Abstract
Background:
Lobular endocervical glandular hyperplasia (LEGH) is a rare lesion of the uterine cervix. It has been proposed that LEGH may represent a precursor lesion to a group of mucinous adenocarcinoma with gastric phenotype (GA) that is independent of high-risk human papillomavirus (H-HPV) infection. Carbonic anhydrase-IX (CA-IX) is highly expressed in conventional glandular lesions (CGLs). However, expression of CA-IX in LEGH or GA has not been studied.
Methods:
In all, 12 CGLs, 7 LEGHs, 6 LEGHs with coexisting adenocarcinoma in situ (AIS, 3) and GA (3) were identified from Japanese women with a cytological diagnosis of atypical glandular cells of undetermined significance. Immunostaining was used to detect CA-IX and p16INK4a (hereafter termed p16) protein expression in the tissues and CA-IX protein expression in the Papanicolaou smears (PSs). Polymerase chain reaction was used to detect H-HPV DNA in liquid-based cytology.
Results:
Out of 12 (83%) CGLs, 10 were positive with H-HPV and high levels of CA-IX expression were seen in all (100%) cases. P16 protein expression was observed in 11 out of 12 (92%) cases. None of the LEGHs, LEGHs with AIS or GA were positive for H-HPV and only 8 out of 13 (62%) showed focal weak (1+) p16 expression. In contrast, all cases (100%) exhibited strong CA-IX protein expression.
Conclusion:
Our study suggests that there are different molecular mechanisms of carcinogenesis resulting in CGLs vs LEGHs associated with AIS or GA. There is also a possible link between LEGHs and GAs. Furthermore, CA-IX expression may serve as a useful biomarker for the detection of GAs in the absence of H-HPV infection.
Keywords: CA-IX, H-HPV, lobular endocervical glandular hyperplasia, conventional adenocarcinoma, gastric phenotype, minimal deviation
Widespread cervical screening in developed countries has led to a dramatic decline in the incidence of cervical squamous cell carcinoma; however, the rate of endocervical adenocarcinomas remains the same or is on the rise (Smith et al, 2000; Bray et al, 2005). The false negative rate in the cytological screening for cervical adenocarcinomas overall is higher than that for squamous lesions (Makino et al, 1995). The overlapping cytological features between reactive and neoplastic glandular cells may have an important role in the underdiagnosis of glandular neoplasia, and this is particularly true in the diagnosis of early or well-differentiated adenocarcinoma, such as minimal deviation adenocarcinoma (MDA). The cervical adenocarcinomas encompass a heterogeneous group of tumours, including mucinous and non-mucinous types. The mucinous type includes the prototypical endocervical adenocarcinoma of usual type and other subtypes, such as intestinal, villoglandular and MDA. The non-mucinous tumours include endometrioid, clear-cell, serous and mesonephric types. Recently, a subtype of mucinous adenocarcinoma expressing gastric phenotype (GA) has also been described. To date, this type of adenocarcinoma has been primarily identified in the Japanese population and the term ‘gastric-type endocervical adenocarcinoma' has been used in the literature (Kojima et al, 2007; Kusanagi et al, 2010; Park et al, 2011). The tumour shows voluminous amounts of gastric-type mucin within the cytoplasm, giving a pale eosinophilic appearance of neoplastic cells. The cytological atypia can be mild to severe. It has been postulated that MDA is an extremely well-differentiated form of the broader category of GA (Ishii et al, 1998; Mikami et al, 2004). Minimal deviation adenocarcinoma of the cervix is a neoplasm distinguished by its deceptively bland histological features. Although the lesion only accounts for 1 to 3% of all endocervical adenocarcinomas, the prognosis of the advanced-stage disease is poor. In fact, delayed diagnosis occurs in most of the cases (Gilks et al, 1989). In 1999, Nucci and co-workers described a new pseudoneoplastic glandular lesion of the cervix, termed lobular endocervical glandular hyperplasia (LEGH) and considered this as a hyperplastic glandular lesion, sharing similar clinical and histological features with MDA. The importance of the separation between LEGH and MDA has been emphasised in the past. However, in recent years it has been speculated that LEGH may represent the precursor of GA, incorporating MDA as its well-differentiated form based on the following observations: (1) up to 19% of cases of LEGH showed coexisting GA (Nara et al, 2007); (2) both LEGH and GA share a similar immunophenotype of gastric pyloric glands (Mikami et al, 2001, 2004); and (3) it has been postulated that MDA with gastric phenotype may represent an extremely well-differentiated form of GA. Lastly, one recent study has shown that copy number gains of chromosome 3q and loss of 1p in 3 of 14 (21%) LEGHs were also common to MDA and mucinous adenocarcinoma (Kawauchi et al, 2008). This observation is consistent with a possible molecular–genetic link between LEGH and GA/MDA. Therefore, it appears that separating LEGHs from other mimics of benign cervical glandular lesions is as important as correctly diagnosing GAs/MDAs.
Infection with oncogenic high-risk human papillomavirus (H-HPV) type(s) is widely accepted to be an important aetiological factor for cervical cancer (zur Hausen, 2002; Bosch et al, 2008). High-risk human papillomavirus has been detected in 97–99% of cervical cancers (Walboomers et al, 1999; Liao et al, 2009) and 83–100% of adenocarcinomas and their precursor glandular lesions (Pirog et al, 2000; Liao et al, 2009). P16INK4a (hereafter termed p16) is a tumour suppressor gene that has a central role in the regulation of the cell cycle. It has been shown that strong p16 expression in cervical carcinomas is associated with H-HPV infection. Thus, p16 has been commonly accepted as a surrogate biomarker for diagnosing cervical dysplasia/neoplasia associated with H-HPV infection (Klaes et al., 2001; Negri et al, 2003). However, in recent years, certain subsets of cervical adenocarcinomas have been identified, namely MDA and mucinous GA, clear-cell carcinoma and mesonephric carcinoma that are not associated with H-HPV infection (Pirog et al, 2000; Kusanagi et al, 2010; Park et al, 2011).
