Abstract
Background:
The aim of this exploratory subgroup analysis of the fluorouracil, oxaliplatin, docetaxel (FLOT)65+ trial was to determine tolerability and feasibility of perioperative chemotherapy in elderly, potentially operable esophagogastric cancer patients.
Methods:
Patients aged ⩾65 with locally advanced esophagogastric adenocarcinoma were randomized to perioperative chemotherapy consisting of four pre- and four postoperative cycles of infusional 5-FU, leucovorin, and oxaliplatin (FLO) without or with docetaxel 50 mg m−2 (FLOT), every 2 weeks.
Results:
Forty-four patients with a median age of 70 years were randomized and 43 patients started preoperative chemotherapy (FLO, 22; FLOT, 21). Thirty-eight (86.4%) patients completed four cycles of preoperative chemotherapy and 32 (74.4%) proceeded to surgery, with 67.4% R0 resections on intent-to-treat analysis (90.1% of the 32 patients who underwent resection). Median overall survival was not reached and median progression-free survival (PFS) was 17.3 months. Compared with the FLO group, the FLOT group showed a trend towards an improved median PFS (21.1 vs 12.0 months; P=0.09), however, associated with increased chemotherapy related toxicity. No perioperative mortality was observed. Postoperative morbidity was observed in 46.9% of patients (FLO, 35.3% FLOT, 60%).
Conclusion:
Neoadjuvant FLO or FLOT may offer a reasonable chance of curative surgery in elderly patients with locally advanced resectable gastroesophageal cancer. However, the increase in side effects with the FLOT regimen and postoperative morbidity should be carefully considered when an intensive chemotherapy regimen is planned.
Keywords: elderly patients, FLOT, FLO, neoadjuvant chemotherapy, gastroesophageal cancer
Only 50–60% of patients with newly diagnosed gastroesophageal cancer are suitable candidates for radical surgery with curative intent (van de Velde and Peeters, 2003). However, even despite potentially curative resections, long-term survival of these patients remains poor due to a high relapse rate after surgery (Briasoulis et al, 2006).
Several randomized studies demonstrated that perioperative chemotherapy provides a survival benefit over surgery alone and should be considered the standard of care in potentially operable gastroesophageal cancer (Cunningham et al, 2006; Ychou et al, 2011), however, 5-year survival rates are still only in the range of 35–45% (Cunningham et al, 2006; Ychou et al, 2011).
As a result of increased life expectancy, there are a constantly growing proportion of elderly patients with GI tract cancer. Elderly patients may show high incidence of comorbidity and have age-associated physical problems, which are sufficient reasons to withhold combination chemotherapy. In most countries, 65 or 70 years of age is a commonly used limit, however, the most appropriate definition of ‘elderly' is still a matter of debate. The age cutoff varies among studies and patients are usually stratified by age (Cunningham et al, 2006; Van Cutsem et al, 2006). However, many experts favour a more functional definition based on patient's functional health status or comorbidities.
Gastroesophageal cancer is the fourth most frequent malignant disease and cause of cancer death, predominantly occurring in patients who are older than 65 years of age (El-Serag, 2002). Therefore, it becomes increasingly more important to understand how best to treat elderly patients.
Particular concerns have emerged regarding the use of neoadjuvant treatment in elderly patients, who are candidates to a major surgical procedure, such as esophagectomy. Nevertheless, in this older population, little is known about the real potential risks and benefits of treatment, especially regarding the investigational use of a taxan-based chemotherapy regimen and its influence on subsequent surgery. The MAGIC trial stratified patients by age, including around 36% of patients age 60–69 years and around 20% of patients >70 years. The authors report that there was no clear evidence of heterogeneity of treatment effect depending on age (P=0.43), unfortunately they don't specify on feasibility (Cunningham et al, 2006).
Studies on the effects of cytoreductive chemotherapy in esophagogastric cancer often exclude patients with an age ⩾65 or ⩾70 years due to the assumed high risk of aggressive multimodality treatments (Hutchins et al, 1999). Interestingly, despite the use of aggressive approaches, there hasn't been any report of significant increase in perioperative morbidity and mortality, whatever the multimodal approach (Kelley et al, 2004; Lin et al, 2004; Cunningham et al, 2006).
In the metastatic setting, improved efficacy in terms of response rate and overall survival (OS) have been reported with the addition of docetaxel to cisplatin- and 5-fluorouracil-based combinations, although therapy-associated toxicity increases (Van Cutsem et al, 2006). Previous studies could demonstrate that a docetaxel-containing chemotherapy regimen is also an effective and tolerable treatment option in the neoadjuvant setting (Lorenzen et al, 2007; Biffi et al 2010; Sym et al, 2010; Homann et al, 2012; Thuss-Patience et al, 2012) where high anti-tumour activity, resulting in effective downstaging is required. In particular, neoadjuvant taxan-containing regimens can achieve promising complete pathological response rates (pCR) between 12 and 18% (Lorenzen et al, 2007; Biffi et al 2010; Homann et al, 2012), which is a known prognostic marker as patients with a pCR tend to have a much better outcome as underlined in recent studies (Ajani et al, 2006; Homann et al, 2012; Fields et al, 2012). Moreover, substitution of oxaliplatin for cisplatin has proven to be more tolerable and has shown improved efficacy in an exploratory subgroup analysis in patients aged ⩾65 years with metastatic gastroesophageal adenocarcinoma (Al-Batran et al, 2008b).
The aim of this trial was to gain a better understanding of the feasibility and potential benefit of perioperative chemotherapy with either oxaliplatin, fluorouracil (FLO) or fluorouracil, oxaliplatin, docetaxel (FLOT) on postoperative mortality and morbidity, quality of life (QoL) and efficacy in elderly (⩾65 years) patients undergoing esophagectomy or gastrectomy for esophagogastric cancer.
Materials and methods
This study is a predefined exploratory subgroup analysis of elderly patients with locally advanced, potentially resectable adenocarcinoma of the esophagogastric junction and the stomach that were included as a prospective stratum in the randomized phase II FLOT 65+ trial (Al-Batran et al, 2012). This study was registered at ClinicalTrials.gov, identifier NCT 00737373. All participants gave written informed consent, which was approved by the ethics committees of the participating institutions.
Eligibility criteria
Eligibility criteria included: Patients aged ⩾65 years with histologically confirmed and measurable locally advanced, potentially resectable (defined as clinical stages ⩾T3 or N+ as determined by CT scans and endoscopic ultrasound) adenocarcinoma of the stomach or esophagogastric junction; no prior chemotherapy; an Eastern Cooperative Oncology Group performance status 0–2; sufficient bone marrow and kidney function, and no concurrent uncontrolled medical illness.
