Abstract
Objectives
Farming and agricultural pesticide use have been associated with two autoimmune rheumatic diseases, rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE). However, risk associated with other residential or workplace insecticide use is unknown.
Methods
We analyzed data from the Women’s Health Initiative Observational Study (n=76,861, post-menopausal, age 50-79 years). Incident cases (n=213; 178 RA, 27 SLE, and 8 both) were identified based on self-report and use of disease modifying anti-rheumatic drugs at year 3 of follow-up. We examined self-reported residential or workplace insecticide use (personally mixing/applying by self and application by others) in relation to RA/SLE risk, overall and in relation to farm history. Hazard ratios (adj.HR) and 95% confidence intervals (CI) adjusted for age, race, region, education, occupation, smoking, reproductive factors, asthma, other autoimmune diseases and co-morbidities.
Results
Compared to never use, personal use of insecticides was associated with increased RA/SLE risk, with significant trends for greater frequency (adj.HR 2.04; 95%CI 1.17, 3.56 for ≥ 6 times/year) and duration (HR 1.97; 95% CI 1.20, 3.23 for ≥ 20 years). Risk was also associated with long-term insecticide application by others (adj.HR=1.85; 95% CI 1.07, 3.20 for ≥20 years), and frequent application by others among women with a farm history (adj.HR 2.73; 95% CI 1.10, 6.78 for ≥ 6 times/year).
Conclusions
These results suggest residential and workplace insecticide exposure is associated with risk of ARD in post-menopausal women. Although these findings require replication in other populations, they support a role for environmental pesticide exposure in development of autoimmune rheumatic diseases.
Keywords: Rheumatoid Arthritis, Systemic Lupus Erythematosus, Prospective Cohort, Environmental exposures, Farming, Insecticide use
Autoimmune rheumatic diseases (ARD) such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) affect as many as 1.6 million adults in the United States (1), and disproportionately impact women, the elderly (RA) and minorities (SLE) (2-4). Established risk factors include family history of autoimmune disease and genetic characteristics. Like most complex diseases, the etiology of ARD also is also influenced by environmental exposures. However, knowledge of specific environmental risk factors is limited (5), with most evidence of associations pertaining to smoking and various occupational exposures (6, 7).
Farming occupation has been associated with ARD in different populations and using different study designs, with several studies suggesting a role of pesticide exposure from farming (8-12). One recent study reported an association between farming occupation and death with systemic autoimmune disease (including RA and SLE), specifically for work in the crop industry (8). Using a job-exposure matrix, the authors also described an association of occupational pesticide exposure and RA that was primarily related to farming and significant only in men. Other studies have shown an association of farming and RA primarily in men (10, 13-15), and a study of female spouses of pesticide applicators did not identify any associations between RA and specific pesticides (16). On the other hand, a recent study described an association of elevated serum organochlorine pesticides and self-reported RA in a population-based sample of adults in the United States (17). Another study reported an association of SLE with agricultural pesticide mixing (18). In population controls, pesticide mixing was also related to antinuclear antibodies, which can be a pre-clinical, though non-specific marker preceding autoimmune disease onset (19).
The low prevalence of professional farm work and occupational pesticide exposure, especially in women, limits the power of many studies to examine these associations with ARD. In contrast, pesticide exposure in other residential and workplace settings is much more common, e.g., use of household insecticides with similar ingredients to those used for agriculture, though at lower concentrations. We tested the hypothesis that insecticide use may increase ARD risk using data from the Women’s Health Initiative Observational Study. We investigated whether self-reported residential and workplace insecticide use (mixing/application by self, and application by others) were associated with development of RA and SLE in postmenopausal women. We also considered the role of farm history.
METHODS
Sample and case classification
The Women’s Health Initiative Observational Study (WHI-OS) is a multi-center cohort of 93,676 post-menopausal women ages 50 to 79, enrolled from 40 clinics throughout the U.S. in 1993 to 1998 (20). Participants completed clinic visits at baseline, were contacted yearly to update health status, and at year 3 completed another in-person clinic visit. Questionnaires collected data on medical conditions and lifestyle factors. Participants were also asked to bring current medications to the clinic visits at baseline and year 3. Institutional Review Boards of participating institutions approved protocols and consent forms, signed by women at enrollment.
An analysis sample included baseline participants with complete data who were at risk of developing RA or SLE (Supplemental Figure). We excluded prevalent cases (n=815; case classification described below) under the assumption they could not become new cases, and those missing data on farm history, self-report of SLE, RA or medication use at baseline or follow-up (n=16,000), for a final sample of 76,861 at risk. The incident case group (N=213) included 186 RA cases and 35 SLE cases, 8 of whom reported both RA and SLE.
A previous study in the WHI determined that self-report was a non-specific indication of SLE or RA diagnosis compared to medical records, but specificity was improved by in those with current use of disease modifying antirheumatic drugs (DMARDs, Supplemental Table 1; specificity = 95.4 for RA and 99.4 for SLE)(21). Thus, we classified prevalent cases based on self-reported RA or SLE and DMARDs use (including prednisone) at baseline, and incident cases based on newly self-reported RA or SLE at year 1, 2, or 3 plus DMARD use at year 3. We evaluated the impact of potential case misclassification in several ways. We first restricted the analysis sample to increase specificity of the case-definition, limiting cases to those taking non-prednisone DMARDs, excluding 58 incident cases reporting prednisone only and including 151 prevalent cases taking prednisone only as non-cases (total N=76,799)(Supplemental Figure). Second, we restricted analyses to the most likely cases and non-cases, excluding those who reported either RA or SLE alone or non-prednisone DMARD use alone (n=3,285 at baseline, 1,521 at year 3, and 725 at both time points; total N=71,117). This sample was also limited to those most likely to be incident cases (n=174), excluding 39 new cases who reported either an ARD or non-prednisone DMARD use at baseline. Finally, we addressed potential bias due to concurrent reporting of insecticide use and ARD status, excluding 43 DMARD-confirmed cases reported in the first year follow-up.
Farm history and insecticide exposure
A baseline questionnaire collected information on farm history, i.e., whether a woman had ever lived or worked on a farm, and for how many years (less than 5, 5-9, 10-14, 15-19, 20 or more years). The first year follow-up questionnaire included questions on insecticide exposure since age 21. Women were asked if they or someone else had ever “poured, mixed, sprayed or applied insecticides” in their immediate surroundings at home, leisure or work. Given examples were bug or flea spray and garden, lawn, or crop insecticides. Women were instructed not to include insect repellents, weed killers, fungus/mildew killers, or flea, tick, or mite treatments applied to pets. Responses were further specified as: (1) at work only, (2) at home or leisure only, (3) at both work and home/leisure. Women with positive responses were then asked if they personally mixed insecticides, personally applied them, a lawn service applied them at their homes, a commercial service applied them in their home, or other. For personal use of insecticides (direct exposure through mixing or applying by self) and application by others (indirect exposure through commercial or other residential applications), women were asked total duration (never or less than 1 year, 1-4, 5-9, 10-14, 15-19, or 20 or more years) and frequency of use (never or less than once, 1-5, 6-12, 13-24, or 25 or more times per year). Responses for direct and indirect exposures were not mutually exclusive.
Covariates
Covariate data were derived from baseline questionnaire responses (Table 1), including potential risk factors for ARD that might be related to farm history or insecticide use, i.e., age, race/ethnicity, education, occupation, pack-years smoking, geographic region at screening, body mass index, pregnancy and breast feeding history, age at menarche and menopause, history of asthma, thyroid disease, other autoimmune disease (Multiple Sclerosis or Crohn’s), Charlson index of chronic diseases. Other covariates considered were region at birth, income, employment and marital status, current smoking, vitamin D (diet/supplemental), coffee, and alcohol use.
