Abstract
Background
ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) is a recently identified family of extracellular metalloproteinases that has been shown to participate in tissue destruction. We hypothesized that ADAMTS-1 and ADAMTS-4 expression is increased in aortic tissues from patients with thoracic aortic aneurysms and dissections.
Methods
We examined ADAMTS-1 and ADAMTS-4 expression in human descending thoracic aortic aneurysms (n=14), chronic descending thoracic aortic dissections (n=16), and descending thoracic aortas from age-matched control organ donors (n=12). In these tissues, we also evaluated the degradation of versican, a proteoglycan substrate of ADAMTS-1 and ADAMTS-4. In cultured macrophages, we examined whether ADAMTS-4 functions in macrophage infiltration by using a transwell assay.
Results
ADAMTS-1 and ADAMTS-4 protein and mRNA expression was significantly higher in thoracic aortic aneurysm and dissection tissues than in control aortic tissues. Double immunofluorescence staining showed the expression of ADAMTS-1 and ADAMTS-4 in smooth muscle cells and macrophages. Consistent with the upregulation of ADAMTS-1 and ADAMTS-4 in thoracic aortic aneurysm and dissection tissues, versican was degraded significantly more in these tissues than in control aortic tissues. In cultured macrophages, transforming growth factor-β increased ADAMTS-4 protein levels and induced macrophage invasion; knocking down ADAMTS-4 reduced cell invasion.
Conclusions
Increased expression of ADAMTS proteins may promote thoracic aortic aneurysm progression by degrading versican and facilitating macrophage invasion.
Keywords: Aneurysm (thoracic aortic), inflammatory cells (macrophages), inflammatory mediators (metalloproteinases), pathology (aorta), vascular science (pathology)
Introduction
Aortic aneurysms and dissections cause more than 10,500 deaths in the United States every year [1]. These diseases are characterized by the infiltration of inflammatory cells, progressive depletion of vascular smooth muscle cells (VSMC), and destruction of extracellular matrix (ECM) in the aorta. Proteinases that degrade ECM are known to play critical roles in the degradation of aortic ECM and in the formation of aortic aneurysms. These proteinases include matrix metalloproteinases [2] (MMPs), which are primarily produced by macrophages. A recently identified family of extracellular metalloproteinases known as ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) has been shown to be involved in ECM turnover [3]. The ADAMTS family of proteins comprises 20 members; ADAMTS-1 and ADAMTS-4 are particularly well-studied members of this family [4]. ADAMTS-4 promotes ECM turnover by digesting proteoglycans including aggrecan, versican, decorin, and biglycan and ECM proteins such as fibronectin [5,6]. ADAMTS-1 functions in ECM remodeling by digesting versican and tissue factor pathway inhibitor-2 [6,7]. Furthermore, it has been shown that ADAMTS-1 inhibits cell proliferation by binding and sequestering growth factors, such as vascular endothelial growth factor and fibroblast growth factor [8,9]. Similar to MMPs, ADAMTS-1 and ADAMTS-4 cause tissue destruction and have been implicated in vascular diseases, such as atherosclerosis [10–14]. Therefore, we hypothesized that expression of these proteinases is increased in aneurysmal thoracic aortic tissue. The purpose of this study was to determine whether ADAMTS-1 and ADAMTS-4 levels are elevated in aortic tissue obtained from patients with thoracic aortic aneurysms and dissections. In addition, we examined potential mechanisms by which these proteinases might contribute to disease progression, including proteoglycan degradation and macrophage invasion.
Material and Methods
Patient Enrollment and Tissue Collection
This study was approved by the Institutional Review Board at Baylor College of Medicine, and all patients gave written informed consent. We enrolled 30 patients who underwent elective surgical repair of a descending thoracic aortic aneurysm without dissection (TAA; n=14) or associated with chronic dissection (TAD; n=16; time interval since dissection onset, 3.7±3.5 yrs). We excluded patients with any of the following: aortic disease caused by a genetic syndrome (eg, Marfan syndrome); a first-degree relative with TAA or TAD; bicuspid aortic valve; or acute symptoms. None of the aneurysms or dissections was induced by trauma, surgery, aortitis, or infection. In all cases, samples of the posterior-lateral aortic wall were excised from the site of maximal descending thoracic aortic dilatation during aneurysm repair. In cases with TAD, this region corresponded with the outer wall of the false lumen. After excision, samples were rinsed with cold saline and any attached thrombus was removed. From each fresh tissue sample, a portion was snap-frozen in liquid nitrogen and stored at −80°C (for protein extraction), a portion was placed in RNAlater (Qiagen, Valencia, CA) overnight at 4°C and then transferred and stored at −80°C (for RNA extraction), and a portion was fixed in formalin and embedded in paraffin (for staining). Control specimens of descending thoracic aorta (n=12) were obtained from age-matched organ donors (International Institute for the Advancement of Medicine, Jessup, PA and LifeNet Health, Virginia Beach, VA). Characteristics of enrolled subjects are presented in Table 1.
Table 1.
