Abstract
The phytohormones cytokinin and auxin are essential for the control of diverse aspects of cell proliferation and differentiation processes in plants. Although both phytohormones have been suggested to play key roles in the regulation of root nodule development, only recently, significant progress has been made in the elucidation of the molecular genetic basis of cytokinin action in the model leguminous species, Lotus japonicus and Medicago truncatula. Identification and functional analyses of the putative cytokinin receptors LOTUS HISTIDINE KINASE 1 and M. truncatula CYTOKININ RESPONSE 1 have brought a greater understanding of how activation of cytokinin signaling is crucial to the initiation of nodule primordia. Recent studies have also started to shed light on the roles of auxin in the regulation of nodule development. Here, we review the history and recent progress of research into the roles of cytokinin and auxin, and their possible interactions, in nodule development.
Keywords: auxin, cytokinin, legume, nodulation, root nodule symbiosis
INTRODUCTION
Legumes (Fabaceae) are well-known for their ability to form nodules on their roots through symbiotic interaction with soil bacteria (rhizobia), a relationship termed “root nodule symbiosis.” Within the nodules, the rhizobia fix gaseous nitrogen and make it available to the host plants as a nitrogen source; in turn, the plants provide a carbon source for the rhizobia. Nodule development is a form of cellular reprogramming in which host receptors in the root epidermis respond to rhizobia-derived nodulation (Nod) factors by ultimately inducing the dedifferentiation of some root cortical cells (Szczyglowski et al., 1998; Oldroyd et al., 2011). These activated cortical cells subsequently proliferate to form nodule primordia. Nodule organogenesis proceeds further following the invasion of nodule primordia by rhizobia via specialized structures called infection threads (Murray, 2011). Thus, the analysis of nodulation is not only of interest to researchers studying plant–microbe interactions, but also may contribute to our understanding of mechanisms underlying de novo organogenesis in plants.
Elucidation of the roles and functions of phytohormones is crucial to understanding plant development (Durbak et al., 2012). Two of these phytohormones, cytokinin and auxin, are well-known as key players in the regulation of cell proliferation and differentiation processes. In Arabidopsis thaliana, the roles of these phytohormones and their crosstalk during lateral root (LR) development have been broadly characterized (Benková and Bielach, 2010; Bishopp et al., 2011). Auxin is involved in the positive regulation of LR development: establishment of a local auxin response at LR founder cells results from polar auxin transport and maintenance of the local auxin maximum at the root apex (Benková et al., 2003; Marhavý et al., 2013). In contrast, cytokinin acts as a negative regulator of LR initiation through promoting the expression of auxin signaling inhibitors (Laplaze et al., 2007; Bielach et al., 2012).
Most of the early studies on the hormonal control of nodulation adopted a physiological approach using a variety of leguminous and rhizobial species. More recently, the advances in genetic techniques have led to a greater focus on model legumes such as Lotus japonicus and Medicago truncatula. In this review, we summarize past and recent studies, mainly from the latter species, on the actions of cytokinin and auxin in the control of nodule development.
ROLE OF CYTOKININ DURING NODULE DEVELOPMENT
Forty years ago, Libbenga et al. (1973) reported that exogenous application of cytokinin and auxin to pea root cortical explants induced cell proliferation at positions where nodules were expected to initiate. Other early studies found that some rhizobial species could secrete cytokinin-like compounds affecting plant development in soybean (Phillips and Torrey, 1972; Sturtevant and Taller, 1989). Later, Cooper and Long (1994) reported the important observation that the nodulation-deficient phenotype of a Rhizobium mutant could be partially suppressed by the introduction of a gene involved in trans-zeatin secretion. In their experiment, they found that nodules formed by alfalfa roots were devoid of bacteria, suggesting that while cytokinin has the ability to form nodules, bacterial infection is not affected by cytokinin. Thus, cytokinin may specifically function in nodule organogenesis and not in the rhizobial infection process. After the identification of the Nod factor as a bona fide regulator of nodulation (Spaink et al., 1991; Truchet et al., 1991), various studies investigated the similarities among cytokinin, rhizobial-inoculation, and Nod factor with respect to their effects on nodulation. With respect to the expression patterns of some early nodulin genes, the identities of the proliferating cortical cells induced by cytokinin appear identical to those induced by rhizobial-inoculation or Nod factor treatment in alfalfa and white clover (Bauer et al., 1996; Fang and Hirsch, 1998; Mathesius et al., 2000). Expression of EARLY NODULIN 40 (ENOD40), the first gene reported to have the ability to induce cortical cell division in M. truncatula (Charon et al., 1997), is also activated by cytokinin. These early studies carried out in various legume species reported that nodulation did not progress any further following the stimulation of cortical cell proliferation by cytokinin treatment. Under particular experimental conditions, however, it is possible to stimulate formation of bulges with the appearance of nodule-like primordia by application of cytokinin to roots of L. japonicus (Heckmann et al., 2011). Interestingly, the frequency of formation of these structures varies among Lotus species, suggesting that there may be an inter-species difference in cytokinin responses.