Table 1.
Characteristics of the cohort and incident RA and SLE cases in the WHI Observational Study
|
OS cohort N=76,648 n (%) |
RA/SLE N=213 n (%) |
P-value * |
RA cases N=186 n (%) |
P-value * |
SLE cases N=35 n (%) |
P-value * | |
|---|---|---|---|---|---|---|---|
| INSECTICIDE USE AND FARM HISTORY | |||||||
| Insecticide exposure † | 0.30 | 0.45 | 0.047 | ||||
| Never | 21981 (29.6) | 48 (23.0) | 43 (23.6) | 5 (14.3) | |||
| At work only | 1199 (1.6) | 4 (1.9) | 2 (1.1) | 2 (5.7) | |||
| At home or leisure only | 35653 (48.1) | 107 (51.2) | 96 (52.8) | 17 (48.6) | |||
| Both at work and home | 8207 (11.1) | 25 (12.0) | 22 (12.1) | 4 (11.4) | |||
| Don’t know | 6305 (8.5) | 22 (10.5) | 17 (9.3) | 6 (17.1) | |||
| Missing | 804 (1.1) | 3 (1.4) | 2 (1.1) | 1 (2.9) | |||
| Personally used † | 0.010 | 0.020 | 0.07 | ||||
| Ever | 28137 (38.0) | 94 (45.0) | 83 (45.6) | 16 (45.7) | |||
| Missing | 7509 (10.1) | 27 (12.9) | 20 (11.0) | 8 (22.9) | |||
| Others applied † | 0.87 | 0.86 | 0.84 | ||||
| Ever | 23426 (31.6) | 65 (31.1) | 58 (31.9) | 10 (28.6) | |||
| Missing | 7509 (10.1) | 27 (12.9) | 20 (11.0) | 8 (22.9) | |||
| Lived/worked on farm | 0.025 | 0.08 | 0.06 | ||||
| Never | 56642 (73.9) | 143 (66.2) | 127 (68.3) | 21 (60.0) | |||
| Ever | 20006 (26.1) | 70 (33.8) | 59 (31.7) | 14 (40.0) | |||
| SOCIODEMOGRAPHIC AND ENVIRONMENTAL FACTORS | |||||||
| Age | 0.81 | 0.36 | 0.09 | ||||
| 50-54 | 10001 (13.0) | 26 (12.2) | 19 (10.2) | 8 (22.9) | |||
| 55-59 | 14313 (18.7) | 36 (16.9) | 31 (16.7) | 6 (17.1) | |||
| 60-64 | 16859 (22.0) | 47 (22.1) | 38 (20.4) | 12 (34.3) | |||
| 65-69 | 17339 (22.6) | 46 (21.6) | 44 (23.7) | 2 (5.7) | |||
| 70-74 | 12638 (16.5) | 38 (17.8) | 34 (18.3) | 5 (14.3) | |||
| 75-79 | 5498 (7.2) | 20 (9.4) | 20 (10.8) | 2 (5.7) | |||
| Race/ethnicity | 0.39 | 0.38 | 0.77 | ||||
| White | 65171 (85.0) | 175 (82.2) | 154 (82.8) | 28 (80.0) | |||
| Black | 5456 (7.1) | 21 (9.9) | 18 (9.7) | 4 (11.4) | |||
| Hispanic | 2461 (3.2) | 10 (4.7) | 9 (4.8) | 1 (2.9) | |||
| American Indian | 295 (0.4) | 0 (0) | 0 (0) | 0 (0) | |||
| Asian/Pacific Islander | 2248 (2.9) | 5 (2.3) | 3 (1.6) | 2 (5.7) | |||
| Unknown | 1017 (1.3) | 2 (0.9) | 2 (1.1) | 0 (0) | |||
| Geographic residence | 0.25 | 0.370 | 0.26 | ||||
| Northeast | 17466 (22.8) | 39 (18.3) | 35 (18.8) | 4 (11.4) | |||
| South | 19381 (25.3) | 59 (27.7) | 52 (28.0) | 11 (31.4) | |||
| Midwest | 17084 (22.3) | 56 (26.3) | 48 (25.8) | 11 (31.4) | |||
| West | 22737 (29.7) | 59 (27.7) | 51 (27.4) | 9 (25.7) | |||
| Education | 0.008 | 0.06 | 0.024 | ||||
| 0-8 years | 931 (1.2) | 2 (0.9) | 2 (1.1) | 0 (0) | |||
| Some high school | 2293 (3.0) | 11 (5.2) | 10 (5.4) | 2 (5.7) | |||
| High school /GED | 12023 (15.7) | 42 (19.7) | 35 (18.8) | 8 (22.9) | |||
| School after high school | 27466 (35.8) | 89 (41.8) | 75 (40.3) | 19 (54.3) | |||
| College degree or higher | 33354 (43.5) | 69 (32.4) | 64 (34.4) | 6 (17.1) | |||
| Missing | 581 ( 0.8) | 0 | 0 | 0 | |||
| Occupation | <0.001 | <0.001 | 0.24 | ||||
| Managerial/Professional | 32831 (42.8) | 58 (27.2) | 51 (27.4) | 9 (25.7) | |||
| Technical/Sales/Admin | 20923 (27.3) | 75 (35.2) | 66 (35.5) | 12 (34.3) | |||
| Service/Labor | 12179 (15.9) | 42 (19.7) | 37 (19.9) | 8 (22.9) | |||
| Homemaker only | 7652 (10.0) | 30 (14.1) | 26 (14.0) | 4 (11.4) | |||
| Missing | 3063 (4.0) | 8 (3.8) | 6 (3.2) | 2 (5.7) | |||
| HEALTH HISTORY AND REPRODUCTIVE FACTORS | |||||||
| Asthma | <0.001 | 0.002 | 0.039 | ||||
| No | 70643 (92.2) | 182 (85.4) | 160 (86.0) | 29 (82.9) | |||
| Yes | 5965 (7.8) | 31 (14.6) | 26 (14.0) | 6 (17.1) | |||
| Missing | 40 (0.1) | 0 (0) | 0 (0) | 0 (0) | |||
| Other autoimmune | <0.001 | <0.001 | 0.48 | ||||
| No | 75157 (98.1) | 203 (95.3) | 177 (95.2) | 34 (97.1) | |||
| Yes | 1087 (1.4) | 9 (4.2) | 8 (4.3) | 1 (2.9) | |||
| Missing | 404 (0.5) | 1 (0.5) | 1 (0.5) | 0 (0) | |||
| Thyroid problems ‡ | 0.12 | 0.12 | 0.49 | ||||
| No | 57134 (74.5) | 147 (69.0) | 128 (68.8) | 23 (65.7) | |||
| Yes | 19104 (24.9) | 62 (29.1) | 55 (29.6) | 10 (28.6) | |||
| Missing | 410 (0.5) | 4 (1.9) | 3 (1.6) | 2 (5.7) | |||
| Charlson Index (modified) | 0.02 | 0.036 | 0.81 | ||||
| 0 | 50505 (65.9) | 123 (57.7) | 109 (58.6) | 20 (57.1) | |||
| 1 | 12923 (16.9) | 52 (24.4) | 46 (24.7) | 7 (20.0) | |||
| 2 | 8965 (11.7) | 24 (11.3) | 20 (10.8) | 5 (14.3) | |||
| ≥ 3 | 3298 (4.3) | 11 (5.2) | 9 (4.8) | 2 (5.7) | |||
| Missing | 957 (1.2) | 3 (1.4) | 2 (1.1) | 1 (2.9) | |||
| Pack-years smoking | 0.20 | 0.06 | 0.58 | ||||
| Never smoked | 39095 (51.0) | 105 (49.3) | 89 (47.8) | 18 (51.4) | |||
| < 5 | 11046 (14.4) | 23 (10.8) | 18 (9.7) | 8 (22.9) | |||
| 5 - < 20 | 10581 (13.8) | 29 (13.6) | 26 (14.0) | 4 (11.4) | |||
| ≥ 20 | 13368 (17.4) | 47 (22.1) | 44 (23.7) | 5 (14.3) | |||
| Missing | 2558 (3.3) | 9 (4.2) | 9 (4.8) | 0 | |||
| Body Mass Index, kg/m2 | 0.030 | 0.007 | 0.59 | ||||
| < 25 | 31967 (41.7) | 69 (32.4) | 56 (30.1) | 15 (42.9) | |||
| 25- <30 | 25819 (33.7) | 80 (37.6) | 76 (40.9) | 9 (25.7) | |||
| ≥ 30 | 17995 (23.5) | 59 (27.7) | 50 (26.9) | 10 (28.6) | |||
| Missing | 867 (1.1) | 5 (2.3) | 4 (2.2) | 1 (2.9) | |||
| Age at menarche | >0.99 | 0.15 | 0.002 | ||||
| ≤ 10 | 4903 (6.4) | 17 (8.0) | 14 (7.5) | 3 (8.6) | |||
| 11 | 11992 (5.6) | 37 (17.4) | 34 (18.3) | 4 (11.4) | |||
| 12 | 20082 (26.2) | 47 (22.1) | 43 (23.1) | 7 (20.0) | |||
| 13 | 22384 (29.2) | 56 (26.3) | 45 (24.2) | 12 (34.3) | |||
| 14 | 10000 (13.0) | 32 (15.0) | 31 (16.7) | 3 (8.6) | |||
| 15 | 4115 (5.4) | 16 (7.5) | 14 (7.5) | 2 (5.7) | |||
| ≥ 16 | 3001 (4.0) | 8 (3.8) | 5 (2.7) | 4 (11.5) | |||