Patient Characteristics
| Characteristics | Control (n=12) | TAA (n=14) | TAD (n=16) |
|---|---|---|---|
| Age (y) | 59.1±8.2 | 64.8±5.5 | 63.8±5.6 |
| Male | 4 (33%) | 6 (43%) | 11 (69%) |
| History of smoking | 5 (42%) | 14 (100%) | 12 (75%) |
| History of hypertension | 6 (50%) | 13 (93%) | 16 (100%) |
| History of diabetes mellitus | 4 (33%) | 2 (14%) | 1 (6%) |
| History of anti-lipid medication use | 3 (25%) | 5 (36%) | 5 (31%) |
| History of cerebrovascular disease | 7 (58%) | 1 (7%) | 4 (25%) |
| Aortic diameter at sample site (cm) | N/A | 6.4±0.9 | 6.3±1.3 |
| History of chronic aneurysm symptoms | 0 | 10 (71%) | 10 (63%) |
N/A=not available; TAA=thoracic aortic aneurysm; TAD=thoracic aortic dissection.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted from aortic tissues (while on ice) with Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. The mRNA from each tissue sample was reverse-transcribed with a reverse transcription kit (Promega Corp., Madison, WI), and qRT-PCR was performed with the iCycler iQ PCR detection system (Bio-Rad, Hercules, CA). The primers used were as follows: ADAMTS1 forward 5′GGCCGACTGGGAAAGCGGAG 3′ and reverse 5′ TGCCATCGACTGGTCTGCCAC 3′, ADAMTS4 forward 5′TGCCAGCGGTCAAGGTCCCA 3′ and reverse 5′AGGTAGCGCTTTAGCCCCGC3′, and versican V0 forward 5′ AGGCTGGCAACAGTGGGGGA 3′ and reverse 5′ TGGGTGATGCAGTTTCTGCGAGG 3′. Levels of mRNA were acquired by normalizing the threshold cycle to that of 18S. The relative levels of mRNA were normalized with the mRNA level of an internal reference sample.
Western Blot Analysis
Protein extracts from aortic tissues were prepared with tissue lysis buffer (20mM Tris-HCl [pH 7.5], 150mM NaCl, 1mM Na2EDTA, 1mM EGTA, 2M urea, 1% Brij-35, 2.5mM sodium pyrophosphate, 1mM β-glycerophosphate). Protein samples (15 μg per lane) were separated by SDS-PAGE and transferred to PVDF membranes. The membranes were blocked with blocking buffer; incubated with primary antibody anti-ADAMTS-1, anti-ADAMTS-4, or anti-versican (Santa Cruz Biotechnology, Santa Cruz, CA); washed; and then incubated with horseradish peroxidase–labeled anti-goat IgG (Santa Cruz Biotechnology) or anti-mouse IgG (Cell Signaling Technology, Danvers, MA). Protein bands were visualized with enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ). Protein levels were normalized to those of β-actin and expressed as a percentage of the control.
Immunohistochemistry and Immunofluorescence
Aortic sections were deparaffinized and rehydrated before antigen retrieval in citrate buffer (92–98°C for 12 min). For immunohistochemical staining, endogenous peroxidase activity was quenched with 3% hydrogen peroxide. The sections were blocked with 5% horse serum, incubated with primary antibody anti-ADAMTS-1 or anti-ADAMTS-4 (Santa Cruz Biotechnology) at 4°C overnight and then incubated with secondary antibody. The sections were stained with 3, 3-diaminobenzidine by using the VECTASTAIN ABC kit (Vector Laboratories, Burlingame, CA). Nuclei were counterstained with hematoxylin. Slides incubated with normal IgG only were used as negative controls.
For double immunofluorescence staining, paraffin-embedded aortic sections were incubated with primary antibody overnight at 4°C followed by incubation with secondary antibody for 1 h at 37°C. Primary antibodies used were anti-SM22 alpha actin (Abcam, Cambridge, MA), anti-CD68, anti-ADAMTS-4, and anti-ADAMTS-1 (Santa Cruz Biotechnology). Secondary antibodies used were Alexa Fluor 488, Alexa Fluor 568, and Alexa Fluor 647 (Invitrogen). Cell nuclei were visualized with DAPI (4′,6-diamidino-2-phenylindole) staining.
Cell Culture and Transfection
THP-1 cells (ATCC, Manassas, VA) were cultured in RPMI 1640 medium (Invitrogen) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 5% L-glutamine. THP-1 cells were induced to differentiate into macrophages with 100nM phorbol 12-myristate 13-acetate (Alexis Biochemical, Farmingdale, NY) for 72 h. THP-1 macrophages were transfected with siRNA by using Lipofectamine 2000 (Invitrogen). Transfected cells were then treated with 0.3% H2O2, 10 ng/mL tumor necrosis factor-α, 10 ng/mL lipopolysaccharide, 10 ng/mL transforming growth factor (TGF)-β, or 10 ng/mL angiotensin II. ADAMTS-4 siRNA was purchased from Ambion (Ambion, Austin, TX). The siRNA duplex sequences for the ADAMTS-4 gene were as follows: sense, 5′-ACAACAAUGUGGUCACUAUTT-3′; and antisense, 5′AUAGUGACCACAUUGUUGUAT-3′. Silencer negative control siRNA (Ambion) was used as a negative control.