IDENTIFICATION OF KEY COMPONENTS OF NODULATION-RELATED CYTOKININ SIGNALING
In L. japonicus, mutation at any of three spontaneous nodule formation loci (snf1, snf2, or snf4) can cause the formation of nodule-like structures (spontaneous nodules) in the absence of rhizobia (Tirichine et al., 2006b). The histological, physiological, and molecular features of spontaneous nodules resemble those of rhizobia-induced nodules; the major difference is the presence of infection threads and infected cells in the latter. The observation of spontaneous nodules has also been reported in some ecotypes of alfalfa, although the cause remains unknown (Truchet et al., 1989).
The genetic study of cytokinin function during nodule development has been facilitated by use of a mutation at the snf2 locus that is associated with spontaneous nodule development. This dominant snf2 mutant has a gain-of-function mutation of LOTUS HISTIDINE KINASE 1 (LHK1), which encodes a protein closely related to the Arabidopsis cytokinin receptor, CYTOKININ RESPONSE 1 (CRE1)/ARABIDOPSIS HISTIDINE KINASE 4 (Inoue et al., 2001; Tirichine et al., 2007). The mutant histidine kinase receptor can activate an Escherichia coli two-component phosphorelay system without exogenous cytokinin treatment; this observation suggests that cytokinin-induced signaling is constitutively activated in the snf2 mutant. L. japonicus plants carrying a loss-of-function mutation of LHK1 and M. truncatula plants with mutation of CRE1 (MtCRE1), the functional homolog of LHK1, are insensitive to cytokinin and show a nodulation-deficient phenotype (Gonzalez-Rizzo et al., 2006; Murray et al., 2007; Plet et al., 2011). These observations strongly indicate that activation of cytokinin signaling is essential for nodule development.
In a downstream part of the cytokinin receptor pathway, a series of two-component phosphorelay systems activate B-type response regulators (RRs), which have a DNA-binding domain and can directly regulate a number of cytokinin primary response genes. Among the cytokinin primary response genes, A-type RRs are believed to act as negative regulators of cytokinin signaling (Heyl and Schmülling, 2003). In M. truncatula, expression of MtRR1 (B-type) and MtRR4 (A-type) is induced by inoculation with rhizobia (Gonzalez-Rizzo et al., 2006). MtCRE1 and MtRR4 are expressed at proliferating cortical cells during nodule development, and the upregulation of MtRR4 expression is dependent on MtCRE1 (Lohar et al., 2006; Plet et al., 2011), suggesting that MtRR4 is involved in nodule development in a downstream part of the MtCRE1 signaling pathway. At present, no loss-of-function mutants of nodulation-related RRs have been identified. However, in M. truncatula, analyses of the loss- and gain-of-function effects of ETHYLENE RESPONSE FACTOR REQUIRED FOR NODULE DIFFERENTIATION (EFD) showed that it negatively regulates nodulation, potentially through the activation of MtRR4 (Vernié et al., 2008). This is consistent with the suggestion that MtRR4 acts as a negative regulator of nodule development. In addition, the expression of other A-type RRs can be induced by Nod factor treatment in M. truncatula (Op den Camp et al., 2011). Surprisingly, under the experimental conditions used, MtRR4 was not activated by the Nod factor, suggesting that there might be different downstream responses between rhizobial-inoculation and Nod factor treatment. Furthermore, constitutive activation of MtRR9, a newly identified A-type RR, induces cortical cell proliferation, implying that MtRR9 may have a positive role in the formation of nodules (Op den Camp et al., 2011). MtRR9 function in cytokinin signaling should be, however, clarified by investigation of the effects of loss- and gain-of-function mutations on cytokinin sensitivity.
Ariel et al. (2012) recently reported that MtRR1 could bind to the MtRR4 promoter, suggesting that MtRR1 directly controls the expression of MtRR4. Interestingly, electrophoretic mobility shift and chromatin immunoprecipitation assays identified NODULATION SIGNALING PATHWAY 2 (NSP2) as a direct target of MtRR1. NSP2 encodes a GRAS-type transcription factor that is required for the positive regulation of nodule development (Kaló et al., 2005; Heckmann et al., 2006; Murakami et al., 2006; Ariel et al., 2012). Mutation of the putative MtRR1-binding sites of the NSP2 promoter abolished nodulation-related activation of NSP2, suggesting that these cis-elements are essential for NSP2 expression. The regulatory mechanism for NSP2 expression is currently a vibrant area of research in plant–microbe interactions; recent evidence indicates that expression of NSP2 is negatively regulated by microRNA 171 (miR171; De Luis et al., 2012; Lauressergues et al., 2012). Expression of miR171 is induced not only during nodule development but also by cytokinin in an MtCRE1-dependent manner, and the expression pattern is negatively correlated with that of NSP2 (Ariel et al., 2012). Thus, cytokinin signaling may have a dual mode for regulating NSP2 expression: it can directly activate NSP2 transiently and then repress its expression through activation of miR171 expression (Figure 1). Ariel et al. (2012) found that MtRR1 additionally appears to directly regulate a basic helix-loop-helix transcription factor (bHLH476), and that insertion of a Tnt1 retrotransposon into bHLH476 led to reduced nodulation. This observation suggests that bHLH476 positively regulates nodulation. Another candidate MtRR1 target is M. truncatula CYTOKININ OXIDASE 1 (MtCKX1), which is involved in negative regulation of cytokinin signaling (Ariel et al., 2012). MtRR1 binds directly to the MtCKX1 promoter in an MtCRE1-dependent manner. CKX genes have a negative effect onnodule development and their overexpression causes a reduction in the number of nodules (Lohar et al., 2004). Overall, these findings indicate that cytokinin signaling not only positively regulates nodule development but also may control itself through a negative feedback mechanism that may involve CKX1 (Figure 1).