Exclusion criteria included: second malignancy; uncontrolled infection and neuropathy grade>1. All patients gave written informed consent.
Chemotherapy
Patients were randomly assigned to receive oxaliplatin 85 mg m−2, leucovorin 200 mg m−2, and docetaxel 50 mg m−2, each as an IV infusion followed by 5-FU 2600 mg m−2 as a 24-h continuous infusion (FLOT) or the same regimen without docetaxel (FLO). Treatment was administered on day one of two weekly cycles. Antiemetic prophylaxis was given according to local protocols. Patients received four cycles of preoperative FLO or FLOT followed by surgery and then four postoperative cycles of the initial regimen.
Toxicity assessment
Toxic effects were graded according to NCI-CTC version 3.0. Peripheral sensitive neuropathy was graded according to an oxaliplatin-specific scale (Caussanel et al, 1990). The safety analysis included all treatment-emergent adverse events, and those regardless of causality.
Surgery
Surgery was scheduled 3–4 weeks after completion of the last cycle of preoperative chemotherapy. The type of surgical procedure was determined by the location of the primary tumour and was performed according to the local standards. Surgery consisted in a complete excision of the tumour with an extended D2 lymphadenectomy, according to the rules of the Japanese Research Society of Gastric Cancer (Japanese Gastric Cancer A, 1998).
Quality of life assessment
Quality of life was evaluated using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-C30 (EORTC QLQ C30). QoL was assessed after randomisation within seven days before the first cycle and at 8 (before surgery), 16, and 24 weeks thereafter. According to EORTC guidelines, patients completed the QoL questionnaires before the tumour assessment was performed.
Efficacy evaluation
Responses were classified according to the World Health Organisation criteria (Miller et al, 1981). Clinical staging consisted of endoscopy including endoluminal ultrasound and computed tomography scans of the chest abdomen and pelvis, which were carried out within 3 weeks before the start of treatment and after preoperative chemotherapy. R0 resection was defined as no tumour identified on microscopic examination of proximal, distal, or circumferential margins. To evaluate downstaging of tumour after preoperative chemotherapy, the pathologic stage was compared with the endosonographic stage before treatment.
Patients who had ended treatment but had not experienced disease progression were observed every 8 weeks until progressive disease and every 3 months thereafter. Progression-free survival (PFS) was measured from the date of randomisation until disease progression or death of any cause. Overall survival was measured from date of randomisation until death of any cause.
Statistical analysis
The analysis was exploratory. Differences in proportions of patients were analysed by the Fischer's exact test or X2-test. Survival rates were estimated according to Kaplan–Meier. Statistical comparisons between the different groups of patients were performed with a log-rank test and the proportional hazard model. Continuous data were expressed as mean and s.d. or median and interquartile range. Continuous data were compared between FLO and FLOT groups using Student t-test and Mann–Whitney test. All tests are two-sided and are performed at the 5% level of significance by using SPSS 16.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
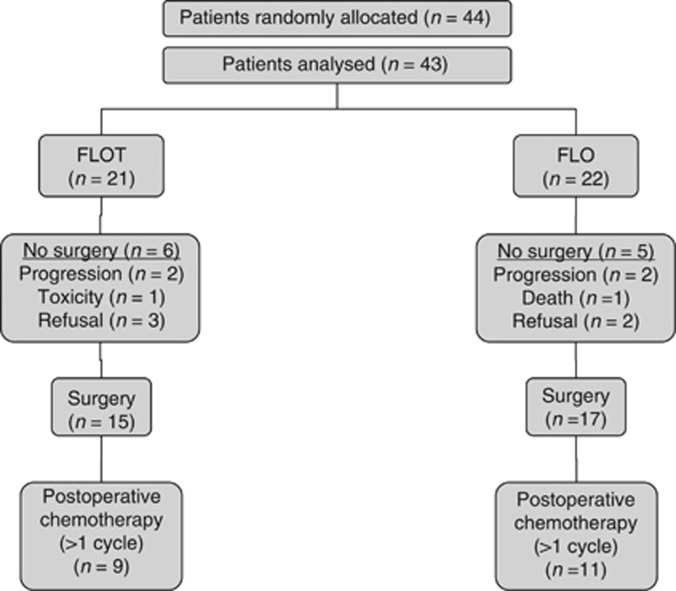
Between February 2007 and October 2008, 44 patients were enrolled from 13 institutions in Germany into the locally advanced, potentially operable stratum of the FLOT 65+ study. Twenty-two patients were randomly assigned to FLO, and 22 patients to FLOT chemotherapy. One patient in the FLOT was excluded from the analysis because of consent withdrawal before study treatment. A trial profile, conforming to the Consolidated Standards of Reporting Trials is shown in Figure 1. The median age of the patient sample was 70 years (maximum 82 years). Patient's tumour and treatment characteristics are summarised in Table 1.
Figure 1.
Consolidated Standards of Reporting Trials diagram.
Table 1. Patient, tumour and treatment characteristics.
|
FLO |
FLOT |
Total |
||||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| No. of patients |
22 |
|
21 |
|
43 |
|
| Median age, years (IQR) |
71.5 (70–76) | 69 (67–72) |
70 (68–75) |
|||
|
Gender |
||||||
| Male/female |
14/8 |
64/36 |
15/6 |
71/29 |
29/14 |
67/33 |
|
ECOG status, median |
||||||
| 0 | 7 | 32 | 6 | 29 | 13 | 30 |
| 1 | 13 | 59 | 14 | 67 | 27 | 63 |
| 2 |
2 |
9 |
1 |
5 |
3 |
7 |
|
Site of tumour |
||||||
| Lower esophagus | 4 | 18 | 4 | 19 | 8 | 19 |
| Oesophagogastric junction | 5 | 23 | 9 | 43 | 14 | 33 |
| Stomach |
13 |
59 |
8 |
38 |
21 |
49 |
|
Clinical stagea |
||||||
| uT1/2 | 3 | 14 | 0 | 0 | 3 | 7 |
| uT3/4 | 19 | 86 | 21 | 100 | 40 | 93 |
| N0 | 1 | 5 | 2 | 10 | 3 | 7 |
| N+ |
21 |
95 |
19 |
91 |
40 |
93 |
|
Tumour grading |
||||||
| Well differentiated | 1 | 5 | 1 | 5 | 2 | 5 |
| Moderately differentiated | 9 | 41 | 12 | 57 | 21 | 49 |
| Poorly differentiated | 9 | 41 | 7 | 33 | 16 | 37 |
| Unknown |
3 |
14 |
1 |
5 |
4 |
9 |
|
Number of preoperative chemotherapy cycles (median); IQR |
4 (4–4) |
4 (4–4) |
4 (4–4) |
|||
| Number of postoperative chemotherapy cycles (median); IQR | 2 | 2–4 | 3 | 0–4 | 2 | 0–4 |
Abbreviations: CT=computerized tomography; ECOG=Eastern Cooperative Oncology Group; FLO=oxaliplatin, fluorouracil; FLOT=fluorouracil, oxaliplatin, docetaxel; IQR=interquartile range.