In the 1990s, the antigen MN was identified (Závada et al, 1993). MN is a glycoprotein, and was found to be a member of the carbonic anhydrase (CA) gene family, designated CA-IX (Opavský et al, 1996). Carbonic anhydrase-IX is a biomarker of several human tumours, including carcinomas of the cervix and kidney (Liao et al, 1994, 1997). Carbonic anhydrase-IX expression in cancerous tissues, and its absence in normal counterparts, has led to the speculation that it has a role in carcinogenesis (Wykoff et al 2000; Ivanov et al, 2001). Its expression is controlled by the transcription factor, hypoxia-inducible factor-1, and is upregulated in hypoxic regions of tumour tissues (Wykoff et al, 2000; Ivanov et al, 2001; Swietach et al, 2007).
In a survey of benign and neoplastic cervical tissues and PSs, it was observed that high levels of CA-IX were expressed by virtually all atypical glandular cells associated with adenocarcinoma in situ (AIS) and adenocarcinoma, but in the benign cervix CA-IX protein was either undetected or present in low levels, restricted to a few endocervical glandular cells and reserve cells. This suggested that high levels of CA-IX expression in cervical glandular cells may serve as a useful biomarker for diagnosing conventional glandular lesions (CGLs) of the cervix, including AIS and adenocarcinoma (Liao et al, 1994, 2009; Liao and Stanbridge, 1996, 2000). However, CA-IX expression in special subtypes of cervical lesions, such as LEGH and GA/MDA, has not been reported.
In 1998, the Gynaecologic Oncology Group (GOG), an international multi-institutional clinical trials group supported by the US National Cancer Institute, conducted a study of women with a cytological diagnosis of atypical glandular cells of undetermined significance (AGC). A total of 25 Institutions in the United States (Liao et al, 2009) and 11 institutions in Japan participated in the trial (Liao et al, 2010). In the Japanese cohort, H-HPV had a sensitivity of 53% in CGLs and an overall specificity of 86%, whereas CA-IX had a sensitivity of 100% in CGLs and an overall specificity of 50% (Liao et al, 2010). In a retrospective histological review of all cases retrieved from the Japanese cohorts, there were 13 cases of LEGH identified. Among these LEGH cases, three coexisted with AIS and three with GA. All of these three GAs were well differentiated with coexisting morphological spectrum of MDA. Here we report the results of H-HPV testing, using polymerase chain reaction (PCR), and p16 and CA-IX expression, using immunohistochemical staining, in the Japanese cases of LEGH and LEGH with coexisting AIS or GA. The objective of this study was to determine the degree of association between H-HPV infection, p16 and CA-IX expression, in LEGH, and in cases of LEGH with coexisting AIS and GA, and to ascertain their relative diagnostic utility. For comparative purposes, we included cases of CGLs from the same Japanese cohort.
Materials and methods
The criteria of AGC diagnosis for patient enrolment were based on the 1991 TBS classification (Kurman and Solomon, 1994) and conventional PSs were used. The protocol used in this study was approved by the National Cancer Institute, Division of Cancer Prevention and the GOG Human Research Committee, and annually by the Institutional Review Board (IRB) at each of the participating institutions.
Patient selection and tissue samples
Women over the age of 18 years, with a referring diagnosis of AGC and who were expected, on a clinical basis, to undergo complete histological evaluation of the cervical transformation zone, either with LEEP and/or cold knife cone or hysterectomy within 6 months of the initial cytological diagnosis, were enrolled in the study. Before enrollment all patients signed an informed consent form. Sample collection, patient eligibility, clinical management and histological evaluation have been described elsewhere (Liao et al, 2009, 2010). Briefly, all H&E-stained histological slides of the most abnormal lesions from each diagnostic procedure were reviewed centrally by the teams of two gynaecological pathologists from the GOG Pathology Committee who reached a consensus diagnosis. The diagnosis of each case was entered to the Statistical Data Base. Retrospective review of the histology of all H-HPV tested cases enrolled in the Japanese study population was conducted by one of the authors (SYL). The diagnosis of LEGH and LEGH with atypical features was established based on the criteria proposed by Nucci et al (1999) and Mikami et al (2004). Briefly, LEGH without atypia was defined as follows: (1) proliferation of small glands in a lobular manner; (2) abundant intracytoplasmic mucin giving a pale eosinophic appearance of glandular cells; (3) basally located round nuclei without anaplasia; and (4) absence of distinct evidence of destructive stroma (Figure 1A). Lobular endocervical glandular hyperplasia with atypical features was defined as follows: (1) nuclear enlargement, with irregularity and conspicuous nucleoli; (2) loss of polarity and papillary growth; and (3) the presence mitotic figures and apoptotic bodies and/or nuclear debris in the lumen (Figures 1C, D and 3A, D). The novel criteria outlined by Kojima et al (2007) were used to define the diagnosis of GA. Those criteria are: (1) clear or pale eosinophilic cytoplasm; (2) voluminous cytoplasm; and (3) distinct cell borders (Figures 1F and 2D). For conventional endocervical adenocarcinoma, AIS, the World Health Organisation (WHO) classification (Tavassoli and Devilee, 2003) was used. To be consistent with our previous reports (Liao et al, 2009, 2010), in this study we use the term CGL to apply to conventional endocervical adenocarcinoma and AIS without coexisting LEGH, and GA to apply to cases with the morphological spectrum of MDA but with gastric phenotype, associated with LEGH. There were 84 cases with known H-HPV data enrolled in the Japanese cohort, and among these, 12 CGLs, 7 LEGHs and 6 LEGHs with coexisting AIS or GA were identified. All of the cases of CGL, LEGH, LEGH with AIS or GA were independently reviewed by the second gynaecological pathologist (WHR) and consensus was reached in all cases. All 84 cases with known H-HPV data were screened for p16 and CA-IX expression in the tissue sections of the cervix and CA-IX expression in the PSs. Among these, 27 cases with benign cervix were included in the study to serve as the negative control group.
