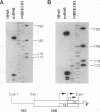
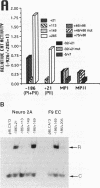
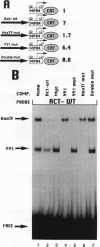
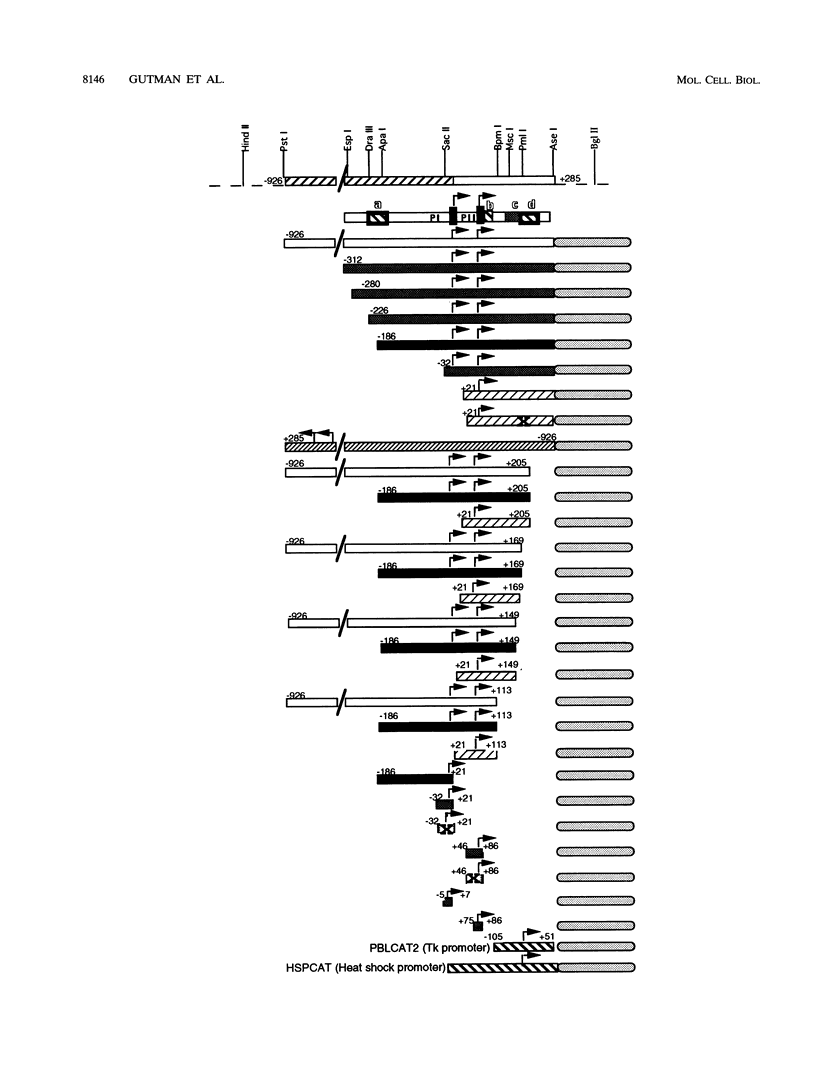
Abstract
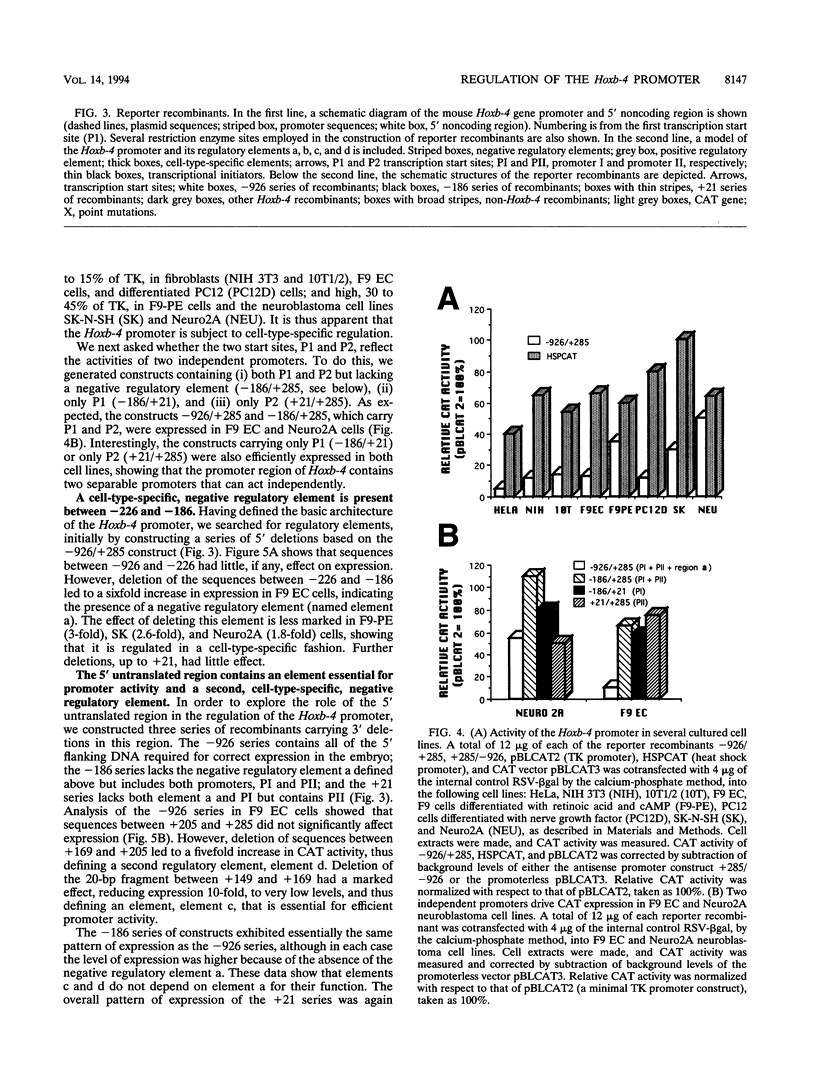
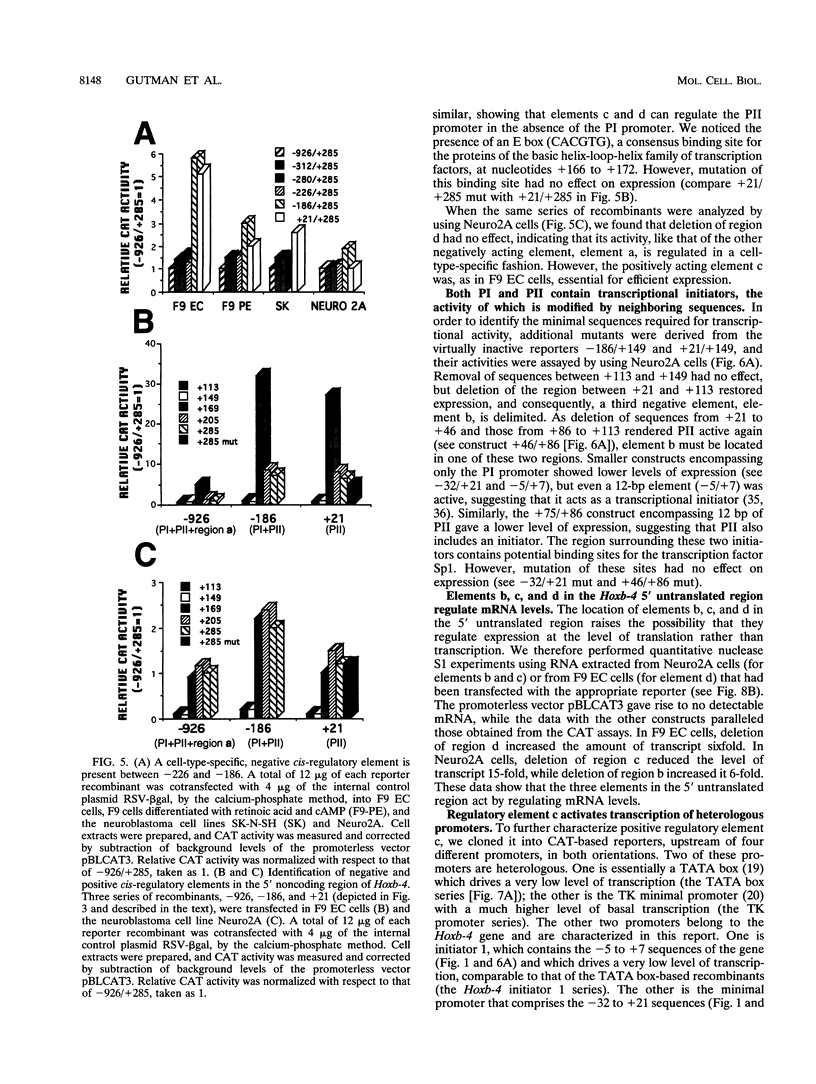
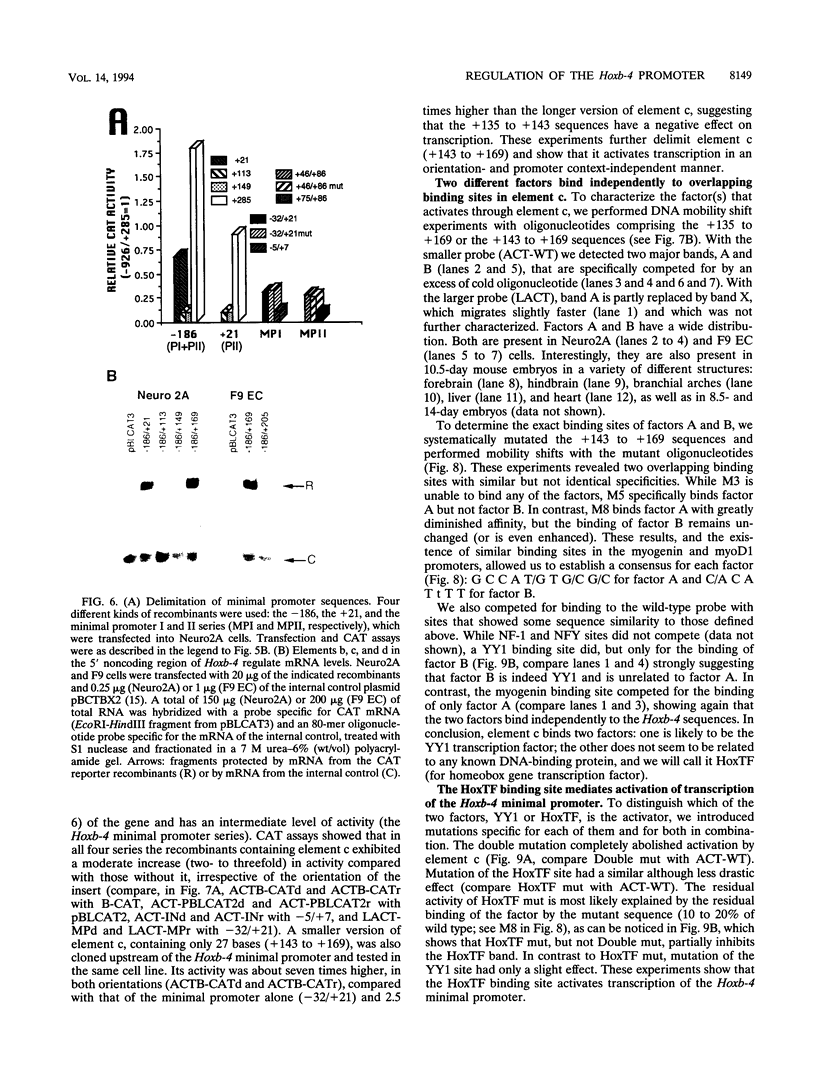
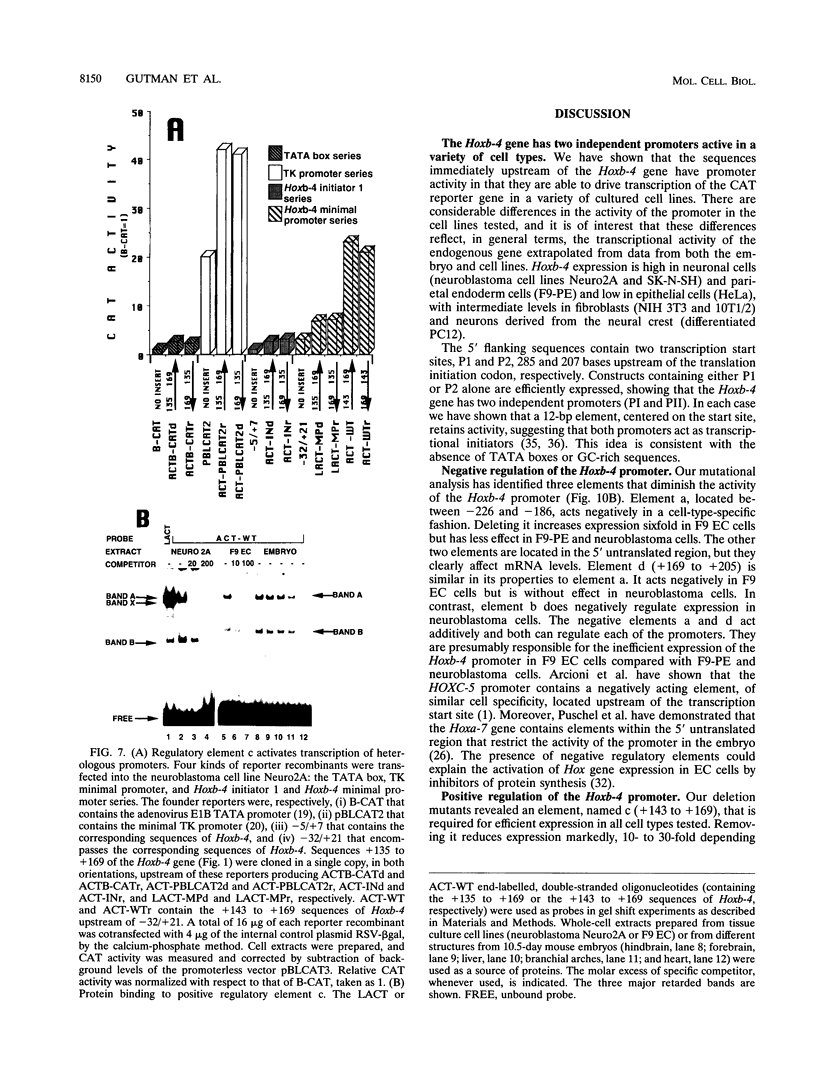
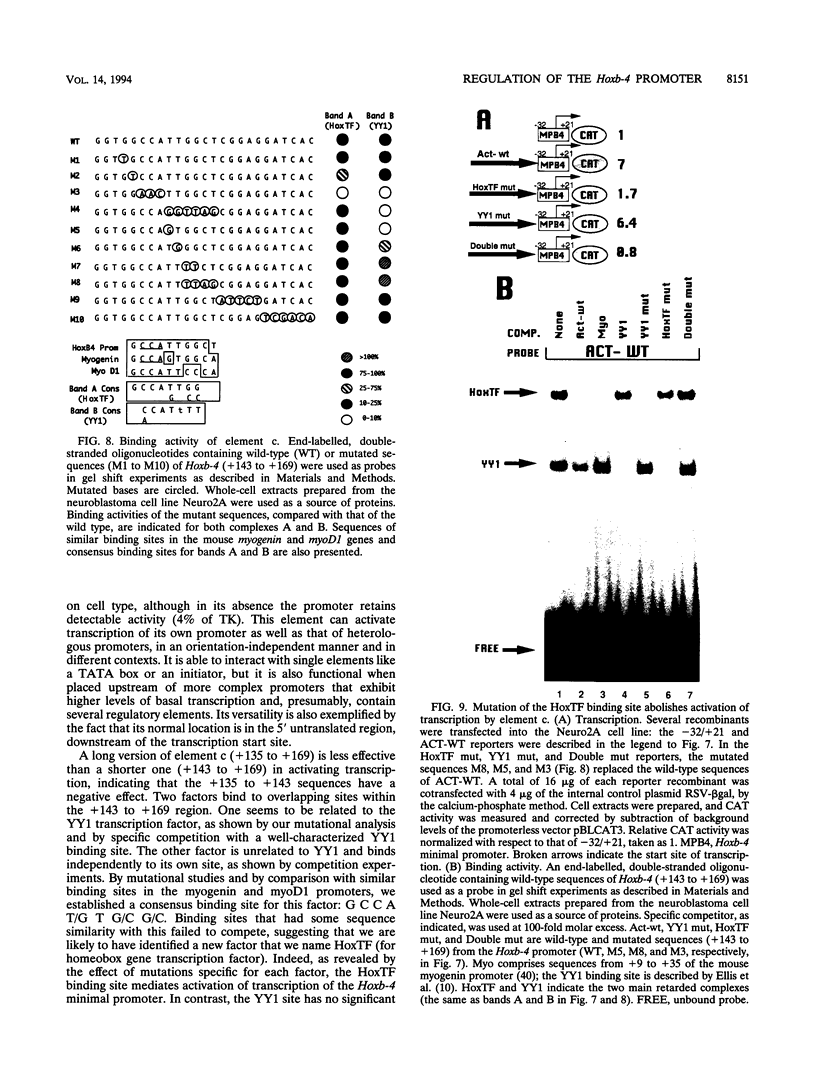
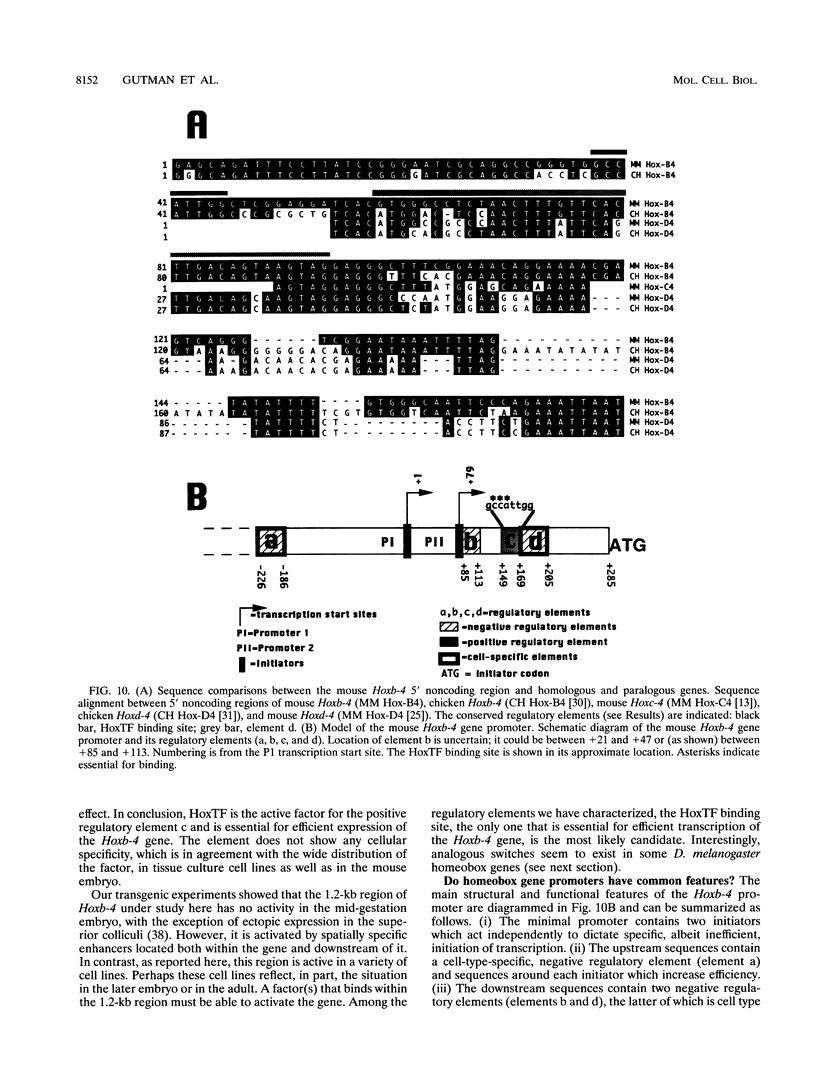
Mouse Hoxb-4 (Hox-2.6) is a homeobox gene that belongs to a family which also includes Hoxa-4, Hoxc-4, and Hoxd-4 and that is related to the Deformed gene in Drosophila melanogaster. We have determined the sequence of 1.2 kb of 5' flanking DNA of mouse Hoxb-4 and by nuclease S1 and primer extension experiments identified two transcription start sites, P1 and P2, 285 and 207 nucleotides upstream of the ATG initiator codon, respectively. We have shown that this region harbors two independent promoters which drive CAT expression in several different cell lines with various efficiencies, suggesting that they are subject to cell-type-specific regulation. Through detailed mutational analysis, we have identified several cis-regulatory elements, located upstream and downstream of the transcription start sites. They include two cell-type-specific negative regulatory elements, which are more active in F9 embryonal carcinoma cells than in neuroblastoma cells (regions a and d at -226 to -186 and +169 