As determined by endoscopic ultrasound and CT scan.
Treatment
Forty-three patients started preoperative chemotherapy and thirty-eight (88.4%) patients completed four cycles of preoperative chemotherapy (FLO, 20 out of 22 (90%); FLOT, 18 out of 21 (85%)) of whom 32 patients proceeded to surgery. The reasons for not completing four preoperative cycles are as follows: progressive disease (2) in the FLO, and progressive disease (2) and toxic effects (1) in the FLOT group.
Among the 43 patients assigned to receive perioperative chemotherapy 20 (46.5%) subsequently began postoperative chemotherapy (FLO, 11; FLOT, 9). Reasons for not starting postoperative chemotherapy were for the FLO regimen: disease progression (two patients), toxicity of preoperative chemotherapy (one patient), and unknown (three patients), and for the FLOT regimen: patient's choice (two patients), postoperative complications (one patient), lack of response to preoperative therapy (one patient), worsening coexisting disease (1 patient), and unknown (one patient). Eleven out of 43 patients (25.6%) randomly assigned to perioperative chemotherapy completed all eight cycles of chemotherapy, 6 out of 22 (27%) in the FLO arm and 5 out of 21 (24%) in the FLOT arm. Table 2 shows the feasibility results of the study.
Table 2. Feasibility; treatment cycles delivered.
|
Preoperative (n=43) |
Postoperative (n=43) |
|||
|---|---|---|---|---|
| Chemotherapy fully evaluable patients (ITT) | FLO (n=22) | FLOT (n=21) | FLO (n=22) | FLOT (n=21) |
|
Cycles received | ||||
| 4 | 90% | 85% | 27% | 24% |
| 3 | 5% | 10% | — | 14% |
| 2 | — | — | 14% | 5% |
| 1 | 5% | 5% | 9% | — |
| 0 |
— |
— |
27% |
29% |
|
Percentage of intended dose delivered (per evaluable patient, ITT)a | ||||
| Docetaxel | — | 100% | — | 68.5% |
| Oxaliplatin | 100% | 98.5% | 74.7% | 50.3% |
| 5-Fluorouracil | 100% | 100% | 75.0% | 75.1% |
Abbreviations: FLO=oxaliplatin, fluorouracil; FLOT=fluorouracil, oxaliplatin, docetaxel; ITT=intention-to-treat.
The sum of the planned doses for all planned cycles compared with the sum of the delivered doses for all 43 patients.
Overall, dose modifications of the chemotherapeutic agents due to grade 3/4 toxicity were performed in 3 out of 22 patients in the FLO arm (13.6%) and 10 out of 21 patients (47.6%) in the FLOT arm (P=0.023). During preoperative treatment, at least one dose attenuation to <80% of the initial dose was required in 1 out of 22 (4.5%) patients treated with FLO and in 9 out of 21 (42.9%) patients treated with FLOT (P=0.0039), including reductions for both docetaxel and oxaliplatin in 3 and 14 patients (13.6% and 66.7%) and fluorouracil in 0 and 4 patients (0% and 19%) for the FLO and FLOT arm, respectively. After surgery, there was no clinically significant increase in grade 3 or grade 4 toxic effects associated with the chemotherapy regimen. No difference between the rate of dose reductions in the FLO arm (2 out of 11 patients; 18%) vs the FLOT arm (2 out of 9 patients; 22%) could be detected (P=1.0).
Safety
Overall, FLOT was associated with significantly more NCI-CTC grade 3 or 4 leucopenia (P=0.0002), neutropenia (P=0.0002), mucositis (P=0.03), nausea (P=0.012) and any grade alopecia (P=0.013). The frequencies of treatment-related adverse events for pre- and postoperative chemotherapy are given in Table 3a and b, respectively. During preoperative chemotherapy, the most frequent grade 3/4 adverse events were: neutropenia (20.9%), leucopenia (16.3%), infection (14%), nausea (14%) sensory neuropathy (16%), and mucositis (7%). More patients treated with preoperative chemotherapy experienced treatment-related grade 3/4 adverse events in the FLOT arm (FLOT, 85.7% FLO, 27.3% P=0.0002). FLOT was associated with significantly more grade 3 or 4 neutropenia (P<.0001), leukopenia (P<.0001), stomatitis (P=0.02), and nausea (P=0.002), as well as a slight increase in complicated neutropenia (neutropenic infection: 9.5% vs 0% P=0.07), however, there was no difference in diarrhoea (4.8% and 4.5%) between the FLO and FLOT arm, respectively (Table 3a).
Table 3. (a) Adverse effects associated with preoperative chemotherapy; (b) Adverse effects associated with postoperative chemotherapy.