| Missing | 171 (0.2) | 0 (0) | 0 (0) | 0 (0) | |||
| Age at menopause | 0.28 | 0.58 | 0.09 | ||||
| < 40 | 6536 (8.5) | 24 (11.3) | 20 (10.8) | 5 (14.3) | |||
| 40-44 | 9413 (12.3) | 28 (13.1) | 22 (11.8) | 8 (22.9) | |||
| 45-49 | 19138 (25.0) | 54 (25.4) | 48 (25.8) | 8 (22.9) | |||
| 50-54 | 28423 (37.1) | 74 (34.7) | 65 (34.9) | 11 (31.4) | |||
| ≥ 55 | 10404 (13.6) | 20 (9.4) | 19 (10.2) | 1 (2.9) | |||
| Missing | 2734 (3.6) | 13 (6.1) | 12 (6.5) | 2 (5.7) | |||
| Term Pregnancies | 0.06 | 0.10 | 0.55 | ||||
| Never pregnant | 7803 (10.2) | 12 (5.6) | 11 (5.9) | 2 (5.7) | |||
| Never term pregnancy | 2093 (2.7) | 9 (4.2) | 8 (4.3) | 1 (2.9) | |||
| 1 | 6895 (9.0) | 21 (9.9) | 20 (10.8) | 1 (2.9) | |||
| 2 | 20158 (26.3) | 44 (20.7) | 39 (21.0) | 7 (20.0) | |||
| 3 | 18567 (24.2) | 56 (26.3) | 46 (24.7) | 12 (34.3) | |||
| 4 | 11021 (14.4) | 37 (17.4) | 31 (16.7) | 7 (20.0) | |||
| ≥ 5 | 9752 (12.7) | 34 (16.0) | 31 (16.7) | 5 (14.3) | |||
| Missing | 359 (0.5) | 0 (0) | 0 (0) | 0 (0) | |||
| Months breastfed | 0.65 | 0.75 | 0.007 | ||||
| Never breastfed | 37004 (48.3) | 96 (45.1) | 88 (47.3) | 11 (31.4) | |||
| 1-6 | 19508 (25.5) | 61 (28.6) | 53 (28.5) | 10 (28.6) | |||
| 7-12 | 8584 (11.2) | 21 (9.9) | 19 (10.2) | 3 (8.6) | |||
| 13-23 | 6578 (8.6) | 20 (9.4) | 13 (7.0) | 9 (25.7) | |||
| ≥ 24 | 4013 (5.2) | 14 (6.6) | 12 (6.5) | 2 (5.7) | |||
| Missing | 961 (1.3) | 1 (0.5) | 1 (0.5) | 0 (0) | |||
8 cases were both RA and SLE; Chi-square test comparing cases to non-cases, excluding missing values.
Referent = never; Personal use = mixed or applied; Others applied = commercially applications to home or garden or other; Four (1.9%) cases and 2499 (3.3%) in the cohort did not complete the year 1 questionnaire on insecticide use (Form 48). Missing results are shown for participants completing a Form 48; values include “Don’t know.”
Other variables showed significant associations with RA/SLE, including “underactive thyroid” (assoc. w/SLE, p=0.029) and “overactive thyroid” (assoc. w/RA, p=0.037), but with more missing (4-6%).
Statistical Analysis
Analyses were conducted using SAS version 9.1 (Cary, NC). Following bivariate analyses of RA/SLE and RA or SLE with covariate and insecticide use variables, multivariate analyses were limited to those with complete data on all variables, (excluding 4 incident cases (1.9%) and 2499 in the cohort (3.3%) with missing data on the insecticide questionnaire). Cox regression was used to estimate hazard ratios (HR) and 95% confidence intervals (CI) in adjusted models. The model building process was established a priori, first including potential confounders (related to exposure and disease), then adding other potential risk factors, and finally considering farm history as a possible explanatory variable for insecticide-related effects. Models were constructed as follows: Model 1 = exposure variable + age; Model 2 = model 1 + environmental and socio-demographic covariates (pack-years smoking, education level, occupational class, geographic region); Model 3 = model 2+ physiological and medical covariates (including body mass index, pregnancy and breast feeding history, age at menarche and menopause, history of asthma, thyroid, and other autoimmune disease, Charlson index of chronic diseases); Model 4=model 3+farm history. Models assessing frequency and duration of personal use of insecticides were adjusted for application by others, and visa versa. Other covariates were not included in the final models either because they were not independently associated with disease risk or they did not contribute additional information to the models. Income and employment were marginally associated with RA/SLE status, but were dropped due to having a larger proportion of missing data.
We modeled associations for each of the two types of exposure (personal use or application by others), both for duration and frequency separately and for cumulative measures. Trend test p-values were obtained from models including categorical variables with different levels of duration/frequency, assuming a monotonic increase in effect across levels (significance p<0.05). To reduce the influence of small cell sizes, some categories were combined for duration (none/<1, 1-4, 5-19, 20 or more years) and frequency (never/<1, 1-5, 6 or more times per year). Cumulative scores for “application-years” were constructed as the product of years and frequency of use. Each category was assigned the midpoint value (i.e., 7 for 5-9 years) or 50% above the highest category (i.e., 20 or more years was assigned a value of 30). If duration or frequency was missing, a value was assigned for use in cumulative estimates based on the most common level reported by others in the same duration or frequency stratum among non-cases. Analyses were also run excluding participants with missing frequency or duration data (<1% cases and non-cases; for personal use n=3 cases, n=618 non-cases, for application by others n=1 case, n=590 non-cases). These cumulative scores were then re-grouped for analyses, combining levels with sparse numbers of cases to derive a 3 level variable (0-2 = never/very low, 3-100 = low/moderate, more than 100 = high/very high).
Effect modification was considered in stratified analyses. To quantify apparent differences, we tested interactions by product terms included in a single multivariable model. Specifically we examined whether associations varied by farm history, which might affect pesticide use patterns and the distribution of unmeasured covariates.