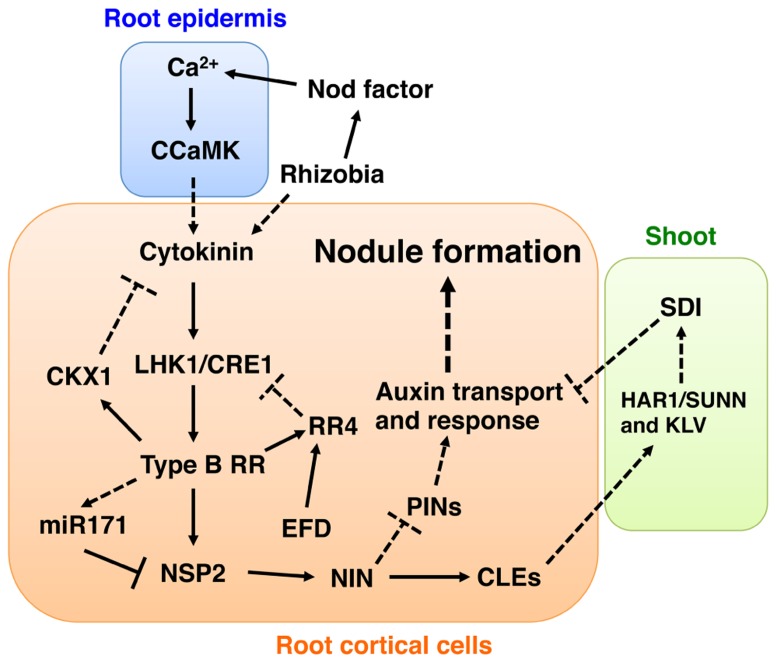
FIGURE 1.
Model depicting auxin- and cytokinin-mediated signaling pathways involved in nodule development. To develop this model, we combined data from studies using L. japonicus and M. truncatula. Based on the observation that NSP2 is required for the induction of NIN (Murakami et al., 2006), we placed NIN downstream of NSP2. Since control of PIN proteins (PINs) localization seems to occur in the downstream part of the cytokinin signaling pathway (Plet et al., 2011) and NIN has an ability to induce localized auxin responses (Suzaki et al., 2012), we suggest that the site of PINs regulation is downstream of NIN. A putative shoot-derived inhibitor (SDI) is proposed as a negative regulatory signal of nodule development mediated by the AON mechanism involving HAR1/SUNN, KLV, and nodulation-related CLE peptides (CLEs) (Ferguson et al., 2010; Kouchi et al., 2010). Here, we suggest that SDI might inhibit cytokinin and auxin actions. Proven and putative regulation points are indicated by intact lines and dotted lines, respectively. See text for more detailed explanations of the model.
Double mutant analyses using snf2 and nodulation-deficient mutants indicate that NODULE INCEPTION (NIN) is also involved in the positive regulation of nodule development in a downstream part of the LHK1-dependent cytokinin signaling pathway. The nin mutation suppresses snf2-dependent spontaneous nodule formation (Tirichine et al., 2007). Expression of NIN is strongly activated during nodulation and is induced by cytokinin in an LHK1/MtCRE1-dependent manner (Schauser et al., 1999; Murray et al., 2007; Heckmann et al., 2011; Plet et al., 2011). In addition, NIN has the ability to induce cortical cell proliferation in L. japonicus; constitutive activation of the geneinduces cortical cell proliferation in the absence of rhizobia (Suzaki et al., 2012; Soyano et al., 2013). Details of the mechanism of the potential interaction between cytokinin signaling and NIN activation await clarification.
RELATIONSHIP BETWEEN CYTOKININ SIGNALING AND AUTOREGULATION OF NODULATION
It has been demonstrated that legumes have a negative regulatory mechanism termed autoregulation of nodulation (AON) that moderates the number of nodules (Caetano-Anollés and Gresshoff, 1991; Oka-Kira and Kawaguchi, 2006; Ferguson et al., 2010; Kouchi et al., 2010). In L. japonicus and M. truncatula, a key component of AON is long-distance communication between the root and shoot that is mediated through the receptor-like kinases HYPERNODULATION ABERRANT ROOT FORMATION 1 (HAR1)/SUPER NUMERIC NODULES (SUNN) and KLAVIER (KLV) in the shoot and the potential root-derived signals L. japonicus CLE-ROOT SIGNAL 1/2 (LjCLE-RS1/2) or MtCLE12/13 (Krusell et al., 2002; Nishimura et al., 2002; Schnabel et al., 2005; Okamoto et al., 2009; Miyazawa et al., 2010; Mortier et al., 2010). Mutation of HAR1 or KLV causes a hypernodulation phenotype in L. japonicus; moreover, these mutations have an additive effect on snf2-dependent spontaneous nodule formation (Wopereis et al., 2000; Tirichine et al., 2007; Miyazawa et al., 2010), suggesting that AON acts in parallel to the cytokinin signaling pathway that includes LHK1. The expression of nodulation-related CLE genes is induced upon rhizobial-inoculation (Okamoto et al., 2009; Mortier et al., 2010), and it has recently been shown that such activation is abolished in the presence of cre1 and nin mutations in M. truncatula (Mortier et al., 2012). Thus, the CLE peptides may be produced in the downstream part of the cytokinin signaling pathway that involves NIN. Several studies have demonstrated that nodulation is strongly suppressed when the CLE genes are constitutively activated (Okamoto et al., 2009; Mortier et al., 2010, 2012). In order to further understand the potential feedback regulation between cytokinin signaling and AON, it will be necessary to determine the effects of CLE expression on snf2-dependent spontaneous nodulation.