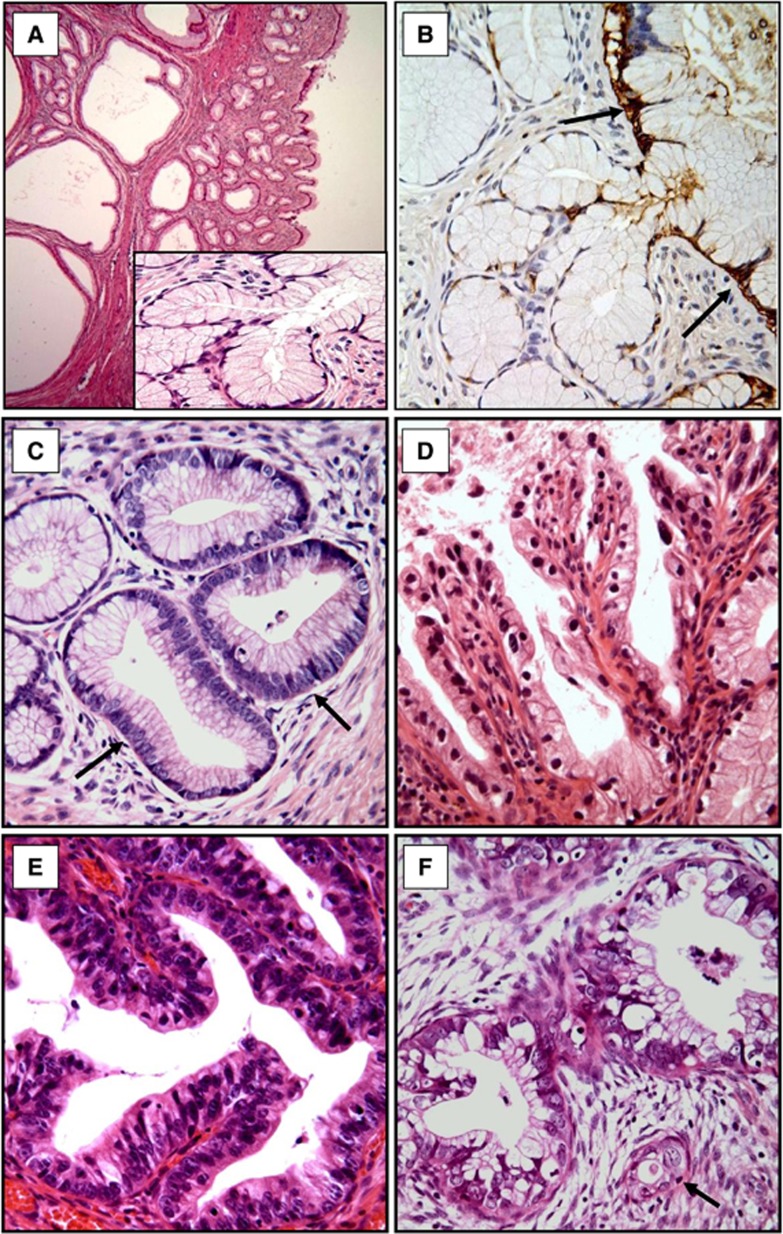
Figure 1.
Histology of LEGH, LEGH with atypia, LEGH with coexisting AIS and mucinous GA and CA-IX expression in LEGH. Lobular endocervical glandular hyperplasia without atypia (A) shows distinct lobular proliferation of the small- to medium-sized round glands surrounding larger central cystic dilated glands. The glands are lined by tall, mucin-rich columnar cells with a basal arrangement of nuclei, similar to pyloric glands (A, inset). Lobular endocervical glandular hyperplasia with atypia (C, D): some show nuclear enlargement, conspicuous nucleoli and apoptosis (C, arrow); some exhibit papillary growth with nuclear irregularity and loss of polarity (D). Adenocarcinoma in situ is defined as when the gland was replaced by cytologically malignant cells with associated apoptosis and/or mitosis (E). Adenocarcinoma with gastric phenotype exhibits voluminous clear cytoplasm with stromal desmoplastic reaction (F). Note the small invasive gland embedded in the desmoplastic stroma (F, arrow). Carbonic anhydrase-IX expression in the LEGH without atypia is limited to the central larger cystic glands (B, arrow). Original magnifications: × 100 in (A) and × 400 (A, inset and B–F).
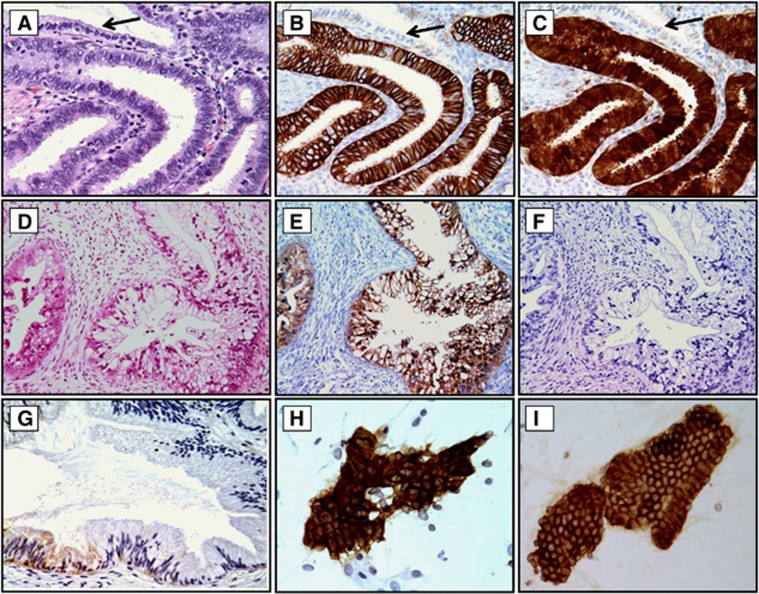
Figure 2.
Examples of CA-IX and p16 immunoreactivity in CGL, GA and CA-IX expression in normal cervix and exfoliated endocervical cells. Strong membranous reaction for CA-IX (B) and cytoplasmic and nuclear staining for p16 (C) was seen in all cases of CGL, but the normal gland was non-reactive for CA-IX or p16 (B and C, arrows). In contrast, in cases of GA only strongly positive CA-IX staining was seen (E), and p16 expression was either weakly cytoplasmic or negative (F). In normal cervical glands, CA-IX expression was limited to a few columnar/reserve cells, and the staining was weak and limited to the cytoplasm (G, arrow). Atypical glandular cells (H) derived from GA and normal looking glandular cells (I) derived from LEGH with atypia showed diffuse strong membranous positivity for CA-IX. The relevant haematoxylin and eosin (H&E)-stained tissue sections are shown in (A) and (D). Original magnifications: × 200 in (D–F) and × 400 in (A–C, G–I).