RESULTS
A new diagnosis of RA/SLE with DMARD-use was reported by 213 women, for a total of 0.28% of the cohort in 3 years. Insecticide use, farm history, and characteristics of cohort participants and cases are shown in Table 1. The majority of cases (63%) reported insecticide exposure at home, while fewer (14%) also reported exposure at work, and there was no significant difference in exposure venue compared to other cohort participants. Cases were more likely to report personal use of insecticides (mixed/applied by self) than non-cases (45% versus 38%, p=0.010), but there was no significant difference in application by others. Having lived or worked on a farm was more common in cases than non-cases (34% versus 26%, p=0.025). Cases also had less education, were less likely to report professional or managerial occupations, and were more likely to report asthma or other autoimmune diseases. Compared to non-cases, RA cases reported higher smoking pack years, higher body mass index, and more comorbidities (higher Charlson Index), while SLE cases were younger, reported earlier menopause, differences in age at menarche, and were more likely to have breastfed.
Table 2 lists exposure-specific RA/SLE rates and adjusted hazard ratios. Women reporting no insecticide exposure had the lowest 3-year risk (0.22%) and rates were similar for women with exposure at home (0.30) or work (0.31). RA/SLE risk was also higher in women who reported not knowing about their insecticide exposures (0.35), and for those reporting personally mixing (0.32) and personally applying (0.34) insecticides, and other types of insecticide use (0.36). Compared to women reporting no exposures, personal use of insecticides (mixing or applying) was significantly associated with increased risk (age-adjusted HR=1.57). Women reporting frequent personal use had the highest risk of ARD (0.50% for 6 or more times per year), with significant dose-responses for duration, frequency, and cumulative use. Adding Model 3 covariates slightly diminished these associations (10-20%). For application by others, frequency and duration trends were non-significant, but higher risk was seen for the longest duration category (age-adjusted HR=1.85 for 20 years or more). In models including farm history, associations persisted for both personal use of insecticides and long-term application by others. Sensitivity analyses showed little impact of varying case and cohort definitions (not shown).
Table 2.
Associations of farm exposure and insecticide use with risk of developing RA/SLE in the WHI Observational Study
| Hazard Ratios (95% CI)* | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| 3-year risk RA/SLE |
Cases % |
OS Cohort % |
Age-adjusted (95% CI) |
Fully-adjusted† (95% CI) |
Farm-adjusted‡ (95% CI) |
|
| Insecticide use | N=206 | N=73,551 | ||||
| Never | 0.22 | 23 | 30 | 1.0 | 1.0 | 1.0 |
| At work only or home and work | 0.31 | 14 | 13 | 1.52 (0.96, 2.43) | 1.42 (0.85, 2.38) | 1.39 (0.83, 2.34) |
| At home only | 0.30 | 52 | 49 | 1.44 (1.02, 2.02) | 1.35 (0.92, 1.96) | 1.34 (0.92, 1.95) |
| Don’t know | 0.35 | 11 | 9 | 1.60 (0.97, 2.66) | 1.70 (1.00, 2.91) | 1.70 (1.00, 2.91) |
| Type of insecticide use | ||||||
| Reference=never or other method | 1.0 | 1.0 | 1.0 | |||
| Personally mixed | 0.32 | 15 | 13 | 1.28 (0.86, 1.89) | 1.36 (0.89, 2.08) | 1.34 (0.88, 2.06) |
| Personally applied | 0.34 | 46 | 38 | 1.57 (1.18, 2.11) | 1.51 (1.09, 2.09) | 1.50 (1.08, 2.07) |
| Lawn service applied | 0.29 | 20 | 19 | 1.09 (0.77, 1.55) | 1.23 (0.84, 1.79) | 1.24 (0.85, 1.81) |
| Commercial application | 0.22 | 15 | 19 | 0.78 (0.53, 1.15) | 0.73 (0.47, 1.14) | 0.74 (0.47, 1.15) |
| Other | 0.36 | 12 | 9 | 1.43 (0.93, 2.20) | 1.29 (0.79, 2.09) | 1.28 (0.79, 2.07) |
|
| ||||||
| Personally mixed or applied | ||||||
|
| ||||||
| Years | N=181 | N=66,627 | ||||
| Never used insecticides | 0.22 | 27 | 33 | 1.0 | 1.0 | 1.0 |
| Never/ < 1 | 0.21 | 21 | 27 | 1.05 (0.65, 1.70) | 0.95 (0.56, 1.62) | 0.94 (0.55, 1.60) |
| 1-4 | 0.36 | 16 | 12 | 1.82 (1.12, 2.95) | 1.52 (0.88, 2.64) | 1.50 (0.87, 2.61) |
| 5-19 | 0.29 | 15 | 14 | 1.45 (0.89, 2.36) | 1.26 (0.72, 2.19) | 1.24 (0.71, 2.16) |
| 20 or more | 0.43 | 21 | 13 | 2.07 (1.31, 3.25) | 1.97 (1.20, 3.23) | 1.94 (1.18, 3.19) |
| P-value for trend | <0.001 | 0.003 | 0.003 | |||
| Times per year | N=180 | N=66,508 | ||||
| Never used insecticides | 0.22 | 27 | 33 | 1.0 | 1.0 | 1.0 |
| Never/ < 1 | 0.24 | 24 | 28 | 1.24 (0.78, 1.98) | 0.98 (0.58, 1.66) | 0.97 (0.57, 1.65) |
| 1-5 | 0.30 | 34 | 31 | 1.52 (1.02, 2.29) | 1.49 (0.96, 2.33) | 1.47 (0.94, 2.30) |
| 6 or more | 0.50 | 15 | 8 | 2.47 (1.51, 4.03) | 2.04 (1.17, 3.56) | 2.01 (1.15, 3.51) |
| P-value for trend | <0.001 | 0.003 | 0.003 | |||
| Cumulative use | N=182 | N=66,878 | ||||
| Never/very low | 0.23 | 50 | 60 | 1.0 | 1.0 | 1.0 |
| Low/moderate | 0.30 | 37 | 34 | 1.35 (0.98, 1.85) | 1.48 (1.04, 2.09) | 1.46 (1.03, 2.07) |
| High/very high | 0.53 | 13 | 6 | 2.36 (1.49, 3.74) | 2.20 (1.31, 3.71) | 2.17 (1.29, 3.67) |
| P-value for trend | <0.001 | 0.001 | 0.002 | |||
|
| ||||||
| Application by others | ||||||
|
| ||||||
| Years | N=182 | N=66,796 | ||||
| Never used insecticides | 0.22 | 26 | 33 | 1.0 | 1.0 | 1.0 |
| Never/ < 1 | 0.34 | 25 | 20 | 1.26 (0.74, 2.12) | 1.10 (0.62, 1.97) | 1.10 (0.61, 1.96) |
| 1-4 | 0.22 | 13 | 15 | 0.89 (0.51, 1.56) | 0.89 (0.48, 1.64) | 0.89 (0.48, 1.64) |
| 5-19 | 0.23 | 18 | 21 | 0.95 (0.57, 1.57) | 0.93 (0.53, 1.63) | 0.93 (0.53, 1.63) |
| 20 or more | 0.45 | 18 | 11 | 1.85 (1.13, 3.04) | 1.85 (1.07, 3.20) | 1.86 (1.07, 3.21) |
| P-value for trend | 0.18 | 0.14 | 0.13 | |||
| Times per year | N=181 | N=66,543 | ||||
| Never used insecticides | 0.22 | 27 | 33 | 1.0 | 1.0 | 1.0 |
| Never/ < 1 | 0.36 | 27 | 21 | 1.39 (0.83, 2.31) | 1.20 (0.68, 2.12) | 1.19 (0.67, 2.11) |
| 1-5 | 0.29 | 37 | 35 | 1.19 (0.77, 1.85) | 1.19 (0.73, 1.92) | 1.19 (0.73, 1.93) |
| 6 or more | 0.23 | 9 | 11 | 0.98 (0.54, 1.80) | 0.99 (0.51, 1.91) | 0.99 (0.51, 1.91) |
| P-value for trend | 0.85 | 0.90 | 0.89 | |||
| Cumulative use | N=182 | N=66,965 | ||||
| Never/very low | 0.27 | 51 | 52 | 1.0 | 1.0 | 1.0 |
| Low/moderate | 0.29 | 43 | 41 | 1.01 (0.74, 1.37) | 1.06 (0.75, 1.49) | 1.06 (0.75, 1.50) |
| High/very high | 0.23 | 5 | 6 | 0.81 (0.42, 1.56) | 0.89 (0.44, 1.80) | 0.90 (0.44, 1.82) |
| P-value for trend | 0.70 | 0.98 | 0.99 | |||
Hazard ratios and 95% confidence intervals estimated from a Cox proportional hazards regression. Models analyzing frequency, duration and cumulative dose of personal use were also adjusted for any commercial use; similarly, models analyzing commercial exposure were also adjusted for any personal use.