Macrophage Invasion Assay
Macrophage invasion was measured by using a modified Boyden chamber assay with an 8-μm pore size membrane coated with a thin layer of ECMatrix as a barrier (Chemicon International, Billerica, MA). Forty-eight hours after transfection, macrophages (2.5×105) were added to the upper chamber and were allowed to attach for 2 to 3 h. Unattached cells were removed by washing with phosphate-buffered saline, and the attached cells were incubated with 10 ng/mL TGF-β. The lower chamber contained 20 ng/mL monocyte chemoattractant protein-1 (EMD Chemicals, Inc., Gibbstown, NJ). Plates were incubated at 37°C for 48 h. The nonmigrating cells on the upper surface were removed by gentle abrasion with a cotton bud, and the cells on the underside (invaded cells) were fixed and stained. The mean number of cells on the lower side of the surface was calculated from 5 randomly selected fields (magnification ×100).
Statistical Analysis
All quantitative variables are presented as the mean ± standard deviation. The differences among 3 or more groups were compared by one-way analysis of variance. Correlation between ADAMTS-1 or ADAMTS-4 protein levels and versican degradation was analyzed by calculating the Pearson product-moment correlation coefficient. Two-tailed p-values are reported.
Results
Elevated ADAMTS-1 and ADAMTS-4 Expression in TAA and TAD Tissues
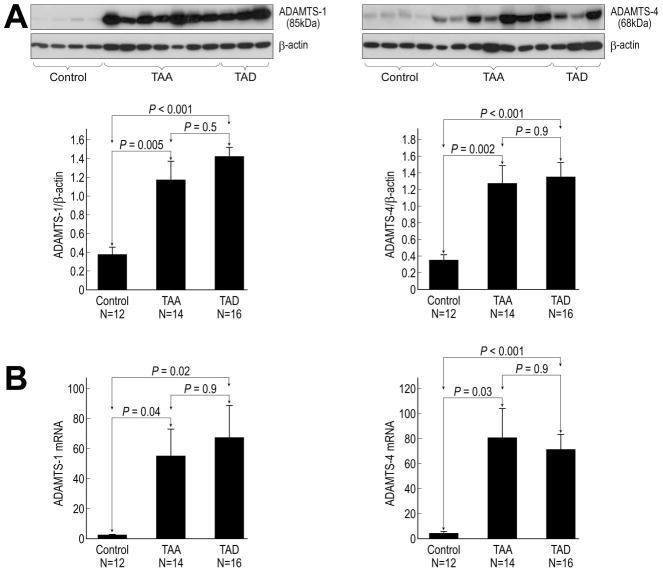
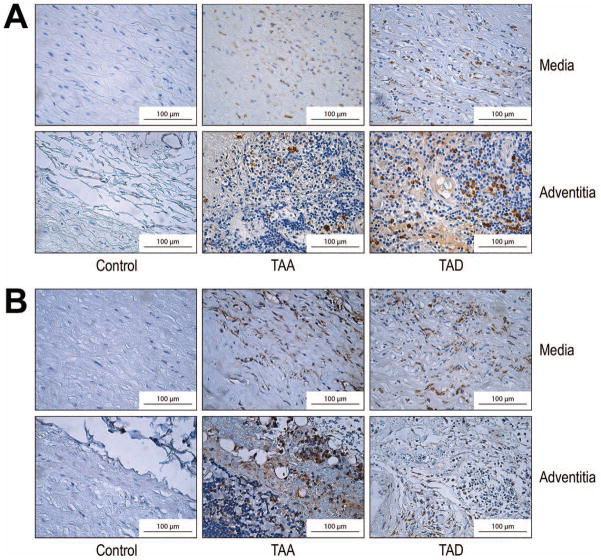
ADAMTS-1 and ADAMTS-4 protein levels were significantly higher in TAA and TAD tissues than in control aortic tissues (Fig 1A). Consistent with these results, ADAMTS-1 and ADAMTS-4 mRNA levels were also significantly increased in TAA and TAD tissues (Fig 1B). Moreover, immunohistochemical analysis revealed that ADAMTS-1 (Fig 2A) and ADAMTS-4 (Fig 2B) protein was abundant in the media and adventitia of the aortic wall in TAA and TAD tissues.
Fig 1.
Increased expression of ADAMTS-1 and ADAMTS-4 in aneurysm and dissection tissues. (A) Western blot and quantitative analysis of ADAMTS-1 and ADAMTS-4 proteins in control, thoracic aortic aneurysm (TAA), and thoracic aortic dissection (TAD) tissues. (B) Quantitative real-time PCR analysis of ADAMTS-1 and ADAMTS-4 mRNA levels in control, TAA, and TAD tissues.
Fig 2.
Increased abundance of ADAMTS-1 and ADAMTS-4 protein in the media and adventitia of aortic aneurysm and dissection tissues. Representative immunohistochemical staining of ADAMTS-1 (A) and ADAMTS-4 (B) in the media and adventitia of the aortic wall in control, thoracic aortic aneurysm (TAA), and thoracic aortic dissection (TAD) tissues (original magnification ×400).