to +205, respectively). An additional negative regulatory element has been delimited (region b between +22 and +113). Positive regulation is achieved by binding of HoxTF, a previously unknown factor, to the sequence GCCATTGG (+148 to +155) that is essential for efficient Hoxb-4 expression. We have also defined the minimal promoter sequences and found that they include two 12-bp initiator elements centered around each transcription start site. The complex architecture of the Hoxb-4 promoter provides the framework for fine-tuned transcriptional regulation during embryonic development.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcioni L., Simeone A., Guazzi S., Zappavigna V., Boncinelli E., Mavilio F. The upstream region of the human homeobox gene HOX3D is a target for regulation by retinoic acid and HOX homeoproteins. EMBO J. 1992 Jan;11(1):265–277. doi: 10.1002/j.1460-2075.1992.tb05049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awgulewitsch A., Bieberich C., Bogarad L., Shashikant C., Ruddle F. H. Structural analysis of the Hox-3.1 transcription unit and the Hox-3.2--Hox-3.1 intergenic region. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6428–6432. doi: 10.1073/pnas.87.16.6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balling R., Mutter G., Gruss P., Kessel M. Craniofacial abnormalities induced by ectopic expression of the homeobox gene Hox-1.1 in transgenic mice. Cell. 1989 Jul 28;58(2):337–347. doi: 10.1016/0092-8674(89)90848-9. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Tjian R. Transcription factors that activate the Ultrabithorax promoter in developmentally staged extracts. Cell. 1988 Jun 3;53(5):699–711. doi: 10.1016/0092-8674(88)90088-8. [DOI] [PubMed] [Google Scholar]
- Chisaka O., Capecchi M. R. Regionally restricted developmental defects resulting from targeted disruption of the mouse homeobox gene hox-1.5. Nature. 1991 Apr 11;350(6318):473–479. doi: 10.1038/350473a0. [DOI] [PubMed] [Google Scholar]
- Chisaka O., Musci T. S., Capecchi M. R. Developmental defects of the ear, cranial nerves and hindbrain resulting from targeted disruption of the mouse homeobox gene Hox-1.6. Nature. 1992 Feb 6;355(6360):516–520. doi: 10.1038/355516a0. [DOI] [PubMed] [Google Scholar]
- Cho K. W., Goetz J., Wright C. V., Fritz A., Hardwicke J., De Robertis E. M. Differential utilization of the same reading frame in a Xenopus homeobox gene encodes two related proteins sharing the same DNA-binding specificity. EMBO J. 1988 Jul;7(7):2139–2149. doi: 10.1002/j.1460-2075.1988.tb03053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianetti L., Di Cristofaro A., Zappavigna V., Bottero L., Boccoli G., Testa U., Russo G., Boncinelli E., Peschle C. Molecular mechanisms underlying the expression of the human HOX-5.1 gene. Nucleic Acids Res. 1990 Aug 11;18(15):4361–4368. doi: 