| FLO (n=22) | FLOT (n=21) | Total (n=43) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Grade 1/2 |
Grade 3/4 |
Grade 1/2 |
Grade 3/4 |
Grade 1/2 |
Grade 3/4 |
|
||||||
| Adverse event | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | P-valuea |
|
(a) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Haematologic | |||||||||||||
| Leucopenia | 8 | 36.4 | 9 | 42.9 | 7 | 33.3 | 17 | 39.5 | 7 | 16.3 | <0.0001 | ||
| Neutropenia | 7 | 31.8 | 1 | 4.5 | 5 | 23.8 | 8 | 38.1 | 12 | 27.9 | 9 | 20.9 | <0.0001 |
| Thrombocytopenia | 7 | 31.8 | 8 | 38.1 | 15 | 34.9 | 0.37 | ||||||
| Anaemia | 19 | 86.4 | 1 | 4.5 | 12 | 57.1 | 31 | 72.1 | 1 | 2.3 | 0.46 | ||
| Infection | 4 | 18.2 | 2 | 9.5 | 6 | 28.6 | 6 | 14.0 | 6 | 14.0 | 0.05 | ||
| Fever |
2 |
9.1 |
|
|
6 |
28.5 |
|
|
8 |
18.6 |
|
|
0.07 |
|
Non-haematologic | |||||||||||||
| Nausea | 16 | 72.7 | 1 | 4.5 | 16 | 76.2 | 5 | 23.8 | 32 | 74.4 | 6 | 14.0 | 0.002 |
| Vomiting | 6 | 27.3 | 1 | 4.5 | 8 | 38.1 | 2 | 9.5 | 14 | 32.6 | 3 | 7.0 | 0.48 |
| Fatigue | 15 | 68.2 | 13 | 61.9 | 3 | 14.3 | 28 | 65.1 | 3 | 7.0 | 0.13 | ||
| Diarrhoea | 8 | 36.4 | 1 | 4.5 | 14 | 66.7 | 1 | 4.8 | 22 | 51.2 | 2 | 4.7 | 0.15 |
| Mucositis | 7 | 31.8 | 9 | 42.9 | 3 | 14.3 | 16 | 37.2 | 3 | 7.0 | 0.02 | ||
| Constipation | 8 | 36.4 | 5 | 23.8 | 13 | 30.2 | 0.37 | ||||||
| Sensory neuropathy | 15 | 68.2 | 3 | 13.6 | 11 | 52.4 | 4 | 19.0 | 26 | 60.5 | 7 | 16.3 | 0.60 |
| Fluid retention | 3 | 13.6 | 1 | 4.8 | 4 | 9.3 | 0.95 | ||||||
|
(b) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Haematologic | |||||||||||||
| Leucopenia | 5 | 45.5 | 4 | 44.4 | 1 | 11.1 | 9 | 45.0 | 1 | 5.0 | 0.25 | ||
| Neutropenia | 4 | 36.4 | 1 | 9.1 | 2 | 22.2 | 4 | 44.4 | 6 | 30.0 | 5 | 25.0 | 0.11 |
| Thrombocytopenia | 2 | 18.2 | 1 | 11.1 | 3 | 15.0 | 0.86 | ||||||
| Anaemia | 11 | 100 | 9 | 100 | 20 | 100 | 0.20 | ||||||
| Infection | 1 | 9.1 | 1 | 9.1 | 1 | 11.1 | 2 | 10.0 | 1 | 5.0 | 0.54 | ||
| Fever |
3 |
27.3 |
|
|
1 |
11.1 |
|
|
4 |
20 |
|
|
0.29 |
|
Non-haematologic | |||||||||||||
| Nausea | 7 | 63.6 | 4 | 44.4 | 1 | 11.1 | 11 | 55.0 | 1 | 5.0 | 0.87 | ||
| Vomiting | 4 | 36.4 | 3 | 33.3 | 7 | 35.0 | 0.56 | ||||||
| Fatigue | 8 | 72.8 | 1 | 9.1 | 5 | 55.6 | 1 | 11.1 | 13 | 65 | 2 | 10.0 | 0.17 |
| Diarrhoea | 4 | 36.4 | 2 | 22.2 | 1 | 11.1 | 6 | 30 | 1 | 5.0 | 0.43 | ||
| Mucositis | 1 | 9.1 | 1 | 11.1 | 2 | 22.2 | 2 | 10.0 | 2 | 10.0 | 0.17 | ||
| Constipation | 1 | 9.1 | 1 | 9.1 | 1 | 11.1 | 2 | 10.0 | 1 | 5.0 | 0.37 | ||
| Sensory neuropathy | 8 | 72.7 | 4 | 44.4 | 12 | 60.0 | 0.23 | ||||||
| Fluid retention | |||||||||||||
Abbreviation: FLO=oxaliplatin, fluorouracil; FLOT=fluorouracil, oxaliplatin, docetaxel.
Fisher's exact test was used. P-values are related to the groupings grade 0–2 vs grade 3–4 for FLO vs FLOT.
As with preoperative chemotherapy, patients treated with postoperative chemotherapy experienced more treatment-related grade 3/4 adverse events in the FLOT arm (FLOT, 88.9%) compared with the FLO arm (27.3% P=0.001). During postoperative treatment, the most common treatment-related grade 3/4 adverse events were neutropenia (25%), mucositis (10%), and fatigue (10%); however, the difference between both treatment arms was not statistically significant (Table 3b).
Regarding pre- and postoperatively administered chemotherapy, similar rates of serious adverse events were observed among patients in both the treatment arms (FLO, 40.9% FLOT, 47.6%). No toxic death with suspected relation to study treatment was observed with either chemotherapy regimen.
Quality of life analysis
There were no differences regarding QoL scores between treatment arms.
In the group of 43 operable patients, assessable QoL questionnaires were available for 36 patients at baseline, for 33 patients at 8 weeks, for 12 patients at 16 weeks, and for 3 patients at 24 weeks, and were similar in both the arms. Owing to the low number of assessable patients at 16- and 24 weeks, QoL global health status scores (means±s.d.) were only reported for baseline and for the time point at 8 weeks showing 56.3±16.8 and 45.8±19.2, for FLOT and 56.3±24.6 and 58.9±20.6, for FLO (P=0.07). The proportion of patients with a moderate to large (⩾10-points) deterioration of QoL global health status scores during the first 8 weeks of treatment was significantly higher with FLOT (54%) compared with FLO (18% P=0.045).
Response to preoperative chemotherapy
All patients were evaluated for response to preoperative chemotherapy. Response rates (PR+CR) were 18.2% (95% CI: 5.19%–40.28%) and 59.1% (95% CI: 36.35%–79.29% P=0.012) for the FLO/FLOT group, respectively. In the FLO group two patients (9%) experienced progressive disease, four patients (18%) showed partial remission and sixteen patients (73%) stable disease at radiological and endoscopic evaluation. One patient was not evaluable. In the FLOT group two patients showed progressive disease (M1) and, therefore, received palliative treatment off-study. Ten patients (48%) showed partial-, three patients (14%) a complete tumour remission, and six patients (29%) stable disease.
Surgical resection
The median time from random assignment to surgery was 73 and 87 days for the FLO and FLOT groups, respectively (P=0.0006).
In total, 32 patients (74.4%) among the 43 enrolled underwent tumour resection with curative intent (17 out of 22 in the FLO group (77.3%) and 15 out of 21 in the FLOT group (71.4%)). The reasons for not being operated are shown in Figure 1. The type of surgery performed and the pathological tumour stage and nodal status are shown in Table 4. On an intent-to-treat basis, twenty-nine patients (67.4%) achieved a R0 resection (90.1% of the 32 patients who underwent resection), 15 patients in the FLO group (68.2% 88.2% of the resected patients), and 14 patients in the FLOT group (66.6% 93.3% of the resected patients). Nodal downstaging (uN+ to ypN0) was detected in 7 out of 22 patients (31.8%) and in 6 out of 21 patients (28.6%) and the T-stage was downstaged in 15 out of 22 patients (68.2%) and in 12 out of 21 patients (57.1%) in the FLO/FLOT group, respectively. Of note, two patients (9.5%) treated with neoadjuvant FLOT achieved a complete remission (ypT0; ypN0) after being initially staged as T3 tumours.