Expression of ADAMTS-1 and ADAMTS-4 Protein in Macrophages and VSMCs in TAA Tissues
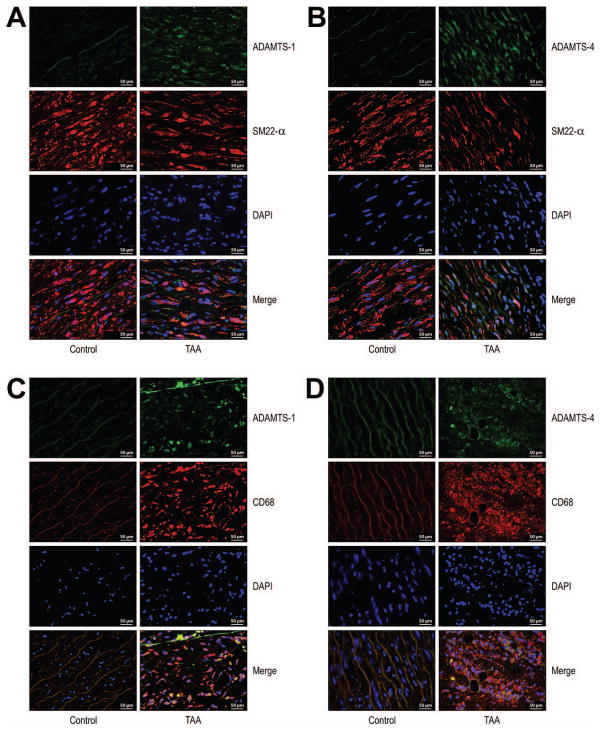
ADAMTS-1 (Fig 3A) and ADAMTS-4 (Fig 3B) colocalized with smooth muscle cell (SMC) marker SM22 alpha in the medial layer of the aortic wall in TAA tissue. ADAMTS-1 and ADAMTS-4 were detected inside SMCs, suggesting the expression/production of these two proteases by SMCs. In addition, ADAMTS-1 (Fig 3C) and ADAMTS-4 (Fig 3D) were also expressed in the CD68+ macrophages in macrophage-rich areas (primarily the adventitia) of TAA tissue.
Fig 3.
Localization of ADAMTS-1 and ADAMTS-4 protein in vascular smooth muscle cells (VSMCs) and macrophages in aneurysm and dissection tissues. Double immunofluorescence staining showing (A-B) colocalization of ADAMTS-1 and ADAMTS-4 with SMC marker SM22 alpha in the medial layer of the aortic wall in thoracic aortic aneurysm (TAA) tissue and (C-D) colocalization of ADAMTS-1 and ADAMTS-4 with macrophage marker CD68 in a macrophage-rich area in adventitia of TAA tissue (original magnification ×400). Nuclei were counterstained with DAPI (blue).
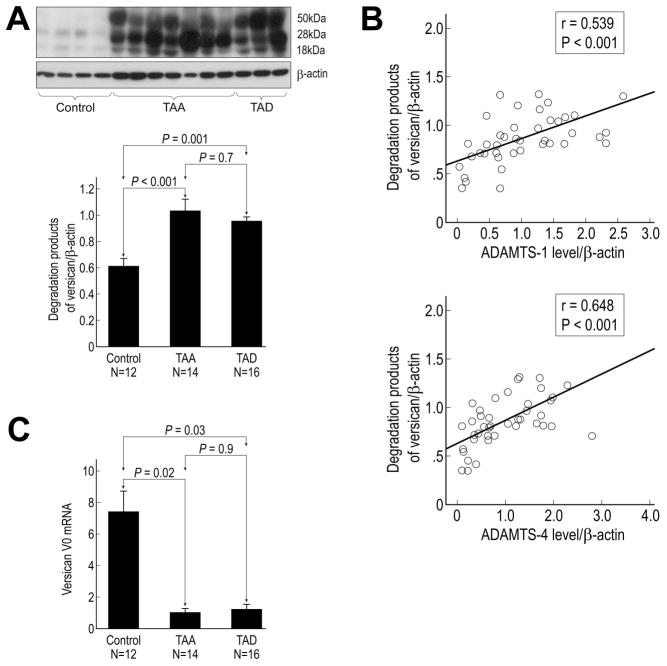
Increased Degradation of Versican in TAA and TAD Tissues
Levels of versican degradation products (ie, fragment bands of 50 kDa, 28 kDa, and 18 kDa) were significantly higher in TAA and TAD tissues than in control aortic tissues (Fig 4A). Levels of versican degradation products strongly correlated with levels of ADAMTS-1 and ADAMTS-4 protein (Fig 4B), suggesting crucial roles for ADAMTS-1 and ADAMTS-4 in the degradation of versican in aortic aneurysms and dissections. Additionally, mRNA levels of the versican isoform V0 were significantly lower in TAA and TAD tissues than in control aortic tissues (Fig 4C), suggesting decreased expression of versican in patient samples.