Immunohistochemistry
Detection of CA-IX in a conventional study PS
Immunodetection of CA-IX was performed on conventional study PSs using the anti CA-IX mouse monoclonal antibody, M75, as described previously (Liao et al, 1994). Specific immunostaining was defined by the presence of a brown reaction product along the plasma membrane under × 40 magnification. Faint staining of the cytoplasm was considered negative. Immunostaining was scored as positive or negative as described previously (Liao and Stanbridge, 1996; Liao et al, 2010). Briefly, the positive reaction was defined as when any immunoreactivity was identified in atypical cells or strong dark brown membranous positivity was observed in the normal looking endocervical cells. No immunoreactivity or faint staining was considered as negative.
Detection of CA-IX and p16 in tissue sections
Carbonic anhydrase-IX and p16 immunostaining was performed on 5-μm-thick sections of formalin-fixed paraffin-embedded tissues, using CA-IX and p16 antibodies (clone G175-405; BD Pharmingen, Erembodegem-Aalst, Belgium). The Ventana amplification ultraview DAB detection kit in a Ventana BenchMark XT processor (Ventnana, Tucson, AZ, USA) was used for detecting CA-IX and p16 protein expression. The appropriate antigen retrieval methodology was used according to the manufacturer's recommendation. The dilutions of antibodies used were as follows: CA-IX (1 : 5000) and p16 (1 : 25). The extent and the intensity of immunostaining were evaluated independently by two pathologists (SYL and WHR) and consensus was reached in all cases. The extent was recorded semiquantitatively as the percentage of the cells that showed positive staining, as follows: negative (<5% of the cells), focal (5–49% of the cells) and diffuse (⩾50% of the cells). The intensity was graded as negative (0), weak (1+) and moderate to strong (2+ to 3+). For p16, both cytoplasmic and/or nuclear staining was recorded. Faint cytoplasmic staining (+/−) was considered as a nonspecific reaction.
HPV genotyping
The detection method employed a modified E6/E7-specific consensus PCR, using mixed primers (pU-1M, pU-1M-L/pU-2R and pU-2R-N). The PCR method used was a minor modification of published procedures (Inoue et al, 1995; Yamazaki et al, 2001), where modification of the primer sequences made it possible to amplify 13 H-HPV types (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 58, 67, 68 and 70). The PCR-based procedure was performed by the Takara Bio Corporation (Otsu, Shiga, Japan). The sequence of the PCR reactions was 94 oC for 30 s, 55 °C for 60 s and 72 oC for 60 s, and each cycle was repeated 35 times. Polymerase chain reaction product sizes ranged from 231 to 271 base pairs and represent the E6 and E7 regions of the H-HPV types. Genotyping of H-HPV DNA was performed according to the restriction fragment length polymorphism method described by Lungu et al (1992). In situations where genotyping was not detected by restriction enzyme analysis, it was determined by direct sequencing of the amplified products.
Results
There were 84 cases with known H-HPV data enrolled in the Japanese cohort, and among these, 13 LEGHs were identified. Among these 13 cases of LEGH, 6 had coexisting mucinous adenocarcinoma: 3 were in situ and 3 were invasive. All three invasive adenocarcinomas were extremely well differentiated and exhibited histological features of mucinous GA as described by Kojima et al (2007). For comparative purposes, 12 cases of H-HPV-positive CGLs, including AIS (n=8) and adenocarcinoma (n=4), and 27 cases with benign cervix identified in the Japanese cohort were also included in the study. The 27 benign cases were used as the negative control group. The ages of the patients ranged from 36 to 80 (median: 55 years) in all cases of LEGH with or without AIS or GA, and from 32 to 56 (median: 45 years) in those patients with CGLs. Characteristic histological features of LEGH, described as well-demarcated proliferation of glands with a lobular architecture, with the glands lined by tall, mucin-rich columnar cells with a basal arrangement of nuclei, were seen in all cases of LEGH (Figure 1A). Out of 13 LEGHs, 12 exhibited variable degrees of atypical features either focal or diffuse, including nuclear enlargement, conspicuous nucleoli, loss of polarity, papillary growth and mitosis/apoptosis (Figures 1C, D and 3A, D). Among these, three had coexisting AIS (Figure 1E) and three had coexisting GA (Figures 1F and 2D).
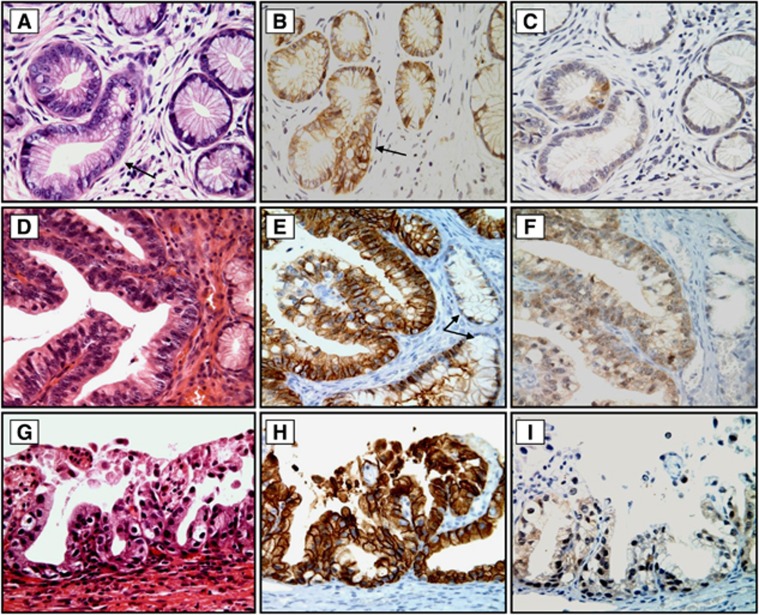
Figure 3.