Adjusted for age (5 year intervals), race/ethnicity, education, occupation, region and pack-years of smoking), BMI, parity, months breastfed, age at menarche, age at menopause, history of asthma, history of other autoimmune disease, history of thyroid disease, and modified Charlson index.
Adjusted for covariates in fully-adjusted model plus farm history.
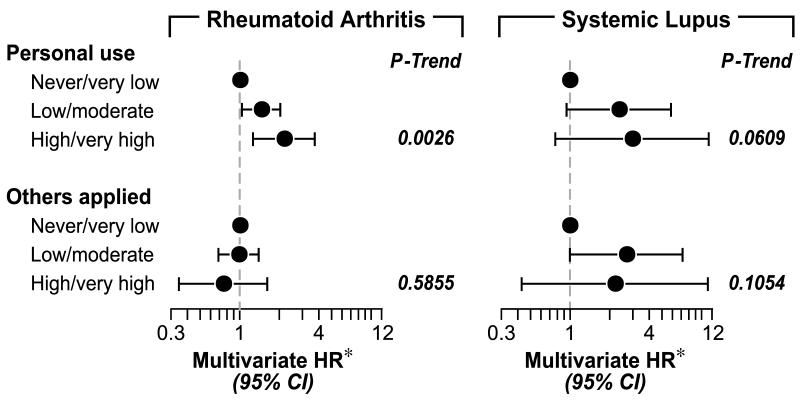
Disease-specific risks ranged from 0.02% for SLE and 0.20% for RA in those reporting no or low personal exposure-years to 0.12% for SLE and 0.44% for RA in those reporting high or very high cumulative personal use. Figure 1 shows associations for insecticide use in relation to RA or SLE, with similar findings for personal use as for RA/SLE combined. Application by others was non-significantly associated with SLE (trend, p=0.10). Other significant associations in multivariate models for RA included older age, higher BMI, non-professional/managerial occupation, asthma and other reported autoimmune diseases, while SLE-specific models showed significant associations with lower education (not shown).
Figure 1.
Disease-specific models of insecticide exposure and risk of RA or SLE
*Hazard ratios and 95% confidence intervals estimated from a Cox proportional hazards regression model, adjusted for age (5 year intervals), race/ethnicity, education, occupation, region, pack-years of smoking, BMI, parity, months breastfed, age at menarche, age at menopause, history of asthma, history of other autoimmune disease, history of thyroid disease, modified Charlson index and farm exposure). Models analyzing frequency, duration and cumulative dose of personal use were also adjusted for any application by others, and visa versa.
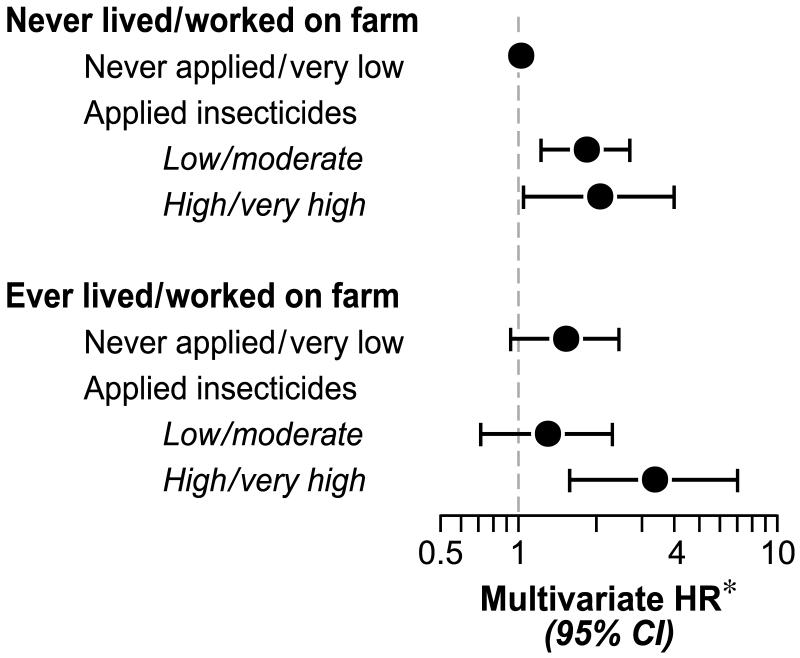
RA/SLE risk was elevated in women with longer duration farm experience (0.48% for 20 years or more living or working on a farm), with an age-adjusted HR of 1.97 for 20 years or more (95% CI 1.14, 3.42). This diminished after adjusting for Model 3 covariates and was substantially attenuated in models accounting for cumulative personal use of insecticides and application by others (fully adjusted HR=1.22, 95%CI 0.58, 2.56). The association of RA/SLE with application by others differed significantly by farm history of the woman (Table 3; interaction p=0.004): In women without a farm history, frequent application by others was not associated with increased RA/SLE risk (adj.HR=0.35 for 6 or more times per year), whereas among women with a farm history the adj.HR was 2.95 for 6 or more times per year. The highest risk (0.81%) was seen in women with a farm history who reported higher cumulative personal use of insecticides, and the lowest risk (0.20%) was seen in women with no reported farm history or insecticide use. The risk associated with farm history plus higher insecticide use (age-adj. HR=4.02) was somewhat reduced (adj. HR=3.36) adjusting for Model 3 covariates (Figure 2).
Table 3.