10.1093/nar/18.15.4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coletta P. L., Shimeld S. M., Chaudhuri C., Müller U., Clarke J. P., Sharpe P. T. Characterisation of the murine Hox-3.3 gene and its promoter. Mech Dev. 1991 Sep;35(2):129–142. doi: 10.1016/0925-4773(91)90063-c. [DOI] [PubMed] [Google Scholar]
- Ellis J., Talbot D., Dillon N., Grosveld F. Synthetic human beta-globin 5'HS2 constructs function as locus control regions only in multicopy transgene concatamers. EMBO J. 1993 Jan;12(1):127–134. doi: 10.1002/j.1460-2075.1993.tb05638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliot B., Dollé P., Vigneron M., Featherstone M. S., Baron A., Duboule D. The mouse Hox-1.4 gene: primary structure, evidence for promoter activity and expression during development. Development. 1989 Oct;107(2):343–359. doi: 10.1242/dev.107.2.343. [DOI] [PubMed] [Google Scholar]
- Gaunt S. J. Expression patterns of mouse Hox genes: clues to an understanding of developmental and evolutionary strategies. Bioessays. 1991 Oct;13(10):505–513. doi: 10.1002/bies.950131004. [DOI] [PubMed] [Google Scholar]
- Geada A. M., Gaunt S. J., Azzawi M., Shimeld S. M., Pearce J., Sharpe P. T. Sequence and embryonic expression of the murine Hox-3.5 gene. Development. 1992 Oct;116(2):497–506. doi: 10.1242/dev.116.2.497. [DOI] [PubMed] [Google Scholar]
