and have found that the disease process is highly penetrant and by the age of 60 all patients would have developed the clinical phenotype with a diagnosis of DCM being rare below the age of 20 years. In line with previous publications, they found that the incidence of adverse cardiac events were high, and within the median five-year follow-up period, as many as twothirds of the affected patients had a life-threatening arrhythmia. Meune et al7 also showed that during a threeyear follow-up period, 42% of patients with the LMNA mutations and implantable cardiac defibrillators had received an appropriate shock for life-threatening ventricular arrhythmia. None of these patients had a depressed ejection fraction, demonstrating that the risk of sudden cardiac death was high before myocardial dysfunction was demonstrated.
Current international guidelines on implantable defibrillators and primary prevention suggest the use of ICD only when left ventricular dysfunction has been established with little consideration to the possible influence of the underlying molecular aetiology.2,3 Published literature suggests that patients with the LMNA mutation are at high risk of malignant arrhythmias irrespective of myocardial function8. In this present case, the resting ECG was highly abnormal showing both AV nodal disease and widespread Twave abnormalities suggesting a pathological electrophysiological process before demonstrable onset of myocardial systolic impairment. Although the occurrence of ventricular tachycardia a month post implant could be viewed as a fortunate co-incidence, there is growing evidence of the potential pro-arrhythmic effects of ICDs.9, 10 With more widespread availability of genetic testing, Lamin A and C deficiencies may become an increasingly recognized cause of previously undiagnosed or idiopathic cardiomyopathy.
This case study highlights that in certain clinical scenarios, consideration should be given to the early implantation of ICDs even though left ventricular function may still be preserved contrary to current recommended guidelines.
Footnotes
PEER REVIEW
Not commissioned. Externally peer reviewed.
CONFLICTS OF INTEREST
The authors declare that they have no competing interests.
PATIENT CONSENT
The authors, Ng KK and Kaye G, declare that:
- They have obtained written, informed consent for the publication of the details relating to the patient(s) in this report.
- All possible steps have been taken to safeguard the identity of the patient(s).
- This submission is compliant with the requirements of local research ethics committees.
Please cite this paper as: Ng KK, Kaye G. A case of Lamin C gene-mutation with preserved systolic function and ventricular dysrrhythmia. AMJ 2013, 6, 2, 75-78. http//dx.doi.org/10.4066/AMJ.2013.1546
References
- 1.Bécane HM, Bonne G, Varnous S, Muchir A, Ortega V, Hammouda EH, Urtizberea JA, Lavergne T, Fardeau M, Eymard B, Weber S, Schwartz K, Duboc D. High incidence of sudden death with conduction system and myocardial disease due to lamins A and C gene mutation. Pacing Clin Electrophysiol. 2000 Nov;23(11 Pt 1):1661–6. doi: 10.1046/j.1460-9592.2000.01661.x. [DOI] [PubMed] [Google Scholar]
- 2.Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Smith SC Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B, Blanc JJ, Budaj A, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL. American College of Cardiology/American Heart Association Task Force; European Society of Cardiology Committee for Practice Guidelines; European Heart Rhythm Association; Heart Rhythm Society. ACC/AHA/ESC 2006 guidelines on the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation. 2006 Sep 5;114(10):e385–484. doi: 10.1161/CIRCULATIONAHA.106.178233. [DOI] [PubMed] [Google Scholar]
- 3.van Rijsingen IA, Arbustini E, Elliott PM, Mogensen J, Hermans-van Ast JF, van der Kooi AJ, van Tintelen JP, van den Berg MP, Pilotto A, Pasotti M, Jenkins S, Rowland C, Aslam U, Wilde AA, Perrot A, Pankuweit S, Zwinderman AH, Charron P, Pinto YM. Risk factors for malignant ventricular arrhythmias on Lamin A/C Mutation Carriers. J Am Coll Cardiol. 2012 Jan 31;59(5):493–500. doi: 10.1016/j.jacc.2011.08.078. [DOI] [PubMed] [Google Scholar]
- 4.Taylor MR, Fain PR, Sinagra G, Robinson ML, Robertson AD, Carniel E, Di Lenarda A, Bohlmeyer TJ, Ferguson DA, Brodsky GL, Boucek MM, Lascor J, Moss AC, Li WL, Stetler GL, Muntoni F, Bristow MR, Mestroni L. Familial Dilated Cardiomyopathy Registry Research Group. Natural history of dilated cardiomyopathy due to lamin A/C gene mutations. J Am Coll Cardiol. 2003 Mar 5;41(5):771–80. doi: 10.1016/s0735-1097(02)02954-6. [DOI] [PubMed] [Google Scholar]
- 5.Parks SB, Kushner JD, Nauman D, Burgess D, Ludwigsen S, Peterson A, Li D, Jakobs P, Litt M, Porter CB, Rahko PS, Hershberger RE. Lamin A/C mutation analysis in a cohort of 324 unrelated patients with idiopathic or familial dilated cardiomyopathy. Am Heart J. 2008 Jul;156(1):161–9. doi: 10.1016/j.ahj.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pasotti M, Klersy C, Pilotto A, Marziliano N, Rapezzi C, Serio A, Mannarino S, Gambarin F, Favalli V, Grasso M, Agozzino M, Campana C, Gavazzi A, Febo O, Marini M, Landolina M, Mortara A, Piccolo G, Viganò M, Tavazzi L, Arbustini E. Long-term outcome and risk stratification in dilated cardiolaminopathies. J Am Coll Cardiol. 2008 Oct 7;52(15):1250–60. doi: 10.1016/j.jacc.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 7.Meune C, Van Berlo JH, Anselme F, Bonne G, Pinto YM, Duboc D. Primary prevention of sudden death in patients with lamin A/C gene mutations. N Engl J Med. 2006 Jan 12;354(2):209–10. doi: 10.1056/NEJMc052632. [DOI] [PubMed] [Google Scholar]
- 8.van Berlo JH, de Voogt WG, van der Kooi AJ, van Tintelen JP, Bonne G, Yaou RB, Duboc D, Rossenbacker T, Heidbüchel H, de Visser M, Crijns HJ, Pinto YM. Meta-analysis of clinical characteristics of 299 carriers of LMNA gene mutations: do Lamin A/C mutations portend a high risk of sudden death. J Mol Med (Berl). 2005 Jan;83(1):79–83. doi: 10.1007/s00109-004-0589-1. [DOI] [PubMed] [Google Scholar]
- 9.Tung R, Zimetbaum P, Josephson M. A critical appraisal of implantable cardioverter-defibrillator therapy for the prevention of sudden cardiac death. J Am Coll Cardiol. 2008 Sep 30 14;52:1111–21. doi: 10.1016/j.jacc.2008.05.058. [DOI] [PubMed] [Google Scholar]
- 10.Pinski SL, Fahy GJ. The proarrhythmic potential of implantable cardioverter-defibrillators. Circulation. 1995 Sep 15;92(6):1651–64. doi: 10.1161/01.cir.92.6.1651. [DOI] [PubMed] [Google Scholar]