Associations of RA/SLE with personal insecticide use and application by others, stratified by farm history
| Never lived or worked on a farm | Ever lived or worked on a farm | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 3 year risk RA/SLE |
Cases % |
OS Cohort % |
Adjusted HR* (95% CI) |
3 year risk RA/SLE |
Cases % |
OS Cohort % |
Adjusted HR* (95% CI) |
|
| Personally mixed/applied | ||||||||
| Years | N=124 | N=49,255 | N=57 | N=17,372 | ||||
| Never used insecticides | 0.20 | 26 | 33 | 1.0 | 0.28 | 28 | 33 | 1.0 |
| Never/ < 1 | 0.19 | 22 | 28 | 1.01 (0.53, 1.92) | 0.26 | 19 | 24 | 0.80 (0.30, 2.11) |
| 1-4 | 0.39 | 19 | 12 | 1.92 (1.01, 3.63) | 0.28 | 11 | 12 | 0.74 (0.25, 2.24) |
| 5-19 | 0.25 | 14 | 14 | 1.31 (0.66, 2.57) | 0.41 | 19 | 16 | 1.09 (0.41, 2.88) |
| 20 or more | 0.41 | 12 | 12 | 2.40 (1.33, 4.33) | 0.49 | 23 | 15 | 1.22 (0.49, 3.05) |
| P-value for trend | 0.002 | 0.47 | ||||||
| Times per year | N=124 | N=49,175 | N=56 | N=17,333 | ||||
| Never used insecticides | 0.20 | 26 | 33 | 1.0 | 0.28 | 29 | 33 | 1.0 |
| Never/ < 1 | 0.22 | 25 | 29 | 1.00 (0.53, 1.90) | 0.31 | 23 | 24 | 0.89 (0.34, 2.30) |
| 1-5 | 0.31 | 37 | 30 | 1.91 (1.24, 3.21) | 0.26 | 27 | 33 | 0.72 (0.30, 1.74) |
| 6 or more | 0.40 | 12 | 8 | 2.01 (0.99, 4.08) | 0.71 | 23 | 10 | 1.93 (0.76, 4.90) |
| P-value for trend | 0.003 | 0.43 | ||||||
| Cumulative use | N=125 | N=49,447 | N=57 | N=17,431 | ||||
| Never/very low | 0.21 | 50 | 61 | 1.0 | 0.30 | 51 | 56 | 1.0 |
| Low/moderate | 0.31 | 41 | 33 | 1.85 (1.22, 2.80) | 0.27 | 30 | 37 | 0.80 (0.41, 1.56) |
| High/very high | 0.40 | 10 | 6 | 2.15 (1.08, 4.29) | 0.81 | 19 | 8 | 2.18 (0.96, 4.91) |
| P-value for trend | 0.002 | 0.28 | ||||||
|
| ||||||||
| Others applied | ||||||||
| Years | N=124 | N=49,401 | N=58 | N=17,395 | ||||
| Never used insecticides | 0.20 | 26 | 33 | 1.0 | 0.28 | 28 | 32 | 1.0 |
| Never/ < 1 | 0.38 | 29 | 19 | 1.23 (0.61, 2.46) | 0.25 | 17 | 23 | 0.72 (0.24, 2.21) |
| 1-4 | 0.19 | 12 | 16 | 0.76 (0.31, 1.61) | 0.31 | 14 | 15 | 1.21 (0.42, 3.49) |
| 5-19 | 0.17 | 14 | 21 | 0.64 (0.31, 1.34) | 0.42 | 24 | 19 | 1.77 (0.71, 4.40) |
| 20 or more | 0.43 | 18 | 11 | 1.79 (0.92, 3.46) | 0.54 | 17 | 11 | 2.05 (0.75, 5.55) |
| P-value for trend | 0.73 | 0.028 | ||||||
| Times per year | N=124 | N=49,236 | N=57 | N=17,307 | ||||
| Never used insecticides | 0.20 | 26 | 33 | 1.0 | 0.28 | 28 | 33 | 1.0 |
| Never/ < 1 | 0.40 | 31 | 20 | 1.30 (0.66, 2.58) | 0.25 | 18 | 23 | 0.83 (0.27, 2.51) |
| 1-5 | 0.28 | 40 | 36 | 1.14 (0.63, 2.05) | 0.31 | 32 | 34 | 1.25 (0.52, 3.01) |
| 6 or more | 0.07 | 3 | 11 | 0.34 (0.11, 1.02) | 0.69 | 23 | 11 | 2.95 (1.16, 7.52) |
| P-value for trend | 0.14 | 0.019 | ||||||
| Cumulative use | N=124 | N=49,523 | N=58 | N=17,422 | ||||
| Never/very low | 0.27 | 55 | 52 | 1.0 | 0.27 | 43 | 54 | 1.0 |
| Low/moderate | 0.26 | 43 | 42 | 0.56 (0.57, 1.30) | 0.37 | 45 | 40 | 1.75 (0.91, 3.35) |
| High/very high | 0.09 | 2 | 7 | 0.35 (0.11, 1.13) | 0.63 | 12 | 6 | 2.93 (1.13, 7.64) |
| P-value for trend | 0.10 | 0.017 | ||||||
Hazard ratios and 95% confidence intervals estimated from a Cox proportional hazards regression model, adjusted for age (5 year intervals), race/ethnicity, education, occupation, region, pack-years of smoking, BMI, parity, months breastfed, age at menarche, age at menopause, history of asthma, history of other autoimmune disease, history of thyroid disease, modified Charlson index and farm exposure). Models analyzing frequency, duration and cumulative dose of personal use were also adjusted for any application by others, and visa versa.
Figure 2.
Joint effect of farm history and personal insecticide exposure on RA/SLE risk.
*Hazard ratios and 95% confidence intervals estimated from a Cox proportional hazards regression model, adjusted for age (5 year intervals), race/ethnicity, education, occupation, region, pack-years of smoking, BMI, parity, months breastfed, age at menarche, age at menopause, history of asthma, history of other autoimmune disease, history of thyroid disease, modified Charlson index and farm exposure). Models analyzing frequency, duration and cumulative dose of personal use were also adjusted for any application by others.
DISCUSSION
Our findings are consistent with the hypothesis that insecticide exposure may increase ARD risk in post-menopausal women. Our study extends previous evidence of an association of farming by showing an association of RA/SLE with self-reported personal use of insecticides, primarily in residential settings. These findings suggest exposure to residential insecticides may play a role in RA/SLE etiology in the general population as well as in those occupationally exposed. The plausibility of our findings is strengthened by the dose-response seen for increasing frequency or duration of personal use, and similar findings for RA and SLE. The observed associations also did not appear to be explained by farm history or other risk factors examined.
In contrast, an apparent association of self-reported farm history (ever lived or worked) and RA/SLE risk was partly explained by covariate adjustment and substantially attenuated in models including insecticide exposure variables. However, risk was highest in women who reported both a farm history and higher personal use of insecticides or application by others. Farm women may report insecticide use more accurately, have higher exposures within the same use categories, or apply different products. Compared to non-farmers, measurement studies have shown higher exposures in farmers who applied pesticides and among family members living in a farm environment where pesticides were used (22, 23). Other farming practices or exposures (e.g., inorganic and organic dusts) also might impact ARD risk (24, 25).
Our study is subject to limitations inherent to its design and use of self-reported data in a cohort not optimized for either these specific exposures or outcomes. Nevertheless, our case definition was likely to identify actively treated RA/SLE patients with a first diagnosis during follow-up. Although self-report of ARD is non-specific, this definition was based on a previous validation in WHI participants showing high specificity of cases identified by self-reported RA or SLE plus current DMARD use compared to the gold standard of chart review (21). With this definition, estimated occurrence of new RA/SLE in the cohort (0.28% in 3 years) was within an expected range for this population (e.g., incidence of 1 per 1000 per year for RA)(26). DMARD use in self-reported cases varied by race and education status (not shown); if such factors were related to disease diagnosis or DMARD treatment in true cases, our findings may not represent the full range of incident RA/SLE. Also, while we expected our case definition to be highly specific, some true cases were likely included in the baseline sample due to the lack of concurrent DMARD use. New cases not reporting DMARDs may have been missed by our case definition. Corticosteroid use, e.g., prednisone, was included as a DMARD in our case definition, but is widely used for other conditions and was the most common DMARD reported by non-cases and incident cases at baseline. The influence of a small proportion of false-negative cases in the cohort is probably minimal, however, and sensitivity analyses showed no substantive differences in main effects based on various alternative definitions of case and non-case groups.