Table 4. Surgical and pathologic results (intention-to-treat population; n=43).
|
FLO (n=22) |
FLOT (n=21) |
Total (n=43) |
||||
|---|---|---|---|---|---|---|
| Parameter | No. | % | No. | % | No | % |
| Time from random assignment to surgery, days, median; IQR |
73
68–78 |
87 79–90 | 78 70–86 | |||
|
Type of surgery | ||||||
| No Surgery |
5 |
22.7 |
6 |
28.6 |
11 |
25.6 |
|
Transhiatal extended | ||||||
| Gastrectomy |
5 |
22.7 |
5 |
23.8 |
10 |
23.3 |
| Esophagogastrectomy |
3 |
13.6 |
6 |
28.6 |
9 |
20.9 |
| Total/subtotal Gastrectomy |
9 |
40.9 |
4 |
19.4 |
13 |
30.2 |
|
Extend of resection | ||||||
| No Resection |
5 |
22.7 |
6 |
28.6 |
11 |
25.6 |
| R0 |
15 |
68.2 |
14 |
66.6 |
29 |
67.4 |
| R1/2 |
2 |
9.1 |
1 |
4.8 |
3 |
7.0 |
|
Tumour stage | ||||||
| ypT0 |
0 |
|
2 |
9.5 |
2 |
4.7 |
| ypT1/2 |
15 |
68.2 |
10 |
47.6 |
25 |
58.1 |
| ypT3/4 |
1 |
4.5 |
1 |
4.8 |
2 |
4.7 |
| Not evaluable |
1 |
4.5 |
2 |
9.5 |
3 |
7.40 |
|
Nodal status | ||||||
| ypN0 |
7 |
31.8 |
6 |
28.6 |
13 |
30.2 |
| ypN+ |
9 |
40.9 |
8 |
38.1 |
17 |
39.5 |
| Not evaluable |
1 |
4.5 |
1 |
4.8 |
2 |
4.7 |
|
Metastatic status | ||||||
| M0 |
16 |
72.7 |
15 |
71.4 |
31 |
72.1 |
| M1 |
1 |
4.5 |
0 |
|
1 |
2.3 |
|
Median no. of nodes removed | ||||||
| Median IQR (1st quartile–3rd quartile) | 22.5 20–26 | 20 (18.5–21.5) | 21 18.5–24 | |||
Abbrevations: FLO=oxaliplatin, fluorouracil; FLOT=fluorouracil, oxaliplatin, docetaxel; IQR=interquartile range.
No intra- or postoperative deaths occurred during a 30-day postoperative course. The median hospital stay was 20 days (range 13–32) in the FLO and 20 days (range 13–97) in the FLOT arm. Postoperative morbidity was observed in 46.9% of patients. One-third of patients (35.3%) in the FLO and almost two-third (60.0%) of patients in the FLOT group had one or more severe complications, predominantly pneumonia (FLO, 17.6% FLOT, 20%) and wound infections (FLO, 0% FLOT, 20%) as shown in Table 5.
Table 5. Postoperative morbidity and mortality.
| Operated patients n=32 | ||||||
|---|---|---|---|---|---|---|
| |
FLO |
FLOT |
Total |
|||
| Type of complication | n=17 | % | n=15 | % | n=32 | % |
| Anastomotic leakage |
1 |
5.9 |
|
|
1 |
3.1 |
| Pulmonary (pneumonia, lung failure, pleural effusion) |
3 |
17.6 |
3 |
20.0 |
6 |
18.8 |
| Cardiovascular (myocardial infarction) |
|
|
1 |
6.7 |
1 |
3.1 |
| Bleeding |
1 |
5.9 |
1 |
6.7 |
2 |
6.3 |
| Enterothorax |
1 |
5.9 |
|
|
1 |
3.1 |
| Wound infection |
|
|
3 |
20.0 |
3 |
9.4 |
| Renal failure |
|
|
1 |
6.7 |
1 |
3.1 |
|
Total morbidity |
6 |
35.3 |
9 |
60.0 |
15 |
46.9 |
| 30-day-mortality | 0 | — | 0 | — | 0 | — |
Abbrevations: FLO=oxaliplatin, fluorouracil; FLOT=fluorouracil, oxaliplatin, docetaxel.
Progression-free and overall survival
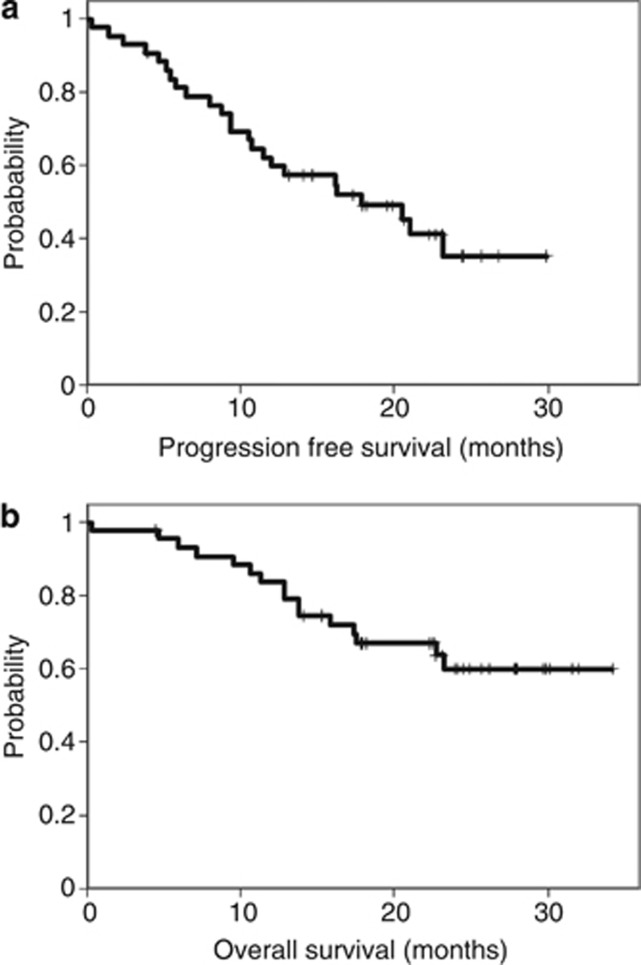
With a median follow-up of 22.4 months (range 0.3 to 34.2), the median PFS was 17.3 months and the median OS was not reached (Figures 2A and B).