RELATIONSHIP BETWEEN AUXIN AND GENETIC PATHWAYS THAT CONTROL NODULE DEVELOPMENT
Allen et al. (1953) were the first to show that exogenous application of polar auxin transport inhibitors to alfalfa roots induced formation of nodule-like structures in the absence of rhizobia. Subsequent investigations on the expression of early nodulin genes and of their expression profiles during pseudonodules development suggest that they are similar to rhizobia-induced nodules in the genus Medicago (Hirsch et al., 1989; Hirsch and Fang, 1994; Rightmyer and Long, 2011). An auxin reporter analysis using the GH3 promoter showed that the Nod factor is able to perturb auxin flow in white clover (Mathesius et al., 1998). Furthermore, deficiency in flavonoids, which act to inhibit auxin transport, causes a reduction in nodule number in M. truncatula (Wasson et al., 2006). Overall, these observations suggest that alteration of the auxin flow affects nodule development, thereby implicating auxin in this process.
Recently, the highly active synthetic auxin-responsive element DR5 has been used in combination with a nuclear-localized green fluorescent protein (GFP) as a reporter to examine auxin response patterns during L. japonicus nodule development (Suzaki et al., 2012). The analysis revealed that auxin responses during nodule development exclusively occur in proliferating cortical cells, as also reported by previous studies using the GH3 promoter(Pacios-Bras et al., 2003; Takanashi et al., 2011). An auxin response was also observed in cyclops mutants, in which infection threads fail to reach cortical cells (Yano et al., 2008). Thus, formation of infection threads may not be required for initiation of the auxin response. During actinorhizal nodule formation in Casuarina glauca, the localized accumulation of auxin is mediated by AUX1-like carriers and is correlated with the cellular infection by bacteria (Péret et al., 2007; Perrine-Walker et al., 2010). Localized auxin responses are induced during snf2-dependent spontaneous nodule formation, suggesting that cytokinin signaling has a role in the production of these responses (Suzaki et al., 2012). A localized auxin response is also observed during spontaneous nodule development mediated by a gain-of-function mutation of the Ca2+/calmodulin-dependent protein kinase (CCaMK; Suzaki et al., 2013), which is responsible for decoding Ca2+ signals during nodulation (Gleason et al., 2006; Tirichine et al., 2006a; Hayashi et al., 2010; Madsen et al., 2010; Shimoda et al., 2012). Since accumulation of MtPIN proteins, coding putative auxin efflux carriers, appears to be negatively regulated by MtCRE1-dependent cytokinin signaling(Plet et al., 2011), the regulation of the polar localization of some PIN proteins may be required for the establishment of localized auxin responses. In A. thaliana, cytokinin inhibits the initiation of LR development by blocking the expression of PIN genes in LR founder cells (Laplaze et al., 2007). Thus, the negative regulation of PIN auxin carriers by cytokinin might be conserved in nodule and LR development. In legumes, cytokinin promotes nodule development (as described above) but also inhibits LR formation (Lohar et al., 2004; Gonzalez-Rizzo et al., 2006). During formation of spontaneous structures induced by constitutive activation of NIN, localized auxin responses are also induced in L. japonicus (Suzaki et al., 2012). Thus, it is highly likely that localized auxin responses occur not only downstream of CCaMK and LHK1 but also of NIN (Figure 1). This interpretation is consistent with the observation that the nin mutation has no effect on pseudonodule formation induced by auxin transport inhibitors (Rightmyer and Long, 2011).
Recently, a DR5 reporter analysis in har1 mutants of L. japonicus indicated that HAR1 may negatively regulate auxin responses during nodule development (Suzaki et al., 2012). Abnormal auxin transport may underlie the higher auxin response in har1 mutants as sunn mutants have an increased auxin transport from the shoot to the root (van Noorden et al., 2006). In addition, in nodulation-deficient roots resulting from the constitutive activation of LjCLE genes, perturbation of cortical cell proliferation is accompanied by the disappearance of auxin responses (Suzaki et al., 2012). Thus, it is possible that AON may negatively regulate nodule development through controlling auxin responses (Figure 1).