Histology and immunohistochemical staining for CA-IX and p16 protein expression in LEGH with atypia. In contrast to Figure 1B, CA-IX immunoreactivity was not only limited to the central larger glands but was also observed in the atypical glands (B, arrow, and E). Diffuse CA-IX membranous positivity was also seen in LEGH with atypical papillary growth (H). Note that the small glands without atypia were CA-IX negative (E, arrow). In contrast, p16 expression in all cases of LEGH with atypia was either negative or exhibited very weak cytoplasmic staining (C, Fand I). The relevant haematoxylin and eosin (H&E)-stained tissue sections are shown in (A, D and G). Original magnification: × 400.
H-HPV infection
All cases of CGL, LEGH and LEGH with AIS or GA/MDA were tested for H-HPV infection. The results are summarised in Tables 1 and 2. High-risk human papillomavirus DNA was detected in 83% (10 out of 12) of the CGLs (Table 1). All but one contained a single genotype: 7 (3 AIS, 4 adenocarcinomas) were type 18; 2 AIS were type 16; and 1 AIS contained both HPV18 and HPV16 DNA. High-risk human papillomavirus was not detected in any cases of LEGH or LEGH with AIS or GA.
Table 1. Detection of the expression of CA-IX and p16 proteins and H-HPV DNA in CGLs.
| CA-IX | p16 | H-HPV | ||
|---|---|---|---|---|
| Pos (D/F) | Pos (D/F) | Pos (type) | ||
| 12/12 | 11/12 | 10/12 | ||
|
Case no. |
Diagnosis |
(100%) |
(92%) |
(83%) |
| 1 |
AIS |
3+ (D) |
3+ (D) |
Negative |
| 2 |
AIS |
3+ (D) |
0 |
Negative |
| 3 |
AIS |
3+ (D) |
3+ (D) |
Pos (16) |
| 4 |
AIS |
3+ (D) |
3+ (D) |
Pos (16) |
| 5 |
AIS |
3+ (D) |
3+ (D) |
Pos (18) |
| 6 |
AIS |
3+ (D) |
3+ (D) |
Pos (18) |
| 7 |
AIS |
3+ (D) |
3+ (D) |
Pos (18) |
| 8 |
AIS |
3+ (D) |
3+ (D) |
Pos (16; 18) |
| 9 |
Adenocarcinoma |
3+ (D) |
3+ (D) |
Pos (18) |
| 10 |
Adenocarcinoma |
3+ (D) |
3+ (D) |
Pos (18) |
| 11 |
Adenocarcinoma |
3+ (D) |
3+ (D) |
Pos (18) |
| 12 | Adenocarcinoma | 3+ (F) | 3+ (F) | Pos (18) |
Abbreviations: AIS=adenocarcinoma in situ; CA-IX=carbonic anhydrase-IX; CGL=conventional glandular lesion; H-HPV=high-risk human papillomavirus; Neg=negative; Pos=positive; D=diffuse positive (⩾50% of cells); F=focal; (16;18)=HPV type.
Table 2. Detection of expression of CA-IX and p16 proteins and H-HPV DNA in cases of LEGH, and well-differentiated mucinous GA.
| CA-IX | p16 | H-HPV | |||
|---|---|---|---|---|---|
| Pos (D/F) | Neg | Pos (D/F) | |||
| Case no. | Diagnosis | 13/13 | 5/13 | 8/13 | 0/13 |
| (100%)a | (38%) | (62%)a | |||
| 1 |
LEGH |
2+ (F) |
— |
|
None |
| 2 |
LEGH+atypia |
2+ (D) |
— |
|
None |
| 3 |
LEGH+atypia |
3+ (D) |
|
1+ (F) |
None |
| 4 |
LEGH+atypia |
3+ (F) |
|
1+ (F) |
None |
| 5 |
LEGH+atypia |
2+ (F) |
— |
|
None |
| 6 |
LEGH+atypia |
3+ (D) |
|
1+ (F) |
None |
| 7 |
LEGH+atypia |
2+ (D) |
|
1+ (F) |
None |
| 8 |
LEGH+AIS |
3+ (D) |
— |
|
None |
| 9 |
LEGH+AIS |
3+ (D) |
|
1+ (F) |
None |
| 10 |
LEGH+AIS |
2+ (F) |
— |
|
None |
| 11 |
LEGH+GA |
2+ (D) |
|
1+ (F) |
None |
| 12 |
LEGH+GA |
3+ (D) |
|
1+ (F) |
None |
| 13 | LEGH+GA | 3+ (D) | 1+ (F) | None |
Abbreviations: AIS=adenocarcinoma in situ; CA-IX=carbonic anhydrase-IX; D=diffuse (positivity in ⩾50% of cells); F=focal; GA, adenocarcinoma with gastric phenotype; H-HPV=high-risk human papillomavirus; LEGH=lobular endocervical glandular hyperplasia.
The immunoreactivity of p16-positive cases was weak (1+) and focal. In contrast, CA-IX positivity was always intense (2+, 3+).
P16 and CA-IX expression
Carbonic anhydrase-IX immunoreactivity in the tissue sections was observed in 100% (12 out of 12) of CGLs and p16 in 92% (11 out of 12). With the exception of one poorly differentiated adenocarcinoma, the positive staining for both CA-IX and p16 was intense (2+ to 3+) and diffuse (Figure 2B and C). Carbonic anhydrase-IX expression was also seen in all 13 cases of LEGH with or without AIS or GA. Carbonic anhydrase-IX expression in those glands without typical features was limited to central larger glands (Figure 1B). In contrast, in those cases of LEGH with atypical features diffuse CA-IX immunoreactivity was seen, not only limited to the central larger glands but also in those glands with atypical cytological features (Figure 3B and E) and the glands with papillary configuration (Figure 3H). For those cases of AIS or GA with coexisting LEGH, all malignant glands exhibited diffuse strong CA-IX immunoreactivity (Figure 2E). In contrast, only 62% (8 out of 13) of LEGH, LEGHs with atypia and LEGHs with AIS or GA expressed p16, and the immunoreactivity in all cases was either negative (Figure 2F) or very weak cytoplasmic staining (Figure 3C, F and I). For the negative control group, CA-IX expression was either negative or limited to few reserve cells or columnar cells. Furthermore, the intensity of staining was weak and cytoplasmic (Figure 2G). These findings are consistent with our previous observation (Liao et al, 1994). Carbonic anhydrase-IX expression was also tested on exfoliative cervical cells in PS from all cases of CGL, LEGH, LEGH with atypia and LEGH with coexisting AIS or GA. With the exception of one LEGH without atypia, diffuse strong immunopositive staining was seen in exfoliative endocervical glandular cells in all cases (data not shown and Figure 2H and I).