Figure 2.
Kaplan–Meier analysis of (A) progression-free survival and (B) overall survival in the ITT population.
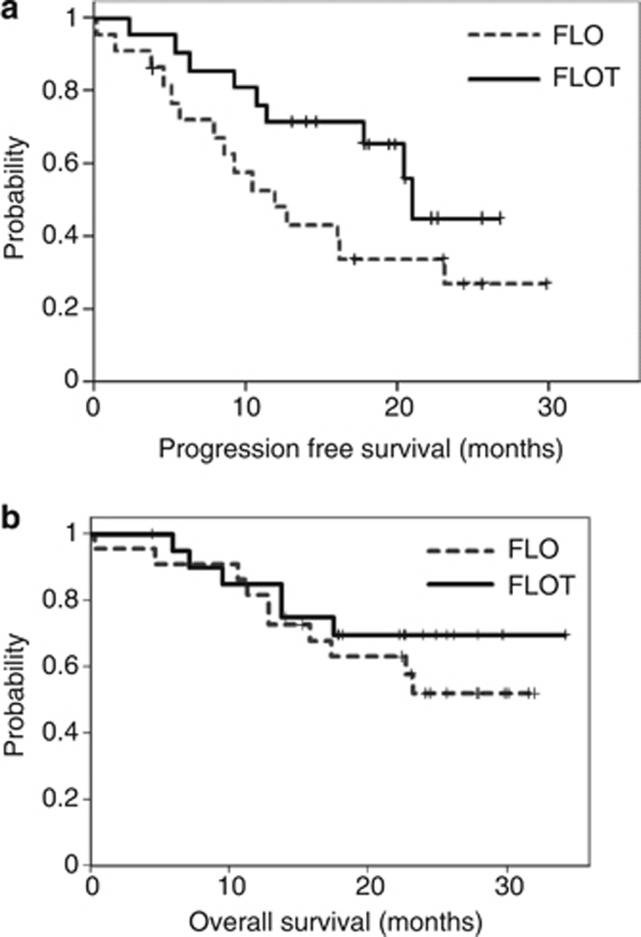
Patients treated with FLOT showed a trend towards longer median PFS (21.1 months) compared with patients in the FLO group (12.0 months; HR: 2.02; 95% CI 1.2 to 2.9; P=0.09) (Figure 3A). Median OS was not reached in both the arms (P=0.399). Among the 32-operated patients, survival at 18 and 24 months was 70% and 56% in the FLO arm vs 78% and 78% in the FLOT arm, respectively (Figure 3B). The median PFS was 12.8 months for FLO and was not reached for FLOT (P=0.059).
Figure 3.
Kaplan–Meier analysis of (A) progression-free survival and (B) overall survival in patients treated with either FLO or FLOT regimen.
Discussion
The aim of this study was to evaluate the feasibility and the potential benefit of perioperative chemotherapy with or without the addition of docetaxel to 5-FU/leucovorin and oxaliplatin in elderly patients with locally advanced esophagogastric cancer. Our results showed high levels of adherence to preoperative treatment with 86.4% of patients completing all planned four cycles. These results did not differ between the taxan- and the non-taxan-containing treatment regimen. In terms of feasibility we did not register any mortality compared with 5.6 and 4.6% in the MAGIC (Cunningham et al, 2006)- and the FFCD (Ychou et al, 2011) trial and the overall morbidity rate of 46.9% compared well with those of the MAGIC- and the FFCD trials, where a 45% and a 28% morbidity rate was reported, respectively. Results from our study confirm previous reports, that elderly patients receiving neoadjuvant cytoreductive therapy are not burdened by a significantly higher risk of developing major or fatal postoperative complications as compared with their younger counterparts (Lin et al, 2004; Rice et al, 2005; Ruol et al, 2007) and, in the metastatic setting, do benefit from chemotherapy to the same degree as younger patients (Trumper et al, 2006; Jatoi et al, 2010).
The present study additionally provided relevant information regarding the safety of a preoperative docetaxel-based three-drug chemotherapy regimen followed by surgery in elderly patients. The FLOT combination used in this study has previously demonstrated a high degree of efficacy and relative safety in the perioperative and metastatic setting in gastroesophageal adenocarcinoma (Al-Batran et al, 2008a, 2008b; Homann et al, 2012).
In this analysis of perioperative chemotherapy in elderly patients, the FLOT regimen was associated with a high incidence of grade 3 or 4 neutropenia and leucopenia, which occurred in 38% and 33%, respectively. However, this was in the range of previous non-, age-specific taxan-containing triple regimens (Van Cutsem et al, 2006; Lorenzen et al, 2007; Al-Batran et al, 2008a; Homann et al, 2012; Sym et al, 2010). Of note, febrile neutropenia did not occur and there were no toxicity related deaths recorded. Except for a significant increase of nausea and mucositis in patients treated with the addition of docetaxel, non-haematological toxicities were generally moderate and predictable. However, the number of patients experiencing a ⩾10-points deterioration of EORTC QoL global health status scores at 8 weeks was tripled in the FLOT group (FLOT, 54% FLO 18%). As rates of primary disease progression were similar in both the arms, the deterioration of QoL is most likely related to the toxicity associated with FLOT.
Albeit the potentially troublesome toxicity profile of taxan-based three-drug combination chemotherapy, there was no increased rate of early cessation of treatment in the FLOT compared with the FLO regimen, with a median number of four preoperatively administered cycles in both the treatment arms, however, dose reductions due to grade 3/4 toxicity were more frequent with the FLOT regimen. Of note, the postoperative morbidity rate in patients treated with neoadjuvant FLOT was nearly twice as high compared with FLO (FLOT, 60% FLO, 35%), mainly due to wound infection. This toxic effect cannot be linked directly to the toxicity profile of docetaxel, and should be interpreted with caution due to the low number of patients.
Our data demonstrate that only 75% of patients assigned for surgery are willing and fit enough to finally undergo resection. A decreased likelihood of having surgery has been reported with increasing age in several studies (Sabel et al, 2002; Cronin-Fenton et al, 2007; Koppert et al, 2012) and this may be a reflection of the presence of toxicity and comorbidity, also influencing patient's choice.