Fig 4.
Increased degradation of versican in aortic aneurysm and dissection tissues. (A) Western blot and quantitative analysis of versican degradation products in control, thoracic aortic aneurysm (TAA), and thoracic aortic dissection (TAD) tissues. (B) Correlation between versican degradation and levels of ADAMTS-1 and ADAMTS-4 protein. (C) Quantitative real-time PCR analysis of versican V0 mRNA levels in control, TAA, and TAD tissues.
Involvement of ADAMTS-4 in Macrophage Invasion
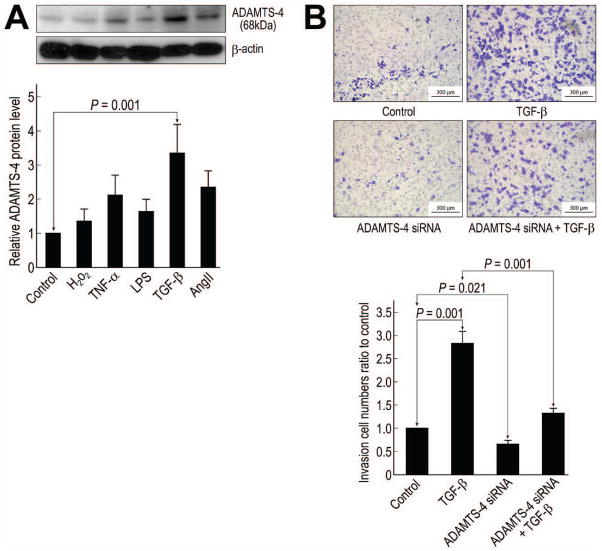
Because we observed high levels of ADAMTS-4 protein in macrophages, we examined whether ADAMTS-4 has a role in macrophage invasion. First, we identified factors that stimulated an increase in ADAMTS-4 protein levels. Macrophages were incubated with H2O2, tumor necrosis factor-α, lipopolysaccharide, TGF-β, or angiotensin II; of these agents, TGF-β induced the highest level of ADAMTS-4 protein (Fig 5A).
Fig 5.
Involvement of ADAMTS-4 in transforming growth factor (TGF)-β–induced macrophage migration. (A) Western blot analysis of ADAMTS-4 in macrophages treated with H2O2, tumor necrosis factor-α (TNF-α), lipopolysaccharide (LPS), TGF-β, or angiotensin II (AngII). (B) Macrophages were transfected with scramble or ADAMTS-4 siRNA before treatment with 10 ng/mL of TGF-β for 24 h (original magnification ×100). Migration of the treated cells was analyzed by using a transwell assay.
To determine the effects of TGF-β and ADAMTS-4 on macrophage migration through the ECM, we used a transwell cell migration (Boyden chamber) assay with monocyte chemoattractant protein-1 as the chemoattractant and the ECMatrix-coated membrane as the barrier. Macrophage migration across the ECM layer was enhanced by TGF-β (Fig 5B). In addition, the siRNA-mediated knockdown of ADAMTS-4 in macrophages significantly reduced the number of basal migrating cells and partially suppressed TGF-β–induced macrophage invasion (Fig 5B), suggesting a critical role of ADAMTS-4 in macrophage invasion.
Comment
In this study, we showed that ADAMTS-1 and ADAMTS-4 expression was significantly increased in the aortic wall of TAA and TAD tissues and that increased ADAMTS-1 and ADAMTS-4 protein levels correlated with the degradation of versican, the main proteoglycan substrate of ADAMTS proteinases in the aorta. In addition, we showed that ADAMTS-1 and ADAMTS-4 were expressed in VSMCs in the media and macrophages in the regions of inflammation. ADAMTS-1 and ADAMTS-4 protein levels strongly correlated with versican degradation products. In cultured cells, ADAMTS-4 was involved in macrophage migration. Our results suggest that ADAMTS-1 and ADAMTS-4 may play a role in inflammation and tissue destruction in the progression of sporadic aneurysms both in aortas without dissection and in those with chronic dissections.
Several other studies have suggested that the dysregulation of ADAMTS expression may contribute to tissue destruction and the development of vascular disease. ADAMTS-1 and ADAMTS-4 were found to be highly expressed in macrophage-rich areas of human atherosclerotic plaque [11,12]. In addition, ADAMTS-4 was shown to be associated with VSMC death and vascular atrophy, and an ADAMTS-1 gene variation was shown to be associated with an increased risk of coronary artery disease events [13,14]. Most notably, Taketani and colleagues reported that ADAMTS1 expression was significantly upregulated in TAA patients in DNA microarray and real-time PCR experiments [10]. Given their role in tissue destruction, ADAMTS-1 and ADAMTS-4 may contribute to aortic destruction during disease progression, particularly when their expression is increased.