Relatively few studies have considered risk factors for RA and SLE in parallel and exclusively among post-menopausal women. SLE has a younger peak of age at onset, so we cannot know if findings are generalizable to the majority of SLE patients. We saw some expected associations, primarily for RA; for example, older age was a significant risk factor for RA. Smoking was uncommon and only modestly associated with RA, whereas a fairly consistent association of smoking and RA has been observed in other studies(27). Other risk factors for RA were higher BMI, and history of diagnosis with other autoimmune diseases and asthma. We also observed associations of potential indicators of lower SES were associated with increased RA/SLE risk; specifically non-professional or managerial occupation was associated with RA and lower education with SLE. Lower socioeconomic status (SES) has previously been associated with RA in previous studies (28-30), though it is unclear whether such findings are influenced by biased case ascertainment methods or cohort participation. Individual measures of SES could plausibly be related to a variety of other risk factors or exposures on the pathway leading to autoimmunity, or to more broadly measured community-level characteristics, such as neighborhood poverty. A recent study described regional differences in RA incidence in a large cohort of nurses, with higher rates in the Northeast compared to the Western U.S.(31). We saw no evidence of this pattern in the WHI; instead, RA and SLE rates were slightly, but non-significantly higher in the South and Midwest compared to the Northeast. We are unaware of variations in diagnosis or treatment practices that might lead to such differences, though one study described clusters of SLE mortality in the South/Southwest partially attributed to geographic differences in poverty (32).
Our findings are primarily based on exposure data collected before report of incident ARD, with limited potential for differential recall or reporting bias. Cases could have had symptoms when they reported their exposure, but we saw little impact of excluding cases reported in the first year of follow-up. Although recall of insecticide use is prone to non-specific error, a recent study showed high reliability of self-reported residential insecticide use (33). Considering likely sources of error, we might expect the observed associations to be attenuated estimates of the underlying relationship. We had no information on types of pests, application practices, or chemicals used; so our results reflect average risks that combine likely differing risks for (unmeasured) specific exposures.
Associations with insecticides were not meaningfully changed in models adjusting for a range of demographic, socioeconomic, health and behavioral covariates. However, some effect estimates were reduced by >10% in these full models, suggesting potential confounding by covariates examined. Although we adjusted for several factors associated with SES (education, occupation, health covariates), there may be residual confounding because SES is a complicated construct encompassing diverse individual and community level characteristics. The observed associations could have been influenced by other unmeasured participant characteristics, such as living conditions favoring insects, use of other household chemical products, or other personal, household, or environmental factors. To confound the observed associations, however, such factors would have to be strongly associated with both ARD risk and insecticide use. We are unaware of known risk factors that might explain the observed associations.
Because two SES measures (education and occupation) were related to RA/SLE risk, we further explored associations of RA/SLE with insecticides in stratified analyses (results not shown). Occupational class might be related to differences in occupational and environmental exposures, while education could impact awareness of insecticides as a potential hazard, use of measures to reduce personal exposures, or accuracy of reported insecticide use. Self-report of “not knowing” about insecticide use (8.5% of the cohort, 9.3% of RA and 17% of SLE cases) was associated with RA/SLE in adjusted models. Both the associations of RA/SLE with personal use of insecticides and with “not knowing” were the least apparent in women with a high school education or less and in those working in technical/sales/administrative jobs. However, both associations were clearly seen in women at higher education levels or in those classified as working in jobs classified as managerial/professional, service/labor or homemakers. These apparent effect differences by SES highlight a need to further understand SES-related differences in RA/SLE risk in the cohort.
We conducted other exploratory analyses to better understand the observed associations (results not shown), speculating that insecticide use and associations might vary regionally or by age. Personal use of insecticides was mostly associated with RA/SLE in younger participants, while the association with longer term application by others was primarily seen in older participants. This may reflect differences in recall, age at exposure, time since use, temporal trends in products available for personal and professional use, as well as the potential for longer duration of insecticide use in the older participants. The association of RA/SLE risk with personal use of insecticides was more apparent in the West and Southeast, where higher insecticide use was more common in both the cohort and cases. Measurement studies show geographic differences in types and amount of insecticides used and detected in environmental sampling (34), but it is premature to speculate on how these might relate to potential regional differences in association.
The mechanisms by which insecticides might lead to development of ARD are diverse, and few studies have been conducted on animal models of autoimmunity. Toxicology studies suggest a complex relationship given the diversity of pesticide effects on the immune system (35, 36): pesticides may impact differentiation and regulation of adaptive and innate immune responses, leading to acute and chronic immune suppression and decreased response to infections, inflammation and autoimmunity. Exposures may directly impact disease risk, such as acceleration of autoimmunity in lupus prone mice (37). Indirect effects could include modification of responses to other exposures, such as interactions of organophosphates and endotoxins, a common combination in agricultural settings(38).
The older age of the WHI OS cohort is in some ways well suited to studies of environmental risk factors for ARD. Older onset and the declining female to male ratio in older onset ARD may reflect a larger component of risk due to non-intrinsic, non-hormonal or environmental factors (39-42). Our findings are consistent with a hypothesis that personal insecticide use, primarily residential, may increase risk of developing ARD in post-menopausal women. Work is needed to further characterize RA and SLE cases in the WHI and conduct a detailed assessment of other environmental exposures. Our findings support the need for replication studies in other populations and research to identify specific chemicals that increase susceptibility or promote ARD risk. Finally, they provide new evidence of a potential role for a common environmental exposure in risk of developing autoimmune diseases.
Supplementary Material
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221.
REFERENCES
- 1.Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part I. Arthritis Rheum. 2008;58:15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 2.Lockshin MD. Sex ratio and rheumatic disease: excerpts from an Institute of Medicine report. Lupus. 2002;11:662–6. doi: 10.1191/0961203302lu274oa. [DOI] [PubMed] [Google Scholar]
- 3.Lau CS, Yin G, Mok MY. Ethnic and geographical differences in systemic lupus erythematosus: an overview. Lupus. 2006;15:715–9. doi: 10.1177/0961203306072311. [DOI] [PubMed] [Google Scholar]
- 4.Tutuncu Z, Kavanaugh A. Rheumatic disease in the elderly: rheumatoid arthritis. Clin Geriatr Med. 2005;21:513–25. vi. doi: 10.1016/j.cger.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Gourley M, Miller FW. Mechanisms of disease: Environmental factors in the pathogenesis of rheumatic disease. Nat Clin Pract Rheumatol. 2007;3:172–80. doi: 10.1038/ncprheum0435. [DOI] [PubMed] [Google Scholar]