A limitation of our trial is that only 28% of patients completed all eight cycles of perioperative chemotherapy, which is far below of what was reported from the MAGIC trial (42%). However, there was no difference regarding the amount of treatment cycles between the two treatment arms. Thirty-eight per cent of patients who completed preoperative chemotherapy and surgery did not start postoperative chemotherapy, which confirms the 34% reported by the MAGIC trial. (Cunningham et al, 2006). Our data are in line with these observations and we suppose that the ability to perform postoperative treatments mainly depends on the morbidity associated with the surgical intervention rather than the type of chemotherapy regimen or the age of the patient.
Data from this trial are of value considering response, PFS and OS. Although data on clinical response assessment in localised gastroesophageal cancer have to be regarded with caution, response rates with FLOT were 59.1%, including two complete remissions, compared with only 18.2% and no complete remission with the FLO regimen. These results are in agreement with the literature, where increased pCR rates are reported with the addition of docetaxel (Fields et al, 2012; Homann et al, 2012; Thuss-Patience et al, 2012). The R0 resection rate of 67% on intent-to-treat analysis was far below from what can be expected after preoperative chemotherapy. Indeed, the R0 resection rate exceeded 90% after removal of the 26% of patients who did not undergo surgical resection, which compares favourably with the rates reported by Thuss-Patience et al (2012), (90.2%).
Progression-free survival rates varied with type of chemotherapy being 21.1 months with FLOT and 12.0 months with FLO therapy. Although tumour remissions and survival seemed improved with the addition of docetaxel, which compares well with other docetaxel-containing neoadjuvant treatment regimens (Homann et al, 2012; Thuss-Patience et al, 2012) it is not clear whether these results are attributable to selection of patients or whether they are an effect of chemotherapy due to the small sample size. Albeit the small sample, we prospectively report for the first time on a homogeneous group of patients, which is usually underrepresented or excluded from larger randomized trials when intensive therapy is applied.
We suggest that addition of docetaxel to a platinum compound is an effective treatment option in the neoadjuvant setting, even in patients aged ⩾65. Nevertheless, the increased toxicity with FLOT and the impaired QoL during the first 8 weeks of therapy has to be considered clinically relevant.
It is also noticeable, that the elderly patients included in this exploratory analysis may represent a group of relatively fit elderly patients and, therefore, it may underestimate what might actually occur in a non-study setting.
In conclusion, neoadjuvant FLO or FLOT may offer a reasonable chance of curative surgery in locally advanced resectable gastroesophageal cancer. Our data strengthen that a selected population of elderly patients who undergo careful preoperative risk analysis, can tolerate an aggressive multimodal treatment approach with a taxan-based triple-drug therapy, followed by a major surgical procedure such as esophagectomy, however, the increase in side effects with the FLOT regimen and postoperative morbidity should be carefully considered when an intensive chemotherapy regimen is planned.
Patients ⩾65 years that fulfil the standard inclusion criteria of clinical trials seem to have a similar advantage from perioperative chemotherapy for esophagogastric cancer as patients ⩽65 years, however, this should be further investigated in large-scale randomized trials. Therefore, it is strongly recommended that specific clinical trials limited to older patients should be planned to evaluate response, benefit treatment tolerability, and the effect of comorbid conditions, so that clinicians may optimise their treatment of older cancer patients.
Acknowledgments
We thank Karin Scheffler (MCA, Berlin, Germany) for study monitoring. We thank Michael Scholz and Martina Güntner (Trium Analysis Online GmbH) for the statistical analysis.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Ajani JA, Winter K, Okawara GS, Donohue JH, Pisters PW, Crane CH, Greskovich JF, Anne PR, Bradley JD, Willett C, Rich TA. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol. 2006;24:3953–3958. doi: 10.1200/JCO.2006.06.4840. [DOI] [PubMed] [Google Scholar]
- Al-Batran SE, Hartmann JT, Hofheinz R, Homann N, Rethwisch V, Probst S, Stoehlmacher J, Clemens MR, Mahlberg R, Fritz M, Seipelt G, Sievert M, Pauligk C, Atmaca A, Jäger E. Biweekly fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) for patients with metastatic adenocarcinoma of the stomach or esophagogastric junction: a phase II trial of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol. 2008a;19:1882–1887. doi: 10.1093/annonc/mdn403. [DOI] [PubMed] [Google Scholar]
- Al-Batran SE, Hartmann JT, Probst S, Schmalenberg H, Hollerbach S, Hofheinz R, Rethwisch V, Seipelt G, Homann N, Wilhelm G, Schuch G, Stoehlmacher J, Derigs HG, Hegewisch-Becker S, Grossmann J, Pauligk C, Atmaca A, Bokemeyer C, Knuth A, Jäger E, Arbeitsgemeinschaft Internistische Onkologie Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008b;26:1435–1442. doi: 10.1200/JCO.2007.13.9378. [DOI] [PubMed] [Google Scholar]
- Al-Batran SE, Pauligk C, Homann N, Hartmann JT, Moehler M, Probst S, Rethwisch V, Stoehlmacher-Williams J, Prasnikar N, Hollerbach S, Bokemeyer C, Mahlberg R, Hofheinz RD, Luley K, Kullmann F, Jager E. The feasibility of triple-drug chemotherapy combination in older adult patients with oesophagogastric cancer: a randomised trial of the Arbeitsgemeinschaft Internistische Onkologie (FLOT65+) Eur J Cancer. 2012. [DOI] [PubMed]
- Biffi R, Fazio N, Luca F, Chiappa A, Andreoni B, Zampino MG, Roth A, Schuller JC, Fiori G, Orsi F, Bonomo G, Crosta C, Huber O. Surgical outcome after docetaxel-based neoadjuvant chemotherapy in locally-advanced gastric cancer. World J Gastroenterol. 2010;16:868–874. doi: 10.3748/wjg.v16.i7.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briasoulis E, Liakakos T, Dova L, Fatouros M, Tsekeris P, Roukos DH, Kappas AM. Selecting a specific pre- or postoperative adjuvant therapy for individual patients with operable gastric cancer. Expert Rev Anticancer Ther. 2006;6:931–939. doi: 10.1586/14737140.6.6.931. [DOI] [PubMed] [Google Scholar]