- Gorman C., Padmanabhan R., Howard B. H. High efficiency DNA-mediated transformation of primate cells. Science. 1983 Aug 5;221(4610):551–553. doi: 10.1126/science.6306768. [DOI] [PubMed] [Google Scholar]
- Gutman A., Wasylyk C., Wasylyk B. Cell-specific regulation of oncogene-responsive sequences of the c-fos promoter. Mol Cell Biol. 1991 Oct;11(10):5381–5387. doi: 10.1128/mcb.11.10.5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel M., Balling R., Gruss P. Variations of cervical vertebrae after expression of a Hox-1.1 transgene in mice. Cell. 1990 Apr 20;61(2):301–308. doi: 10.1016/0092-8674(90)90810-2. [DOI] [PubMed] [Google Scholar]
- Krumlauf R. Evolution of the vertebrate Hox homeobox genes. Bioessays. 1992 Apr;14(4):245–252. doi: 10.1002/bies.950140408. [DOI] [PubMed] [Google Scholar]
- Le Mouellic H., Lallemand Y., Brûlet P. Homeosis in the mouse induced by a null mutation in the Hox-3.1 gene. Cell. 1992 Apr 17;69(2):251–264. doi: 10.1016/0092-8674(92)90406-3. [DOI] [PubMed] [Google Scholar]
- Lillie J. W., Green M. R. Transcription activation by the adenovirus E1a protein. Nature. 1989 Mar 2;338(6210):39–44. doi: 10.1038/338039a0. [DOI] [PubMed] [Google Scholar]
- Luckow B., Renkawitz R., Schütz G. A new method for constructing linker scanning mutants. Nucleic Acids Res. 1987 Jan 26;15(2):417–429. doi: 10.1093/nar/15.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lufkin T., Dierich A., LeMeur M., Mark M., Chambon P. Disruption of the Hox-1.6 homeobox gene results in defects in a region corresponding to its rostral domain of expression. Cell. 1991 Sep 20;66(6):1105–1119. doi: 10.1016/0092-8674(91)90034-v. [DOI] [PubMed] [Google Scholar]