FUTURE PERSPECTIVES
As we show in this mini-review, significant progress has been made recently in our understanding of how and when cytokinin and auxin act in the various genetic pathways that control nodule development. Although auxin has a longer history than cytokinin with respect to research into root nodule symbiosis, there is comparatively little known of its role in nodule development due to a dearth of auxin-related mutants involved in nodulation. In M. truncatula, however, the characterization of the Mtpin1 (smooth leaf margin 1) nodulation-phenotype may help remedy this situation (Zhou et al., 2011). Additionally, characterization of mutants created by retrotransposon mutagenesis (LORE1 in L. japonicus and Tnt1 in M. truncatula; Fukai et al., 2012; Pislariu et al., 2012; Urbański et al., 2012) should accelerate genetic studies of nodule development. In the current model of nodule development, it is proposed that auxin accumulates in the incipient nodule primordia under the control of auxin transport (Figure 1). However, we cannot rule out the possibility of de novo auxin production as expression of a putative auxin biosynthesis gene is activated during nodule development in L. japonicus (Suzaki et al., 2012). With regard to cytokinin, a recent study has shown that activation of some genes involved in cytokinin biosynthesis, degradation, and conjugation is correlated with nodule development in M. truncatula (Moreau et al., 2011). In addition to studies of plant phytohormones, it is possible that investigation of auxin- and cytokinin-like compounds derived from the rhizobia may provide new insights into nodule development. Some species of rhizobia do not possess genes to synthesize Nod factors but instead might use cytokinin-like compounds to establish root nodule symbiosis (Giraud et al., 2007). In order to elucidate how cytokinin and auxin are provided during nodule development, it will be necessary to investigate the functions of host and rhizobial genes involved in the production of these phytohormones.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (23012038 to Takuya Suzaki; 22128006 to Masayoshi Kawaguchi).
REFERENCES
- Allen E. K., Allen O. N., Newman A. S. (1953). Pseudonodulation of leguminous plants induced by 2-bromo-3,5-dichlorobenzoic acid. Am. J. Bot. 40 429–435 [Google Scholar]
- Ariel F., Brault-Hernandez M., Laffont C., Huault E., Brault M., Plet J., et al. (2012). Two direct targets of cytokinin signaling regulate symbiotic nodulation in Medicago truncatula. Plant Cell 24 3838–3852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer P., Ratet P., Crespi M., Schultze M., Kondorosi A. (1996). Nod factors and cytokinins induce similar cortical cell division, amyloplast deposition and MsEnod12A expression patterns in alfalfa roots. Plant J. 10 91–105 [Google Scholar]
- Benková E., Bielach A. (2010). Lateral root organogenesis – from cell to organ. Curr. Opin. Plant Biol. 13 677–683 [DOI] [PubMed] [Google Scholar]
- Benková E., Michniewicz M., Sauer M., Teichmann T., Seifertova D., Jurgens G., et al. (2003). Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115 591–602 [DOI] [PubMed] [Google Scholar]
- Bielach A., Podlesáková K., Marhavý P., Duclercq J., Cuesta C., Müller B., et al. (2012). Spatiotemporal regulation of lateral root organogenesis in Arabidopsis by cytokinin. Plant Cell 24 3967–3981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishopp A., Benková E., Helariutta Y. (2011). Sending mixed messages: auxin–cytokinin crosstalk in roots. Curr. Opin. Plant Biol. 14 10–16 [DOI] [PubMed] [Google Scholar]
- Caetano-Anollés G., Gresshoff P. M. (1991). Plant genetic control of nodulation. Annu. Rev. Microbiol. 45 345–382 [DOI] [PubMed] [Google Scholar]
- Charon C., Johansson C., Kondorosi E., Kondorosi A., Crespi M. (1997). enod40 induces dedifferentiation and division of root cortical cells in legumes. Proc. Natl. Acad. Sci. U.S.A. 94 8901–8906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. B., Long S. R. (1994). Morphogenetic rescue of Rhizobium meliloti nodulation mutants by trans-zeatin secretion. Plant Cell 6 215–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luis A., Markmann K., Cognat V., Holt D. B., Charpentier M., Parniske M., et al. (2012). Two microRNAs linked to nodule infection and nitrogen-fixing ability in the legume Lotus japonicus. Plant Physiol. 160 2137–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbak A., Yao H., McSteen P. (2012). Hormone signaling in plant development. Curr. Opin. Plant Biol. 15 92–96 [DOI] [PubMed] [Google Scholar]
- Fang Y., Hirsch A. M. (1998). Studying early nodulin gene ENOD40 expression and induction by nodulation factor and cytokinin in transgenic alfalfa. Plant Physiol. 116 53–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson B. J., Indrasumunar A., Hayashi S., Lin M. H., Lin Y. H., Reid D. E., et al. (2010). Molecular analysis of legume nodule development and autoregulation. J. Integr. Plant Biol. 52 61–76 [DOI] [PubMed] [Google Scholar]