Discussion
It is widely accepted that H-HPV infection has a crucial role in the aetiology of cervical carcinoma. Almost all cases of squamous cell carcinomas and the vast majority of adenocarcinomas were reported to contain H-HPV genotypes. However, in our recent study, conducted on Japanese women with a cytological diagnosis of AGC, H-HPV infection was identified in only 53% of cervical adenocarcinomas (Liao et al, 2010). In this study, retrospective review of the histology found that 13 of 15 H-HPV-negative cases exhibited histological features of LEGH with or without the coexisting AIS or GA. When these special subsets of glandular lesions are excluded, the prevalence of H-HPV DNA detection in CGLs in this study was 83% (10 out of 12), which falls within the range of 83–100% reported in the literature (Pirog et al, 2000). In the case of the two H-HPV-negative AIS (see Table 1), one exhibited gastric morphology without associated destructive stromal reaction. This raises the possibility that this lesion may represent in situ GA, although this has not been definitively proven. Interestingly, this gastric-type like AIS was also p16 negative. The other HPV-negative case had histological features of conventional AIS, but the lesion in this case was limited to two glands. Thus, the negative result in this case may be due to insufficient H-HPV DNA load. Furthermore, this explanation is supported by the observation of diffuse expression of the p16 protein, a biomarker strongly correlated with H-HPV infection.
LEGH is a rare lesion of the uterine cervix that usually occurs in postmenopausal women, with a reported incidence of 0.7% (Mikami et al, 2001). In this study, we identified 13 out of 84 (15%) of LEGHs and, among these, 6 (46%) had coexisting AIS or GA. The rate of LEGH and LEGH associated with AIS or GA in our study is higher than that reported in the literature (Mikami et al, 2001; Nara et al, 2007) and this discrepancy may be attributed to our focus on the selection of cases where only women with a cytological diagnosis of AGC were included. All 13 cases, and one AIS without associated LEGH, reported here exhibited gastric phenotype, which was further supported by positive immunostaining of the lesions with anti-MUC-6 antibody, a marker of pyloric glands (data not shown). None of these cases contained H-HPV DNA or expressed high levels of p16 protein. As illustrated in Figure 3, variable degrees of atypical features were observed in all LEGHs but one, and the expression of CA-IX protein appears to be closely related to the atypia. Our findings appear to support the notion that LEGH and GA are closely related lesions and arise independently of H-HPV infection. Similar H-HPV-negative cervical lesions have been recently reported that also exhibit special histological features described as LEGH, MDA, mucinous GA, mesonephric and clear-cell carcinoma. Thus, it is possible that separate and distinct molecular genetic events, independent of H-HPV infection, may have a role in the progression to the malignant condition in this category of cervical glandular lesions compared with CGLs.
Our previous studies have shown the diagnostic utility of CA-IX in detecting CGLs in patients with a cytological diagnosis of AGC. In this study, we show that practically all of the exfoliative endocervical glandular cells derived from the glandular cells of all cases of LEGH with atypia, AIS and adenocarcinoma of usual type or gastric phenotype showed diffuse and strong CA-IX immunoreactivity. Correspondingly, high levels of CA-IX expression were also identified in the tissue sections of all cases tested in the study. This novel finding has a significant clinical implication. It has been reported that most cases of GA, including MDA, are diagnosed at an advanced clinical stage and carry a poor prognosis (Gilks et al, 1989; Kojima et al, 2007). The vast majority of cases of GA was H-HPV negative and showed the absence or low levels of p16 expression. Thus, CA-IX protein expression in both exfoliative cells and tissue sections appears to serve as a useful biomarker for diagnosing LEGH-related lesions or the gastric phenotype neoplasms. Confirmation of this possibility awaits further analyses with more cases. Significant CA-IX expression in LEGHs with atypia and without associated carcinoma is an intriguing finding. Molecular genetic analyses of these cases should enable resolution of the speculation that LEGH may be a precursor of GA. Another area that warrants special consideration is the identification of the molecular genetic defects and aberrant signalling pathways that exist in this category of cervical glandular lesions as compared with the CGLs that are associated with H-HPV infection. High-risk human papillomavirus E6 and E7 products effectively abrogate the p53 and Rb tumour suppressor functions. Are these genes mutated in LEGH and/or GA cells or are other pathways operating that effectively bypass these powerful tumour suppressor functions?
In conclusion, our study has supported the following claims: (1) H-HPV DNA, p16 and CA-IX protein expression are useful biomarkers for detecting CGLs; (2) the subset of cervical lesions that encompasses LEGH with coexisting AIS or GA arise independently of H-HPV infection; (3) CA-IX may serve as the optimal surrogate biomarker for identifying this subset of cervical neoplasm with gastric phenotype; (4) the spectrum of histological changes seen in LEGH, LEGH with atypia and LEGH with AIS or GA suggests there is a possible link between LEGH and mucinous GA; and (5) the high rate (46%) of LEGHs associated with AIS and GA observed in this study warrants special attention being paid to identify LEGH with atypia in those women with an AGC cytological diagnosis.
Acknowledgments
We thank Kim Blaser, Jan Barnes and Amy Speaker for their assistance in preparing this manuscript and coordinating the clinical and pathology data collection. We also thank the GOG Publications Subcommittee for their critical review of the manuscript. This study was supported by National Cancer Institute grants to the Gynaecologic Oncology Group (GOG) Administrative Office (CA 27469), the GOG Tissue Bank (CA 11479) and GOG Statistical and Data Centre (CA 37517). Funding was also provided by a supplemental award from the Division of Centre Prevention at the National Cancer Institute (GOG38886). The following member institutions participated in this study: Jikei University School, Kagoshima City Hospital, Keio University, Kinki University, National Kyushu Cancer Centre, Sapporo Medical University, Tottori University, Kobe Medical Centre, Shikoku Cancer Centre, Tohoku University Medical School and Kawasaki Medical School.