- Lufkin T., Mark M., Hart C. P., Dollé P., LeMeur M., Chambon P. Homeotic transformation of the occipital bones of the skull by ectopic expression of a homeobox gene. Nature. 1992 Oct 29;359(6398):835–841. doi: 10.1038/359835a0. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Krumlauf R. Homeobox genes and axial patterning. Cell. 1992 Jan 24;68(2):283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- Perkins K. K., Dailey G. M., Tjian R. In vitro analysis of the Antennapedia P2 promoter: identification of a new Drosophila transcription factor. Genes Dev. 1988 Dec;2(12A):1615–1626. doi: 10.1101/gad.2.12a.1615. [DOI] [PubMed] [Google Scholar]
- Püschel A. W., Balling R., Gruss P. Separate elements cause lineage restriction and specify boundaries of Hox-1.1 expression. Development. 1991 May;112(1):279–287. doi: 10.1242/dev.112.1.279. [DOI] [PubMed] [Google Scholar]
- Ramírez-Solis R., Zheng H., Whiting J., Krumlauf R., Bradley A. Hoxb-4 (Hox-2.6) mutant mice show homeotic transformation of a cervical vertebra and defects in the closure of the sternal rudiments. Cell. 1993 Apr 23;73(2):279–294. doi: 10.1016/0092-8674(93)90229-j. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H., Kuroiwa A. The nucleotide sequence of the cDNA encoding a chicken Deformed family homeobox gene, Chox-Z. Nucleic Acids Res. 1990 Jan 11;18(1):184–184. doi: 10.1093/nar/18.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H., Yokoyama E., Kuroiwa A. Specific DNA binding of the two chicken Deformed family homeodomain proteins, Chox-1.4 and Chox-a. Nucleic Acids Res. 1990 Apr 11;18(7):1739–1747. doi: 10.1093/nar/18.7.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone A., Acampora D., Nigro V., Faiella A., D'Esposito M., Stornaiuolo A., Mavilio F., Boncinelli E. Differential regulation by retinoic acid of the homeobox genes of the four HOX loci in human embryonal carcinoma cells. Mech Dev. 1991 Mar;33(3):215–227. doi: 10.1016/0925-4773(91)90029-6. [DOI] [PubMed] [Google Scholar]
- Simeone A., Mavilio F., Acampora D., Giampaolo A., Faiella A., Zappavigna V., D'Esposito M., Pannese M., Russo G., Boncinelli E. Two human homeobox genes, c1 and c8: structure analysis and expression in embryonic development. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4914–4918. doi: 10.1073/pnas.84.14.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh M. J. A nonchromatographic assay for expression of the chloramphenicol acetyltransferase gene in eucaryotic cells. Anal Biochem. 1986 Jul;156(1):251–256. doi: 10.1016/0003-2697(86)90180-6. [DOI] [PubMed] [Google Scholar]