- Fukai E., Soyano T., Umehara Y., Nakayama S., Hirakawa H., Tabata S., et al. (2012). Establishment of a Lotus japonicus gene tagging population using the exon-targeting endogenous retrotransposon LORE1. Plant J. 69 720–730 [DOI] [PubMed] [Google Scholar]
- Giraud E., Moulin L., Vallenet D., Barbe V., Cytryn E., Avarre J. C., et al. (2007). Legumes symbioses: absence of Nod genes in photosynthetic bradyrhizobia. Science 316 1307–1312 [DOI] [PubMed] [Google Scholar]
- Gleason C., Chaudhuri S., Yang T., Munoz A., Poovaiah B. W., Oldroyd G. E. (2006). Nodulation independent of rhizobia induced by a calcium-activated kinase lacking autoinhibition. Nature 441 1149–1152 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rizzo S., Crespi M., Frugier F. (2006). The Medicago truncatula CRE1 cytokinin receptor regulates lateral root development and early symbiotic interaction with Sinorhizobium meliloti. Plant Cell 18 2680–2693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Banba M., Shimoda Y., Kouchi H., Hayashi M., Imaizumi-Anraku H. (2010). A dominant function of CCaMK in intracellular accommodation of bacterial and fungal endosymbionts. Plant J. 63 141–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann A. B., Lombardo F., Miwa H., Perry J. A., Bunnewell S., Parniske M., et al. (2006). Lotus japonicus nodulation requires two GRAS domain regulators, one of which is functionally conserved in a non-legume. Plant Physiol. 142 1739–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann A. B., Sandal N., Bek A. S., Madsen L. H., Jurkiewicz A., Nielsen M. W., et al. (2011). Cytokinin induction of root nodule primordia in Lotus japonicus is regulated by a mechanism operating in the root cortex. Mol. Plant Microbe Interact. 24 1385–1395 [DOI] [PubMed] [Google Scholar]
- Heyl A., Schmülling T. (2003). Cytokinin signal perception and transduction. Curr. Opin. Plant Biol. 6 480–488 [DOI] [PubMed] [Google Scholar]
- Hirsch A. M., Bhuvaneswari T. V., Torrey J. G., Bisseling T. (1989). Early nodulin genes are induced in alfalfa root outgrowths elicited by auxin transport inhibitors. Proc. Natl. Acad. Sci. U.S.A. 86 1244–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch A. M., Fang Y. (1994). Plant hormones and nodulation: what’s the connection? Plant Mol. Biol. 26 5–9 [DOI] [PubMed] [Google Scholar]
- Inoue T., Higuchi M., Hashimoto Y., Seki M., Kobayashi M., Kato T., et al. (2001). Identification of CRE1 as a cytokinin receptor from Arabidopsis. Nature 409 1060–1063 [DOI] [PubMed] [Google Scholar]
- Kaló P., Gleason C., Edwards A., Marsh J., Mitra R. M., Hirsch S., et al. (2005). Nodulation signaling in legumes requires NSP2, a member of the GRAS family of transcriptional regulators. Science 308 1786–1789 [DOI] [PubMed] [Google Scholar]
- Kouchi H., Imaizumi-Anraku H., Hayashi M., Hakoyama T., Nakagawa T., Umehara Y., et al. (2010). How many peas in a pod? Legume genes responsible for mutualistic symbioses underground. Plant Cell Physiol. 51 1381–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusell L., Madsen L. H., Sato S., Aubert G., Genua A., Szczyglowski K., et al. (2002). Shoot control of root development and nodulation is mediated by a receptor-like kinase. Nature 420 422–426 [DOI] [PubMed] [Google Scholar]
- Laplaze L., Benkova E., Casimiro I., Maes L., Vanneste S., Swarup R., et al. (2007). Cytokinins act directly on lateral root founder cells to inhibit root initiation. Plant Cell 19 3889–3900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauressergues D., Delaux P.-M., Formey D., Lelandais-Brière C., Fort S., Cottaz S., et al. (2012). The microRNA miR171h modulates arbuscular mycorrhizal colonization of Medicago truncatula by targeting NSP2. Plant J. 72 512–522 [DOI] [PubMed] [Google Scholar]
- Libbenga K. R., van Iren F., Bogers R. J., Schraag-Lamers M. F. (1973). The role of hormones and gradients in the initiation of cortex proliferation and nodule formation in Pisum sativum L. Planta 114 29–39 [DOI] [PubMed] [Google Scholar]
- Lohar D. P., Schaff J. E., Laskey J. G., Kieber J. J., Bilyeu K. D., Bird D. M. (2004). Cytokinins play opposite roles in lateral root formation, and nematode and Rhizobial symbioses. Plant J. 38 203–214 [DOI] [PubMed] [Google Scholar]
- Lohar D. P., Sharopova N., Endre G., Peñuela S., Samac D., Town C., et al. (2006). Transcript analysis of early nodulation events in Medicago truncatula. Plant Physiol. 140 221–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen L. H., Tirichine L., Jurkiewicz A., Sullivan J. T., Heckmann A. B., Bek A. S., et al. (2010). The molecular network governing nodule organogenesis and infection in the model legume Lotus japonicus. Nat. Commun. 1 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhavý P., Vanstraelen M., De Rybel B., Zhaojun D., Bennett M. J., Beeckman T., et al. (2013). Auxin reflux between the endodermis and pericycle promotes lateral root initiation. EMBO J. 32 149–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathesius U., Charon C., Rolfe B. G., Kondorosi A., Crespi M. (2000). Temporal and spatial order of events during the induction of cortical cell divisions in white clover by Rhizobium leguminosarum bv. trifolii inoculation or localized cytokinin addition. Mol. Plant Microbe Interact. 13 617–628 [DOI] [PubMed] [Google Scholar]