- Caussanel JP, Levi F, Brienza S, Misset JL, Itzhaki M, Adam R, Milano G, Hecquet B, Mathé G. Phase I trial of 5-day continuous venous infusion of oxaliplatin at circadian rhythm-modulated rate compared with constant rate. J Natl Cancer Inst. 1990;82:1046–1050. doi: 10.1093/jnci/82.12.1046. [DOI] [PubMed] [Google Scholar]
- Cronin-Fenton DP, Sharp L, Carsin AE, Comber H. Patterns of care and effects on mortality for cancers of the oesophagus and gastric cardia: a population-based study. Eur J Cancer. 2007;43 (3:565–575. doi: 10.1016/j.ejca.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, MAGIC Trial Participants Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- El-Serag HB. The epidemic of esophageal adenocarcinoma. Gastroenterol Clin North Am. 2002;31:421–440. doi: 10.1016/s0889-8553(02)00016-x. [DOI] [PubMed] [Google Scholar]
- Fields RC, Busam KJ, Chou JF, Panageas KS, Pulitzer MP, Allen PJ, Kraus DH, Brady MS, Coit DG. Recurrence after complete resection and selective use of adjuvant therapy for stage I through III Merkel cell carcinoma. Cancer. 2012;118 (13:3311–3320. doi: 10.1002/cncr.26626. [DOI] [PubMed] [Google Scholar]
- Homann N, Pauligk C, Luley K, Werner Kraus T, Bruch HP, Atmaca A, Noack F, Altmannsberger HM, Jäger E, Al-Batran SE. Pathological complete remission in patients with oesophagogastric cancer receiving preoperative 5-fluorouracil, oxaliplatin and docetaxel. Int J Cancer. 2012;130:1706–1713. doi: 10.1002/ijc.26180. [DOI] [PubMed] [Google Scholar]
- Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- Japanese Gastric Cancer A Japanese classification of gastric carcinoma – 2nd english edition. Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- Jatoi A, Foster NR, Egner JR, Burch PA, Stella PJ, Rubin J, Dakhil SR, Sargent DJ, Murphy BR, Alberts SR. Older versus younger patients with metastatic adenocarcinoma of the esophagus, gastroesophageal junction, and stomach: a pooled analysis of eight consecutive North Central Cancer Treatment Group (NCCTG) trials. Int J Oncol. 2010;36:601–606. doi: 10.3892/ijo_00000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley ST, Coppola D, Karl RC.2004Neoadjuvant chemoradiotherapy is not associated with a higher complication rate vs. surgery alone in patients undergoing esophagectomy J Gastrointest Surg 8227–231.(discussion 231–232) [DOI] [PubMed] [Google Scholar]
- Koppert LB, Lemmens VE, Coebergh JW, Steyerberg EW, Wijnhoven BP, Tilanus HW, Janssen-Heijnen ML. Impact of age and co-morbidity on surgical resection rate and survival in patients with oesophageal and gastric cancer. Br J Surg. 2012;99 (12:1693–1700. doi: 10.1002/bjs.8952. [DOI] [PubMed] [Google Scholar]
- Lin FC, Durkin AE, Ferguson MK. Induction therapy does not increase surgical morbidity after esophagectomy for cancer. Ann Thorac Surg. 2004;78:1783–1789. doi: 10.1016/j.athoracsur.2004.04.081. [DOI] [PubMed] [Google Scholar]
- Lorenzen S, Hentrich M, Haberl C, Heinemann V, Schuster T, Seroneit T, Röthling N, Peschel C, Lordick F, et al. Split-dose docetaxel, cisplatin and leucovorin/fluorouracil as first-line therapy in advanced gastric cancer and adenocarcinoma of the gastroesophageal junction: results of a phase II trial. Ann Oncol. 2007;18:1673–1679. doi: 10.1093/annonc/mdm269. [DOI] [PubMed] [Google Scholar]
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Rice DC, Correa AM, Vaporciyan AA, Sodhi N, Smythe WR, Swisher SG, Walsh GL, Putnam JB, Komaki R, Ajani JA, Roth JA.2005Preoperative chemoradiotherapy prior to esophagectomy in elderly patients is not associated with increased morbidity Ann Thorac Surg 79391–397.(discussionn 391–397) [DOI] [PubMed] [Google Scholar]
- Ruol A, Portale G, Castoro C, Merigliano S, Cagol M, Cavallin F, Chiarion Sileni V, Corti L, Rampado S, Costantini M, Ancona E. Effects of neoadjuvant therapy on perioperative morbidity in elderly patients undergoing esophagectomy for esophageal cancer. Ann Surg Oncol. 2007;14:3243–3250. doi: 10.1245/s10434-007-9455-z. [DOI] [PubMed] [Google Scholar]
- Sabel MS, Smith JL, Nava HR, Mollen K, Douglass HO, Gibbs JF. Esophageal resection for carcinoma in patients older than 70 years. Ann Surg Oncol. 2002;9 (2:210–214. doi: 10.1007/BF02557376. [DOI] [PubMed] [Google Scholar]
- Sym SJ, Chang HM, Ryu MH, Lee JL, Kim TW, Yook JH, Oh ST, Kim BS, Kang YK. Neoadjuvant docetaxel, capecitabine and cisplatin (DXP) in patients with unresectable locally advanced or metastatic gastric cancer. Ann Surg Oncol. 2010;17:1024–1032. doi: 10.1245/s10434-009-0838-1. [DOI] [PubMed] [Google Scholar]
- Thuss-Patience PC, Hofheinz RD, Arnold D, Florschutz A, Daum S, Kretzschmar A, Mantovani-Loffler L, Bichev D, Breithaupt K, Kneba M, Schumacher G, Glanemann M, Schlattmann P, Reichardt P, Gahn B. Perioperative chemotherapy with docetaxel, cisplatin and capecitabine (DCX) in gastro-oesophageal adenocarcinoma: a phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO) Ann Oncol. 2012;23 (11:2827–2834. doi: 10.1093/annonc/mds129. [DOI] [PubMed] [Google Scholar]
- Trumper M, Ross PJ, Cunningham D, Norman AR, Hawkins R, Seymour M, Harper P, Iveson T, Nicolson M, Hickish T. Efficacy and tolerability of chemotherapy in elderly patients with advanced oesophago-gastric cancer: A pooled analysis of three clinical trials. Eur J Cancer. 2006;42:827–834. doi: 10.1016/j.ejca.2005.08.044. [DOI] [PubMed] [Google Scholar]
- Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, Risse ML, Ajani JA, V325 Study Group Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–4997. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- van de Velde CJ, Peeters KC. The gastric cancer treatment controversy. J Clin Oncol. 2003;21 (12:2234–2236. doi: 10.1200/JCO.2003.91.138. [DOI] [PubMed] [Google Scholar]
- Ychou M, Boige V, Pignon JP, Conroy T, Bouche O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B, Geneve J, Lasser P, Rougier P. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29 (13:1715–1721. doi: 10.1200/JCO.2010.33.0597. [DOI] [PubMed] [Google Scholar]