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Bosch FX, Burchell AN, Schiffman M, Giuliano AR, de Sanjose S, Bruni L, Tortolero-Luna G, Kjaer SK, Munöz N. Epidemiology and natural history of human papillomavirus infections and type specific implications in cervical neoplasia. Vaccine. 2008;26 (Suppl 10:K17–K28. doi: 10.1016/j.vaccine.2008.05.064. [DOI] [PubMed] [Google Scholar]
- Bray F, Carstensen B, Møller H, Zappa M, Žakelj MP, Lawrence G, Hakama M, Weiderpass E. Incidence trends of adenocarcinoma of the cervix in 13 European countries. Cancer Epidemiol Biomarkers Prev. 2005;14:2191–2199. doi: 10.1158/1055-9965.EPI-05-0231. [DOI] [PubMed] [Google Scholar]
- Gilks CB, Young RH, Aguirre P, DeLellis RA, Scully RF. Adenoma malignum (minimal deviation adenocarcinoma) of the uterine cervix: a clinicopathological and immunochemical analysis of 26 cases. Am J Surg Pathol. 1989;13:717–729. doi: 10.1097/00000478-198909000-00001. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Yamashita T, Ishida S, Nishikawa A, Fujinaga Y, Kudo R, Fujinaga K. Detection and typing of genital high-risk HPV DNAs in cervical scrapes using the E6/E7-specific consensus PCR. Tumor Res. 1995;30:1–19. [Google Scholar]
- Ishii K, Hosaka N, Toki T, Momose M, Hidaka E, Tsuchiya S, Katsuyama T. A new view of the so called adenoma malignum of the uterine cervix. Virchows Arch. 1998;432:315–322. doi: 10.1007/s004280050172. [DOI] [PubMed] [Google Scholar]
- Ivanov S, Liao SY, Ivanova A, Danilkovitch-Miaqkova A, Tarasova N, Weirich G, Merrill MJ, Proescholdt MA, Oldfield EH, Lee J, Zavada J, Waheed A, Sly W, Lerman MI, Stanbridge EJ. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol. 2001;158:905–919. doi: 10.1016/S0002-9440(10)64038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi S, Kusuda T, Liu XP, Suehiro Y, Kaku T, Mikami Y, Takeshita M, Nakao M, Chochi Y, Sasaki K. Is lobular endocervical glandular hyperplasia a cancerous precursor of minimal deviation adenocarcinoma? A comparative molecular-genetic and immunohistochemical study. Am J Surg Pathol. 2008;32:1807–1815. doi: 10.1097/PAS.0b013e3181883722. [DOI] [PubMed] [Google Scholar]
- Klaes R, Friedrich T, Spitkovsky D, Ridder R, Rudy W, Petry U, Dallenbach-Hellweg G, Schmidt D, Doeberitz MVK. Overexpression of p16INK4A as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int j Cancer. 2001;92:276–284. doi: 10.1002/ijc.1174. [DOI] [PubMed] [Google Scholar]
- Kojima A, Mikami Y, Sudo T, Yamaguchi S, Kusangi Y, Ito M, Nishimura R. Gastric morphology and immunophenotype predict poor outcome in mucinous adenocarcinoma of the uterine cervix. Am J Surg Pathol. 2007;31:664–672. doi: 10.1097/01.pas.0000213434.91868.b0. [DOI] [PubMed] [Google Scholar]
- Kurman RJ, Solomon D. The Bethesda System: Terminology for Reporting Cervical/Vaginal Cytologic Diagnoses: Definitions, Criteria and Explanatory Notes for Terminology and Specimen Adequacy. Springer: New York; 1994. [Google Scholar]
- Kusanagi Y, Kojima A, Mikami Y, Kiyokawa T, Sudo T, Yamaguchi S, Nishimura R. Absence of high-risk human papillomavirus (HPV) detection in endocervical adenocarcinoma with gastric morphology and phenotype. Am J Pathol. 2010;177:2169–2175. doi: 10.2353/ajpath.2010.100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao SY, Brewer C, Závada J, Pastorek J, Pastorekova S, Manetta A, Berman ML, DiSaia PJ, Stanbridge EJ. Identification of the MN antigen as a diagnostic biomarker of cervical intraepithelial squamous and glandular neoplasia and cervical carcinoma. Am J Pathol. 1994;145:598–609. [PMC free article] [PubMed] [Google Scholar]
- Liao SY, Stanbridge EJ. Expression of the MN antigen in cervical Papanicolaou smears is an early diagnostic biomarker of cervical dysplasia. Cancer Epidemiol Biomarkers Prev. 1996;5:549–557. [PubMed] [Google Scholar]
- Liao SY, Aurelio ON, Jan K, Zá.vada J, Stanbridge EJ. Identification of the MN/CA9 protein as a reliable diagnostic biomarker of clear cell carcinoma of the kidney. Cancer Res. 1997;57:2827–2831. [PubMed] [Google Scholar]
- Liao SY, Rodgers WH, Kauderer J, Bonfiglio TA, Walker JL, Darcy KM, Cater R, Hatae M, Levine L, Spirtos NM, Stanbridge EJ. Carbonic anhydrase IX (CA-IX) and human papillomavirus (HPV) as diagnostic biomarkers of cervical dysplasia/neoplasia in women with a cytologic diagnosis of atypical glandular cells (AGC): a Gynecologic Oncology Group (GOG) Study in United States. Int J Cancer. 2009;25:2434–2440. doi: 10.1002/ijc.24615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao SY, Stanbridge EJ. Expression of MN/CA9 protein in Papanicolaou smears containing atypical glandular cells of undetermined significance is a diagnostic biomarker of cervical dysplasia and neoplasia. Cancer. 2000;88:1108–1121. doi: 10.1002/(sici)1097-0142(20000301)88:5<1108::aid-cncr23>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Liao SY, Rodgers WH, Kauderer J, Bonfiglio TA, Darcy KM, Carter R, Levine L, Spirtos NM, Susumu N, Fujiwara K, Walker JL, Hatae M, Stanbridge EJ. Carbonic anhydrase IX (CA-IX) and high-risk human papillomavirus (H-HPV) as diagnostic biomarkers of cervical dysplasia/neoplasia in Japanese women with a cytologic diagnosis of atypical glandular cells (AGC): a Gynecologic Oncology Group (GOG) Study. Br J Cancer. 2010;104:353–360. doi: 10.1038/sj.bjc.6606049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lungu O, Wright TC, Silverstein S. Typing of human papillomaviruses by polymerase chain reaction amplification with L1 consensus primers and RFLP analysis. Mol Cell Probes. 1992;6:145–152. doi: 10.1016/0890-8508(92)90059-7. [DOI] [PubMed] [Google Scholar]