- Mathesius U., Schlaman H. R., Spaink H. P., Of Sautter C., Rolfe B. G., Djordjevic M. A. (1998). Auxin transport inhibition precedes root nodule formation in white clover roots and is regulated by flavonoids and derivatives of chitin oligosaccharides. Plant J. 14 23–34 [DOI] [PubMed] [Google Scholar]
- Miyazawa H., Oka-Kira E., Sato N., Takahashi H., Wu G. J., Sato S., et al. (2010). The receptor-like kinase KLAVIER mediates systemic regulation of nodulation and non-symbiotic shoot development in Lotus japonicus. Development 137 4317–4325 [DOI] [PubMed] [Google Scholar]
- Moreau S., Verdenaud M., Ott T., Letort S., de Billy F., Niebel A., et al. (2011). Transcription reprogramming during root nodule development in Medicago truncatula. PLoS ONE 6:e16463 10.1371/journal.pone.0016463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortier V., Den Herder G., Whitford R., Van de Velde W., Rombauts S., D’Haeseleer K., et al. (2010). CLE peptides control Medicago truncatula nodulation locally and systemically. Plant Physiol. 153 222–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortier V., De Wever E., Vuylsteke M., Holsters M., Goormachtig S. (2012). Nodule numbers are governed by interaction between CLE peptides and cytokinin signaling. Plant J. 70 367–376 [DOI] [PubMed] [Google Scholar]
- Murakami Y., Miwa H., Imaizumi-Anraku H., Kouchi H., Downie J. A., Kawaguchi M., et al. (2006). Positional cloning identifies Lotus japonicus NSP2, a putative transcription factor of the GRAS family, required for NIN and ENOD40 gene expression in nodule initiation. DNA Res. 13 255–265 [DOI] [PubMed] [Google Scholar]
- Murray J. D. (2011). Invasion by invitation: rhizobial infection in legumes. Mol. Plant Microbe Interact. 24 631–639 [DOI] [PubMed] [Google Scholar]
- Murray J. D., Karas B. J., Sato S., Tabata S., Amyot L., Szczyglowski K. (2007). A cytokinin perception mutant colonized by Rhizobium in the absence of nodule organogenesis. Science 315 101–104 [DOI] [PubMed] [Google Scholar]
- Nishimura R., Hayashi M., Wu G. J., Kouchi H., Imaizumi-Anraku H., Murakami Y., et al. (2002). HAR1 mediates systemic regulation of symbiotic organ development. Nature 420 426–429 [DOI] [PubMed] [Google Scholar]
- Oka-Kira E., Kawaguchi M. (2006). Long-distance signaling to control root nodule number. Curr. Opin. Plant Biol. 9 496–502 [DOI] [PubMed] [Google Scholar]
- Okamoto S., Ohnishi E., Sato S., Takahashi H., Nakazono M., Tabata S., et al. (2009). Nod factor/nitrate-induced CLE genes that drive HAR1-mediated systemic regulation of nodulation. Plant Cell Physiol. 50 67–77 [DOI] [PubMed] [Google Scholar]
- Oldroyd G. E., Murray J. D., Poole P. S., Downie J. A. (2011). The rules of engagement in the legume-rhizobial symbiosis. Annu. Rev. Genet. 45 119–144 [DOI] [PubMed] [Google Scholar]
- Op den Camp R. H. M., De Mita S., Lillo A., Cao Q., Limpens E., Bisseling T., et al. (2011). A phylogenetic strategy based on a legume-specific whole genome duplication yields symbiotic cytokinin type-A response regulators. Plant Physiol. 157 2013–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacios-Bras C., Schlaman H. R., Boot K., Admiraal P., Langerak J. M., Stougaard J., et al. (2003). Auxin distribution in Lotus japonicus during root nodule development. Plant Mol. Biol. 52 1169–1180 [DOI] [PubMed] [Google Scholar]
- Péret B., Swarup R., Jansen L., Devos G., Auguy F., Collin M., et al. (2007). Auxin influx activity is associated with Frankia infection during actinorhizal nodule formation in Casuarina glauca. Plant Physiol. 144 1852–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine-Walker F., Doumas P., Lucas M., Vaissayre V., Beauchemin N. J., Band L. R., et al. (2010). Auxin carriers localization drives auxin accumulation in plant cells infected by Frankia in Casuarina glauca actinorhizal nodules. Plant Physiol. 154 1372–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. A., Torrey J. G. (1972). Studies on cytokinin production by Rhizobium. Plant Physiol. 49 11–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pislariu C. I., Murray J. D., Wen J., Cosson V., Muni R. R. D., Wang M., et al. (2012). A Medicago truncatula tobacco retrotransposon insertion mutant collection with defects in nodule development and symbiotic nitrogen fixation. Plant Physiol. 159 1686–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plet J., Wasson A., Ariel F., Le Signor C., Baker D., Mathesius U., et al. (2011). MtCRE1-dependent cytokinin signaling integrates bacterial and plant cues to coordinate symbiotic nodule organogenesis in Medicago truncatula. Plant J. 65 622–633 [DOI] [PubMed] [Google Scholar]
- Rightmyer A. P., Long S. R. (2011). Pseudonodule formation by wild-type and symbiotic mutant Medicago truncatula in response to auxin transport inhibitors. Mol. Plant Microbe Interact. 24 1372–1384 [DOI] [PubMed] [Google Scholar]
- Schauser L., Roussis A., Stiller J., Stougaard J. (1999). A plant regulator controlling development of symbiotic root nodules. Nature 402 191–195 [DOI] [PubMed] [Google Scholar]