Our findings suggest that ADAMTS-1 and ADAMTS-4 may play an important role in aortic inflammation. Inflammatory cells such as macrophages play critical roles in the progression of aortic aneurysms by producing proinflammatory cytokines and proteolytic enzymes [15]. In diseased aortic tissues, we found that ADAMTS-1 and ADAMTS-4 were present at high levels around inflammatory cells and that they colocalized with macrophages. In cultured cells, ADAMTS-4 facilitated macrophage migration across an ECM barrier, supporting the role of ADAMTS-4 in inflammatory cell infiltration.
Our results also suggest that the decrease in versican levels that resulted from reduced versican expression and increased versican degradation by ADAMTS-1 and ADAMTS-4 may contribute to the loss of structural and functional integrity of the aortic wall in the progression of aneurysms. Versican is predominantly synthesized by VSMCs and can be cleaved by both ADAMTS-1 and ADAMTS-4 [5]. At least 4 isoforms of versican (V0, V1, V2, and V3) are created by alternative splicing of a single gene [16]. Versican interacts with hyaluronan and other link proteins to form large aggregates that can retain water and create reversible compressive structures necessary for smoothing out pressure waves and avoiding blood vessel deformation [17]. Versican is also involved in regulating elastic fiber assembly inhibiting cell apoptosis, and stimulating cell growth and migration [18–25]. Versican has also been shown to regulate VSMC proliferation and angiogenesis [26, 27]. Thus, it has been suggested that versican may play an important role in maintaining the structural and functional integrity of the vascular wall, and its degradation may be responsible for certain vascular diseases [26,28]. In TAA and TAD tissues, we observed decreased versican expression, which may have resulted from the reduced number of SMCs, decreased versican expression in the remaining SMCs, or a combination of these. We also observed significantly increased versican degradation, which correlated with the increase in ADAMTS-1 and ADAMTS-4 protein levels. This strong correlation suggests that the elevated ADAMTS-1 and ADAMTS-4 protein levels that we observed may be partially responsible for the increased versican degradation. Additionally, versican can be cleaved by MMPs, so the versican degradation products we observed in diseased aortas may have been the collective result of cleavage by ADAMTS, MMPs, and potentially, other unidentified extracellular proteases. Consistent with the results of our study, recent reports have also shown decreased synthesis and increased degradation of versican in abdominal aortic aneurysm tissue [29].
In addition to degrading versican, ADAMTS proteins may also destroy the aortic wall by degrading other ECM components. ADAMTS-4 has been shown to digest other proteoglycans, such as decorin and biglycan [6]. Defects in decorin and biglycan have been implicated in the formation of aortic aneurysms and dissections [30,31]. Recent studies showed that ADAMTS-4 can also cleave alpha-2-macroglobulin, matrilin-3, cartilage oligomeric matrix protein, and fibromodulin [32–34]. Furthermore, ADAMTS-4 has been associated with SMC death [35]. Thus, ADAMTS proteins may promote tissue destruction and disease formation through various mechanisms.
There are several limitations in our study. First, because we only evaluated end-stage disease tissue, we cannot speculate whether ADAMTS-1 and ADAMTS-4 play a role in the initial of formation of TAA and TAD. Similarly, in the absence of serial tissue samples spanning the period of disease progression, it is unclear whether diminished levels of versican are a factor in the development of TAA and TAD or are merely an epiphenomenon. In addition, although increased ADAMTS levels were associated with versican degradation, the cause of diminished versican levels within diseased aortic tissue is likely multifactorial. For example, patients with aortic disease may have reduced levels of the substrate at baseline. Furthermore, versican may be degraded by proteases other than ADAMTS-1 and ADAMTS-4, Finally, our control group consisted of organ donors matched for age; although these subjects did not have aortic disease, many had cerebrovascular disease or diabetes. The impact of these latter conditions (as well as other factors that were not present in our TAA and TAD patients, such as recent trauma) on aortic ADAMTS levels in our control subjects is unknown.
In summary, ADAMTS-1 and ADAMTS-4 levels are elevated in patients with TAA and TAD and may contribute to inflammatory cell infiltration and aortic destruction. Additional studies in animal models are needed to determine the specific role of ADAMTS-1 and ADAMTS-4 in the formation of TAA and TAD and whether these proteinases represent a valuable therapeutic target for the treatment of aortic disease.