- 6.Alamanos Y, Drosos AA. Epidemiology of adult rheumatoid arthritis. Autoimmun Rev. 2005;4:130–6. doi: 10.1016/j.autrev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Parks CG, Cooper GS. Occupational exposures and risk of systemic lupus erythematosus: a review of the evidence and exposure assessment methods in population- and clinic-based studies. Lupus. 2006;15:728–36. doi: 10.1177/0961203306069346. [DOI] [PubMed] [Google Scholar]
- 8.Gold LS, Ward MH, Dosemeci M, De Roos AJ. Systemic autoimmune disease mortality and occupational exposures. Arthritis Rheum. 2007;56:3189–201. doi: 10.1002/art.22880. [DOI] [PubMed] [Google Scholar]
- 9.Khuder SA, Peshimam AZ, Agraharam S. Environmental risk factors for rheumatoid arthritis. Rev Environ Health. 2002;17:307–15. doi: 10.1515/reveh.2002.17.4.307. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Sundquist J, Sundquist K. Socioeconomic and occupational risk factors for rheumatoid arthritis: a nationwide study based on hospitalizations in Sweden. J Rheumatol. 2008;35:986–91. [PubMed] [Google Scholar]
- 11.Milham S., Jr. Using multiple cause of death coding in occupational mortality studies. Am J Ind Med. 1988;14:341–4. doi: 10.1002/ajim.4700140311. [DOI] [PubMed] [Google Scholar]
- 12.Olsson AR, Skogh T, Axelson O, Wingren G. Occupations and exposures in the work environment as determinants for rheumatoid arthritis. Occup Environ Med. 2004;61:233–8. doi: 10.1136/oem.2003.007971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee E, Burnett CA, Lalich N, Cameron LL, Sestito JP. Proportionate mortality of crop and livestock farmers in the United States, 1984-1993. Am J Ind Med. 2002;42:410–20. doi: 10.1002/ajim.10131. [DOI] [PubMed] [Google Scholar]
- 14.Lundberg I, Alfredsson L, Plato N, et al. Occupation, occupational exposure to chemicals and rheumatological disease. A register based cohort study. Scand J Rheumatol. 1994;23:305–10. doi: 10.3109/03009749409099278. [DOI] [PubMed] [Google Scholar]
- 15.Olsson AR, Skogh T, Wingren G. Occupational determinants for rheumatoid arthritis. Scand J Work Environ Health. 2000;26:243–9. doi: 10.5271/sjweh.538. [DOI] [PubMed] [Google Scholar]
- 16.De Roos AJ, Cooper GS, Alavanja MC, Sandler DP. Rheumatoid arthritis among women in the Agricultural Health Study: risk associated with farming activities and exposures. Ann Epidemiol. 2005;15:762–70. doi: 10.1016/j.annepidem.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Lee DH, Steffes M, Jacobs DR. Positive associations of serum concentration of polychlorinated biphenyls or organochlorine pesticides with self-reported arthritis, especially rheumatoid type, in women. Environ Health Perspect. 2007;115:883–8. doi: 10.1289/ehp.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper GS, Parks CG, Treadwell EL, et al. Occupational risk factors for the development of systemic lupus erythematosus. J Rheumatol. 2004;31:1928–33. [PubMed] [Google Scholar]
- 19.Cooper GS, Parks CG, Schur PS, Fraser PA. Occupational and environmental associations with antinuclear antibodies in a general population sample. J Toxicol Environ Health A. 2006;69:2063–9. doi: 10.1080/15287390600746165. [DOI] [PubMed] [Google Scholar]
- 20.Langer RD, White E, Lewis CE, et al. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13:S107–21. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 21.Walitt B, Constantiinescu F, Katz J, et al. Validation of the self-report of Rheumatoid Arthritis and Systemic Lupus Erythematosus: The Women’s Health Initiative. J Rheumatol. 2008 in press. [PMC free article] [PubMed] [Google Scholar]
- 22.Curwin BD, Hein MJ, Sanderson WT, et al. Pesticide dose estimates for children of Iowa farmers and non-farmers. Environ Res. 2007;105:307–15. doi: 10.1016/j.envres.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Curwin BD, Hein MJ, Sanderson WT, et al. Urinary and hand wipe pesticide levels among farmers and nonfarmers in Iowa. J Expo Anal Environ Epidemiol. 2005;15:500–8. doi: 10.1038/sj.jea.7500428. [DOI] [PubMed] [Google Scholar]
- 24.Parks CG, Cooper GS, Dooley MA, et al. Childhood agricultural and adult occupational exposures to organic dusts in a population-based case-control study of systemic lupus erythematosus. Lupus. 2008;17:711–9. doi: 10.1177/0961203308089436. [DOI] [PubMed] [Google Scholar]
- 25.Parks CG, Cooper GS, Nylander-French LA, et al. Occupational exposure to crystalline silica and risk of systemic lupus erythematosus: a population-based, case-control study in the southeastern United States. Arthritis Rheum. 2002;46:1840–50. doi: 10.1002/art.10368. [DOI] [PubMed] [Google Scholar]
- 26.Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the incidence of rheumatoid arthritis rising? Results from olmsted county, minnesota, 1955-2007. Arthritis Rheum. doi: 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harel-Meir M, Sherer Y, Shoenfeld Y. Tobacco smoking and autoimmune rheumatic diseases. Nat Clin Pract Rheumatol. 2007;3:707–15. doi: 10.1038/ncprheum0655. [DOI] [PubMed] [Google Scholar]
- 28.Pedersen M, Jacobsen S, Klarlund M, Frisch M. Socioeconomic status and risk of rheumatoid arthritis: a Danish case-control study. J Rheumatol. 2006;33:1069–74. [PubMed] [Google Scholar]
- 29.Bengtsson C, Nordmark B, Klareskog L, Lundberg I, Alfredsson L. Socioeconomic status and the risk of developing rheumatoid arthritis: results from the Swedish EIRA study. Ann Rheum Dis. 2005;64:1588–94. doi: 10.1136/ard.2004.031666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reckner Olsson A, Skogh T, Wingren G. Comorbidity and lifestyle, reproductive factors, and environmental exposures associated with rheumatoid arthritis. Ann Rheum Dis. 2001;60:934–9. doi: 10.1136/ard.60.10.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costenbader KH, Chang SC, Laden F, Puett R, Karlson EW. Geographic variation in rheumatoid arthritis incidence among women in the United States. Arch Intern Med. 2008;168:1664–70. doi: 10.1001/archinte.168.15.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walsh SJ, Gilchrist A. Geographical clustering of mortality from systemic lupus erythematosus in the United States: contributions of poverty, Hispanic ethnicity and solar radiation. Lupus. 2006;15:662–70. doi: 10.1191/0961203306071455. [DOI] [PubMed] [Google Scholar]
- 33.Fortes C, Mastroeni S, Boffetta P, et al. Reliability of self-reported household pesticide use. Eur J Cancer Prev. 2009;18:404–6. doi: 10.1097/CEJ.0b013e32832caab5. [DOI] [PubMed] [Google Scholar]
- 34.Colt JS, Lubin J, Camann D, et al. Comparison of pesticide levels in carpet dust and self-reported pest treatment practices in four US sites. J Expo Anal Environ Epidemiol. 2004;14:74–83. doi: 10.1038/sj.jea.7500307. [DOI] [PubMed] [Google Scholar]
- 35.Holsapple MP. Autoimmunity by pesticides: a critical review of the state of the science. Toxicol Lett. 2002;127:101–9. doi: 10.1016/s0378-4274(01)00489-1. [DOI] [PubMed] [Google Scholar]
- 36.Luebke RW, Parks C, Luster MI. Suppression of immune function and susceptibility to infections in humans: association of immune function with clinical disease. J Immunotoxicol. 2004;1:15–24. doi: 10.1080/15476910490438342. [DOI] [PubMed] [Google Scholar]
- 37.Sobel ES, Gianini J, Butfiloski EJ, et al. Acceleration of autoimmunity by organochlorine pesticides in (NZB x NZW)F1 mice. Environ Health Perspect. 2005;113:323–8. doi: 10.1289/ehp.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duramad P, Harley K, Lipsett M, et al. Early environmental exposures and intracellular Th1/Th2 cytokine profiles in 24-month-old children living in an agricultural area. Environ Health Perspect. 2006;114:1916–22. doi: 10.1289/ehp.9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Schaardenburg D, Breedveld FC. Elderly-onset rheumatoid arthritis. Semin Arthritis Rheum. 1994;23:367–78. doi: 10.1016/0049-0172(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 40.Boddaert J, Huong DL, Amoura Z, et al. Late-onset systemic lupus erythematosus: a personal series of 47 patients and pooled analysis of 714 cases in the literature. Medicine (Baltimore) 2004;83:348–59. doi: 10.1097/01.md.0000147737.57861.7c. [DOI] [PubMed] [Google Scholar]
- 41.Rovensky J, Tuchynova A. Systemic lupus erythematosus in the elderly. Autoimmun Rev. 2008;7:235–9. doi: 10.1016/j.autrev.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 42.Papadopoulos IA, Katsimbri P, Alamanos Y, Voulgari PV, Drosos AA. Early rheumatoid arthritis patients: relationship of age. Rheumatol Int. 2003;23:70–4. doi: 10.1007/s00296-002-0251-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.