- Schnabel E., Journet E. P., de Carvalho-Niebel F., Duc G., Frugoli J. (2005). The Medicago truncatula SUNN gene encodes a CLV1-like leucine-rich repeat receptor kinase that regulates nodule number and root length. Plant Mol. Biol. 58 809–822 [DOI] [PubMed] [Google Scholar]
- Shimoda Y., Han L., Yamazaki T., Suzuki R., Hayashi M., Imaizumi-Anraku H. (2012). Rhizobial and fungal symbioses show different requirements for calmodulin binding to calcium calmodulin-dependent protein kinase in Lotus japonicus. Plant Cell 24 304–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyano T., Kouchi H., Hirota A., Hayashi M. (2013). NODULE INCEPTION directly targets NF-Y subunit genes to regulate essential processes of root nodule development in Lotus japonicus. PLoS Genet. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaink H. P., Sheeley D. M., Vanbrussel A. A. N., Glushka J., York W. S., Tak T., et al. (1991). A novel highly unsaturated fatty-acid moiety of lipo-oligosaccharide signals determines host specificity of Rhizobium. Nature 354 125–130 [DOI] [PubMed] [Google Scholar]
- Sturtevant D. B., Taller B. J. (1989). Cytokinin production by Bradyrhizobium japonicum. Plant Physiol. 89 1247–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzaki T., Ito M., Kawaguchi M. (2013). Induction of localized auxin response during spontaneous nodule development in Lotus japonicus. Plant Signal. Behav. 8 e23359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzaki T., Yano K., Ito M., Umehara Y., Suganuma N., Kawaguchi M. (2012). Positive and negative regulation of cortical cell division during root nodule development in Lotus japonicus is accompanied by auxin response. Development 139 3997–4006 [DOI] [PubMed] [Google Scholar]
- Szczyglowski K., Shaw R. S., Wopereis J., Copeland S., Hamburger D., Kasiborski B., et al. (1998). Nodule organogenesis and symbiotic mutants of the model legume Lotus japonicus. Mol. Plant Microbe Interact. 11 684–697 [Google Scholar]
- Takanashi K., Sugiyama A., Yazaki K. (2011). Involvement of auxin distribution in root nodule development of Lotus japonicus. Planta 234 73–81 [DOI] [PubMed] [Google Scholar]
- Tirichine L., Imaizumi-Anraku H., Yoshida S., Murakami Y., Madsen L. H., Miwa H., et al. (2006a). Deregulation of a Ca2+/calmodulin-dependent kinase leads to spontaneous nodule development. Nature 441 1153–1156 [DOI] [PubMed] [Google Scholar]
- Tirichine L., James E. K., Sandal N., Stougaard J. (2006b). Spontaneous root-nodule formation in the model legume Lotus japonicus: a novel class of mutants nodulates in the absence of rhizobia. Mol. Plant Microbe Interact. 19 373–382 [DOI] [PubMed] [Google Scholar]
- Tirichine L., Sandal N., Madsen L. H., Radutoiu S., Albrektsen A. S., Sato S., et al. (2007). A gain-of-function mutation in a cytokinin receptor triggers spontaneous root nodule organogenesis. Science 315 104–107 [DOI] [PubMed] [Google Scholar]
- Truchet G., Barker D. G., Camut S., de Billy F., Vasse J., Huguet T. (1989). Alfalfa nodulation in the absence of Rhizobium. Mol. Gen. Genet. 219 65–68 [Google Scholar]
- Truchet G., Roche P., Lerouge P., Vasse J., Camut S., Debilly F., et al. (1991). Sulfated lipo-oligosaccharide signals of Rhizobium meliloti elicit root nodule organogenesis in alfalfa. Nature 351 670–673 [Google Scholar]
- Urbański D. F., Malolepszy A., Stougaard J., Andersen S. U. (2012). Genome-wide LORE1 retrotransposon mutagenesis and high-throughput insertion detection in Lotus japonicus. Plant J. 69 731–741 [DOI] [PubMed] [Google Scholar]
- van Noorden G. E., Ross J. J., Reid J. B., Rolfe B. G., Mathesius U. (2006). Defective long-distance auxin transport regulation in the Medicago truncatula super numeric nodules mutant. Plant Physiol. 140 1494–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernié T., Moreau S., de Billy F., Plet J., Combier J.-P., Rogers C., et al. (2008). EFD is an ERF transcription factor involved in the control of nodule number and differentiation in Medicago truncatula. Plant Cell 20 2696–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasson A. P., Pellerone F. I., Mathesius U. (2006). Silencing the flavonoid pathway in Medicago truncatula inhibits root nodule formation and prevents auxin transport regulation by rhizobia. Plant Cell 18 1617–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wopereis J., Pajuelo E., Dazzo F. B., Jiang Q., Gresshoff P. M., De Bruijn F. J., et al. (2000). Short root mutant of Lotus japonicus with a dramatically altered symbiotic phenotype. Plant J. 23 97–114 [DOI] [PubMed] [Google Scholar]
- Yano K., Yoshida S., Muller J., Singh S., Banba M., Vickers K., et al. (2008). CYCLOPS, a mediator of symbiotic intracellular accommodation. Proc. Natl. Acad. Sci. U.S.A. 105 20540–20545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C., Han L., Hou C., Metelli A., Qi L., Tadege M., et al. (2011). Developmental analysis of a Medicago truncatula smooth leaf margin1 mutant reveals context-dependent effects on compound leaf development. Plant Cell 23 2106–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]