- Smale S. T., Baltimore D. The "initiator" as a transcription control element. Cell. 1989 Apr 7;57(1):103–113. doi: 10.1016/0092-8674(89)90176-1. [DOI] [PubMed] [Google Scholar]
- Smale S. T., Schmidt M. C., Berk A. J., Baltimore D. Transcriptional activation by Sp1 as directed through TATA or initiator: specific requirement for mammalian transcription factor IID. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4509–4513. doi: 10.1073/pnas.87.12.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeller W. C., Poole S. J., Kornberg T. In vitro transcription of the Drosophila engrailed gene. Genes Dev. 1988 Jan;2(1):68–81. doi: 10.1101/gad.2.1.68. [DOI] [PubMed] [Google Scholar]
- Whiting J., Marshall H., Cook M., Krumlauf R., Rigby P. W., Stott D., Allemann R. K. Multiple spatially specific enhancers are required to reconstruct the pattern of Hox-2.6 gene expression. Genes Dev. 1991 Nov;5(11):2048–2059. doi: 10.1101/gad.5.11.2048. [DOI] [PubMed] [Google Scholar]
- Wolgemuth D. J., Behringer R. R., Mostoller M. P., Brinster R. L., Palmiter R. D. Transgenic mice overexpressing the mouse homoeobox-containing gene Hox-1.4 exhibit abnormal gut development. Nature. 1989 Feb 2;337(6206):464–467. doi: 10.1038/337464a0. [DOI] [PubMed] [Google Scholar]
- Yee S. P., Rigby P. W. The regulation of myogenin gene expression during the embryonic development of the mouse. Genes Dev. 1993 Jul;7(7A):1277–1289. doi: 10.1101/gad.7.7a.1277. [DOI] [PubMed] [Google Scholar]