Acknowledgments
We gratefully acknowledge Scott A. Weldon, MA, CMI, of Baylor College of Medicine, for assistance with illustrations, and Nicole Stancel, PhD, and Rebecca A. Bartow, PhD, of the Texas Heart Institute at St. Luke’s Episcopal Hospital, for providing editorial support. This study was supported by grant 5R01HL085341 (to S.A.L.). The Thoracic Aortic Disease Tissue Bank at Baylor College of Medicine was supported in part through the Tissue Banking Core of the Specialized Center of Clinically Oriented Research in Thoracic Aortic Aneurysms and Dissections (NIH P50 HL083794).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kochanek KD, Xu JQ, Murphy SL, Miniño AM, Kung H. National Vital Statistics Reports. 3. Vol. 60. Hyattsville, MD: National Center for Health Statistics; 2011. Deaths: final data for 2009. [PubMed] [Google Scholar]
- 2.Davies MJ. Aortic aneurysm formation: lessons from human studies and experimental models. Circulation. 1998;98:193–5. doi: 10.1161/01.cir.98.3.193. [DOI] [PubMed] [Google Scholar]
- 3.Kuno K, Kanada N, Nakashima E, Fujiki F, Ichimura F, Matsushima K. Molecular cloning of a gene encoding a new type of metalloproteinase-disintegrin family protein with thrombospondin motifs as an inflammation associated gene. J Biol Chem. 1997;272:556–62. doi: 10.1074/jbc.272.1.556. [DOI] [PubMed] [Google Scholar]
- 4.Salter RC, Ashlin TG, Kwan AP, Ramji DP. ADAMTS proteases: key roles in atherosclerosis? J Mol Med (Berl) 2010;88:1203–11. doi: 10.1007/s00109-010-0654-x. [DOI] [PubMed] [Google Scholar]
- 5.Sandy JD, Westling J, Kenagy RD, et al. Versican V1 proteolysis in human aorta in vivo occurs at the Glu441-Ala442 bond, a site that is cleaved by recombinant ADAMTS-1 and ADAMTS-4. J Biol Chem. 2001;276:13372–8. doi: 10.1074/jbc.M009737200. [DOI] [PubMed] [Google Scholar]
- 6.Gendron C, Kashiwagi M, Lim NH, et al. Proteolytic activities of human ADAMTS-5: comparative studies with ADAMTS-4. J Biol Chem. 2007;282:18294–306. doi: 10.1074/jbc.M701523200. [DOI] [PubMed] [Google Scholar]
- 7.Torres-Collado AX, Kisiel W, Iruela-Arispe ML, Rodriguez-Manzaneque JC. ADAMTS1 interacts with, cleaves, and modifies the extracellular location of the matrix inhibitor tissue factor pathway inhibitor-2. J Biol Chem. 2006;281:17827–37. doi: 10.1074/jbc.M513465200. [DOI] [PubMed] [Google Scholar]
- 8.Luque A, Carpizo DR, Iruela-Arispe ML. ADAMTS1/METH1 inhibits endothelial cell proliferation by direct binding and sequestration of VEGF165. J Biol Chem. 2003;278:23656–65. doi: 10.1074/jbc.M212964200. [DOI] [PubMed] [Google Scholar]
- 9.Krampert M, Kuenzle S, Thai SN, Lee N, Iruela-Arispe ML, Werner S. ADAMTS1 proteinase is up-regulated in wounded skin and regulates migration of fibroblasts and endothelial cells. J Biol Chem. 2005;280:23844–52. doi: 10.1074/jbc.M412212200. [DOI] [PubMed] [Google Scholar]
- 10.Taketani T, Imai Y, Morota T, et al. Altered patterns of gene expression specific to thoracic aortic aneurysms: microarray analysis of surgically resected specimens. Int Heart J. 2005;46:265–77. doi: 10.1536/ihj.46.265. [DOI] [PubMed] [Google Scholar]
- 11.Jonsson-Rylander AC, Nilsson T, Fritsche-Danielson R, et al. Role of ADAMTS-1 in atherosclerosis: remodeling of carotid artery, immunohistochemistry, and proteolysis of versican. Arterioscler Thromb Vasc Biol. 2005;25:180–5. doi: 10.1161/01.ATV.0000150045.27127.37. [DOI] [PubMed] [Google Scholar]
- 12.Wagsater D, Bjork H, Zhu C, et al. ADAMTS-4 and -8 are inflammatory regulated enzymes expressed in macrophage-rich areas of human atherosclerotic plaques. Atherosclerosis. 2008;196:514–22. doi: 10.1016/j.atherosclerosis.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 13.Kenagy RD, Min SK, Mulvihill E, Clowes AW. A link between smooth muscle cell death and extracellular matrix degradation during vascular atrophy. J Vasc Surg. 2011;54:182–91. e24. doi: 10.1016/j.jvs.2010.12.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabatine MS, Ploughman L, Simonsen KL, et al. Association between ADAMTS1 matrix metalloproteinase gene variation, coronary heart disease, and benefit of statin therapy. Arterioscler Thromb Vasc Biol. 2008;28:562–7. doi: 10.1161/ATVBAHA.107.156653. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu K, Mitchell RN, Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2006;26:987–94. doi: 10.1161/01.ATV.0000214999.12921.4f. [DOI] [PubMed] [Google Scholar]
- 16.Lemire JM, Braun KR, Maurel P, Kaplan ED, Schwartz SM, Wight TN. Versican/PG-M isoforms in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:1630–9. doi: 10.1161/01.atv.19.7.1630. [DOI] [PubMed] [Google Scholar]