- Makino H, Sato S, Yajima A, Komatsu S, Fukao A. Evaluation of the effectiveness of cervical cancer screening: a case–control study in Miyagi, Japan. Tohoku J Exp Med. 1995;175:171–178. doi: 10.1620/tjem.175.171. [DOI] [PubMed] [Google Scholar]
- Mikami Y, Hata S, Melamed J, Fujiwara K, Manabe T. Lobular endocervical glandular hyperplasia is a metaplastic process with a pyloric gland phenotype: Histopathology. 2001;39:364–372. doi: 10.1046/j.1365-2559.2001.01239.x. [DOI] [PubMed] [Google Scholar]
- Mikami Y, Kiyokawa T, Hata S, Fujiwara K, Moriya T, Sasano H, Manabe T, Akahira J-I, Ito K, Tase T, Yaegashi N, Sato I, Tateno H, Naganuma H. Gastrointestinal immunophenotype in adenocarcinoma of the uterine cervix and related glandular lesions: a possible link between lobular endocervical glandular hyperplasia/pyloric gland metaplasia and ‘adenoma malignum'. Mod Pathol. 2004;17:962–972. doi: 10.1038/modpathol.3800148. [DOI] [PubMed] [Google Scholar]
- Nara M, Hashi A, Murata SI, Kondo T, Yuminnamochi T, Nakazawa K, Katoh R, Hoshi K. Lobular endocervical glandular hyperplasia as a precursor of cervical adenocarcinoma independent of human papillomavirus infection. Gynecol Oncol. 2007;106:289–298. doi: 10.1016/j.ygyno.2007.03.044. [DOI] [PubMed] [Google Scholar]
- Negri G, Egarter-Vigl E, Kasal A, Romano F, Haitel A, Mian C. p16INK4a is a useful marker for the diagnosis of adenocarcinoma of the cervix uteri and its precursors: an immunohistochemical study with immunocytochemical correlations: Am J Surg Pathol. 2003;27:187–193. doi: 10.1097/00000478-200302000-00006. [DOI] [PubMed] [Google Scholar]
- Nucci MR, Clement PB, Young RH. Lobular endocervical glandular hyperplasia, not otherwise specified: a clinicopathologic analysis of thirteen cases of a distinctive pseudoneoplastic lesion and comparison with fourteen cases of adenoma malignum. Am J Surg Pathol. 1999;23:886–891. doi: 10.1097/00000478-199908000-00005. [DOI] [PubMed] [Google Scholar]
- Opavský R, Pastoreková S, Zelník V, Gibadulinová A, Stanbridge EJ, Závada J, Kettmann R, Pastorek J. Human MN/CA9 gene, a novel member of the carbonic anhydrase family: structure and exon to protein domain relationships: Genomics. 1996;33:480–487. doi: 10.1006/geno.1996.0223. [DOI] [PubMed] [Google Scholar]
- Park JK, Kiyokawa T, Soslow RA, Lamb CA, Oliva E, Zivanovic O, Juretzka MM, Pirog EC. Unusual endocervical adenocarcinomas: an immunohistochemical analysis with molecular detection of human papillomavirus. Am J Surg Pathol. 2011;35:633–646. doi: 10.1097/PAS.0b013e31821534b9. [DOI] [PubMed] [Google Scholar]
- Pirog EC, Kleter B, Olgac S, Bobkiewicz P, Lindeman J, Quint WG, Richart RM, Isacson C. Prevalence of human papillomavirus DNA in different histological subtypes of cervical adenocarcinoma. Am J Pathol. 2000;157:1055–1062. doi: 10.1016/S0002-9440(10)64619-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HO, Tiffany MF, Qualls CR, Key CR. The rising incidence of adenocarcinoma relative to squamous cell carcinoma of the uterine cervix in the United States – a 24-year population-based study. Gynecol Oncol. 2000;78:97–105. doi: 10.1006/gyno.2000.5826. [DOI] [PubMed] [Google Scholar]
- Swietach P, Vaughan-Jones RD, Harris AL. Regulation of tumor pH and the role of carbonic anhydrase 9. Cancer Metast Rev. 2007;26:299–310. doi: 10.1007/s10555-007-9064-0. [DOI] [PubMed] [Google Scholar]
- Tavassoli FA, Devilee P.eds (2003World Organization Classification of Tumours. Tumours of the Breast and Female Genital Organs IARC Press: Lyon; 260–279. [Google Scholar]
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, CJLM Meijer, Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer world-wide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Wykoff CC, Beasley NJ, Watson PH, Turner KJ, Pastorek J, Sibtain A, Wilson GD, Turley H, Talks KL, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL. Hypoxia- Inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000;60:7075–7083. [PubMed] [Google Scholar]
- Yamazaki H, Sasagawa T, Basha W, Segawa T, Inoue M. Hybrid capture II and LCR-E7 PCR assays for HPV typing in cervical cytologic samples. Int J Cancer. 2001;94:222–227. doi: 10.1002/ijc.1455. [DOI] [PubMed] [Google Scholar]
- Závada J, Závadová Z, Pastoreková S, Ciampor F, Pastorek J, Zelník V. Expression of MaTu-MN protein in human tumor cultures and in clinical specimens. Int J Cancer. 1993;54:268–274. doi: 10.1002/ijc.2910540218. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]