- 17.LeBaron RG, Zimmermann DR, Ruoslahti E. Hyaluronate binding properties of versican. J Biol Chem. 1992;267:10003–10. [PubMed] [Google Scholar]
- 18.Merrilees MJ, Lemire JM, Fischer JW, et al. Retrovirally mediated overexpression of versican v3 by arterial smooth muscle cells induces tropoelastin synthesis and elastic fiber formation in vitro and in neointima after vascular injury. Circ Res. 2002;90:481–7. doi: 10.1161/hh0402.105791. [DOI] [PubMed] [Google Scholar]
- 19.Hinek A, Braun KR, Liu K, Wang Y, Wight TN. Retrovirally mediated overexpression of versican v3 reverses impaired elastogenesis and heightened proliferation exhibited by fibroblasts from Costello syndrome and Hurler disease patients. Am J Pathol. 2004;164:119–31. doi: 10.1016/S0002-9440(10)63103-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang R, Merrilees MJ, Braun K, et al. Inhibition of versican synthesis by antisense alters smooth muscle cell phenotype and induces elastic fiber formation in vitro and in neointima after vessel injury. Circ Res. 2006;98:370–7. doi: 10.1161/01.RES.0000202051.28319.c8. [DOI] [PubMed] [Google Scholar]
- 21.Sheng W, Wang G, Wang Y, et al. The roles of versican V1 and V2 isoforms in cell proliferation and apoptosis. Mol Biol Cell. 2005;16:1330–40. doi: 10.1091/mbc.E04-04-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaPierre DP, Lee DY, Li SZ, et al. The ability of versican to simultaneously cause apoptotic resistance and sensitivity. Cancer Res. 2007;67:4742–50. doi: 10.1158/0008-5472.CAN-06-3610. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Cao L, Yang BL, Yang BB. The G3 domain of versican enhances cellproliferation via epidermial growth factor-like motifs. J Biol Chem. 1998;273:21342–51. doi: 10.1074/jbc.273.33.21342. [DOI] [PubMed] [Google Scholar]
- 24.Ricciardelli C, Russell DL, Ween MP, et al. Formation of hyaluronan- and versican-rich pericellular matrix by prostate cancer cells promotes cell motility. J Biol Chem. 2007;282:10814–25. doi: 10.1074/jbc.M606991200. [DOI] [PubMed] [Google Scholar]
- 25.Dutt S, Kleber M, Matasci M, Sommer L, Zimmermann DR. Versican V0 and V1 guide migratory neural crest cells. J Biol Chem. 2006;281:12123–31. doi: 10.1074/jbc.M510834200. [DOI] [PubMed] [Google Scholar]
- 26.Evanko SP, Angello JC, Wight TN. Formation of hyaluronan- and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:1004–13. doi: 10.1161/01.atv.19.4.1004. [DOI] [PubMed] [Google Scholar]
- 27.Lemire JM, Merrilees MJ, Braun KR, Wight TN. Overexpression of the V3 variant of versican alters arterial smooth muscle cell adhesion, migration, and proliferation in vitro. J Cell Physiol. 2002;190:38–45. doi: 10.1002/jcp.10043. [DOI] [PubMed] [Google Scholar]
- 28.Kenagy RD, Plaas AH, Wight TN. Versican degradation and vascular disease. Trends Cardiovasc Med. 2006;16:209–15. doi: 10.1016/j.tcm.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Theocharis AD, Tsolakis I, Hjerpe A, Karamanos NK. Versican undergoes specific alterations in the fine molecular structure and organization in human aneurysmal abdominal aortas. Biomed Chromatogr. 2003;17:411–6. doi: 10.1002/bmc.263. [DOI] [PubMed] [Google Scholar]
- 30.Pulkkinen L, Kainulainen K, Krusius T, et al. Deficient expression of the gene coding for decorin in a lethal form of Marfan syndrome. J Biol Chem. 1990;265:17780–5. [PubMed] [Google Scholar]
- 31.Heegaard AM, Corsi A, Danielsen CC, et al. Biglycan deficiency causes spontaneous aortic dissection and rupture in mice. Circulation. 2007;115:2731–8. doi: 10.1161/CIRCULATIONAHA.106.653980. [DOI] [PubMed] [Google Scholar]
- 32.Tortorella MD, Arner EC, Hills R, et al. Alpha2-macroglobulin is a novel substrate for ADAMTS-4 and ADAMTS-5 and represents an endogenous inhibitor of these enzymes. J Biol Chem. 2004;279:17554–61. doi: 10.1074/jbc.M313041200. [DOI] [PubMed] [Google Scholar]
- 33.Hills R, Mazzarella R, Fok K, et al. Identification of an ADAMTS-4 cleavage motif using phage display leads to the development of fluorogenic peptide substrates and reveals matrilin-3 as a novel substrate. J Biol Chem. 2007;282:11101–9. doi: 10.1074/jbc.M611588200. [DOI] [PubMed] [Google Scholar]
- 34.Kashiwagi M, Enghild JJ, Gendron C, et al. Altered proteolytic activities of ADAMTS-4 expressed by C-terminal processing. J Biol Chem. 2004;279:10109–19. doi: 10.1074/jbc.M312123200. [DOI] [PubMed] [Google Scholar]
- 35.Kenagy RD, Min SK, Clowes AW, Sandy JD. Cell death-associated ADAMTS4 and versican degradation in vascular tissue. J Histochem Cytochem. 2009;57:889–97. doi: 10.1369/jhc.2009.953901. [DOI] [PMC free article] [PubMed] [Google Scholar]