Abstract
Members of the genus Rhizopus within the class Zygomycetes can cause devastating opportunistic infections. Cutaneous disease arising from direct inoculation of fungal spores has the potential to disseminate widely. Here, we describe a dramatic case of cutaneous Rhizopus infection involving the penis in a patient with acute myelogenous leukemia. Despite aggressive surgical debridement, systemic antifungal therapy, and donor lymphocyte infusion, the infection was ultimately fatal. This case illustrates the unique diagnostic and therapeutic challenges in the clinical management of cutaneous Rhizopus infection.
Keywords: Rhizopus, Zygomycetes, zygomycosis, mucormycosis, Fournier’s gangrene, penile infection
Zygomycetes are ubiquitous in nature and can cause fulminant infections in the immunocompromised host. Known risk factors include hematologic malignancy, neutropenia, diabetes mellitus, steroid use, and trauma (1). A recent increase in the incidence of zygomycosis compared with candidiasis or aspergillosis may be explained by increasing use of antifungal agents lacking activity against Zygomycetes, in addition to the increasing numbers of higher risk bone marrow transplants (2). Organisms in the genus Rhizopus cause the majority of human infections. They typically manifest as cutaneous, rhino-cerebral, pulmonary, or gastrointestinal infection and may progress to disseminated disease.
Cutaneous disease may arise either from direct inoculation of the skin, or less commonly, from hematogenous dissemination. Genital infection is exceedingly uncommon and only 4 cases involving the penis have been reported in the medical literature (3–6). Our case illustrates the diagnostic challenge of penile infection caused by Rhizopus. One of the key diagnostic dilemmas is that the appearance of skin lesions secondary to Rhizopus may be confused with viral eruptions, cutaneous leukemia, and bacterial necrotizing infection. The anatomy, physiology, and lymphatic drainage of the penis and perineum may predispose to rapid dissemination into the deep tissues of the pelvis with nearly universally fatal results. Current management strategies including novel adjuvant therapies are discussed.
Case report
A 53-year-old male with acute myelogenous leukemia (AML) was admitted to the hospital with one day of fever. He reported several months of malaise but no additional localizing symptoms. His past medical history was significant for diffuse large B-cell lymphoma, treated with chemotherapy and autologous bone marrow transplantation 9 years earlier. One month before admission, he was diagnosed with AML with trilineage dysplasia, presumably secondary to his prior chemotherapy.
Medications on admission included norfloxacin and val-acyclovir. He was not receiving antifungal prophylaxis. Before his admission, he had not started any chemotherapy for his AML. On physical exam, his temperature was 38.5°C, heart rate was 106 beats/min, blood pressure was 105/63 mm Hg, respiratory rate was 18 breaths/min, and oxygen saturation was 100% on room air. Physical examination including evaluation of the sinuses, chest, and skin was unremarkable. Laboratory data were significant for a white blood cell count of 3010 cells/mm3 with 33% polymorphonuclear cells (absolute neutrophil count of 1447 cells/mm3), 7% blasts, and 5% young unidentified cells. His hematocrit was 25% and platelet count was 7 K/mm3. Computed tomography of the sinuses and chest were unrevealing. The patient was started on intravenous (IV) broad-spectrum antimicrobial agents including piperacillin-tazobactam (4.5 g IV every 6 h), vancomycin (1 g IV every 12 h), and micafungin (100 mg IV every 24 h).
His fevers abated within the first week without determination of a clear infectious etiology. Micafungin was discontinued but broad-spectrum antibacterial coverage with piperacillin–tazobactam and vancomycin was continued. On hospital day 7, he was initiated on chemotherapy with cytarabine, daunorubicin, and etoposide. On hospital day 10, the patient had new onset chest pain with diffuse ST elevations seen on electrocardiogram, elevated cardiac enzymes (peak troponin I 19.9 ng/mL; normal <0.06 ng/mL), and a depressed cardiac ejection fraction of 45%. He was diagnosed with myopericarditis and was treated with methyl-prednisolone sodium succinate 125 mg IV every 6 h. Blood sugars ranged from 200 to 300 mg/dL and subcutaneous insulin was initiated for steroid-induced diabetes mellitus.
By the second week of hospitalization, the patient had progressive renal dysfunction secondary to acute tubular necrosis. Piperacillin–tazobactam was switched to meropenem out of concern for nephrotoxity. A Foley catheter was placed for documentation of the urinary output. The patient was obese and had a retracted penis, making catheter placement difficult. Within days of catheter placement, the patient complained of dysuria and penile pain. The Foley catheter was removed and a condom catheter was placed for patient comfort. Frequent dislodgement of the catheter occurred because of scrotal edema, and a heating pad and barrier cream were applied to promote adhesion. Four days after placement of the condom catheter, the patient was noted to have 2 purpuric bullae on the glans of the penis and at the coronal sulcus.
Initial urologic consultation suggested that the patient had pressure necrosis secondary to catheter placement and conservative management was recommended. Despite scrotal elevation, the patient developed a blackened eschar extending from the base of the penis to the scrotum within 72 h. Initial bedside impression suggested Fournier’s gangrene. He was brought emergently to the operating room for escharotomy and debridement of the scrotum and perineum. Intraoperatively, necrotic skin was noted in the penis, the scrotum, and the perineum below the scrotum, which was debrided. Adjacent lymph nodes demonstrated black stippling without gross purulence. Postoperatively, liposomal amphotercin B (AmB) (5 mg/kg IV daily) was added to his medications.
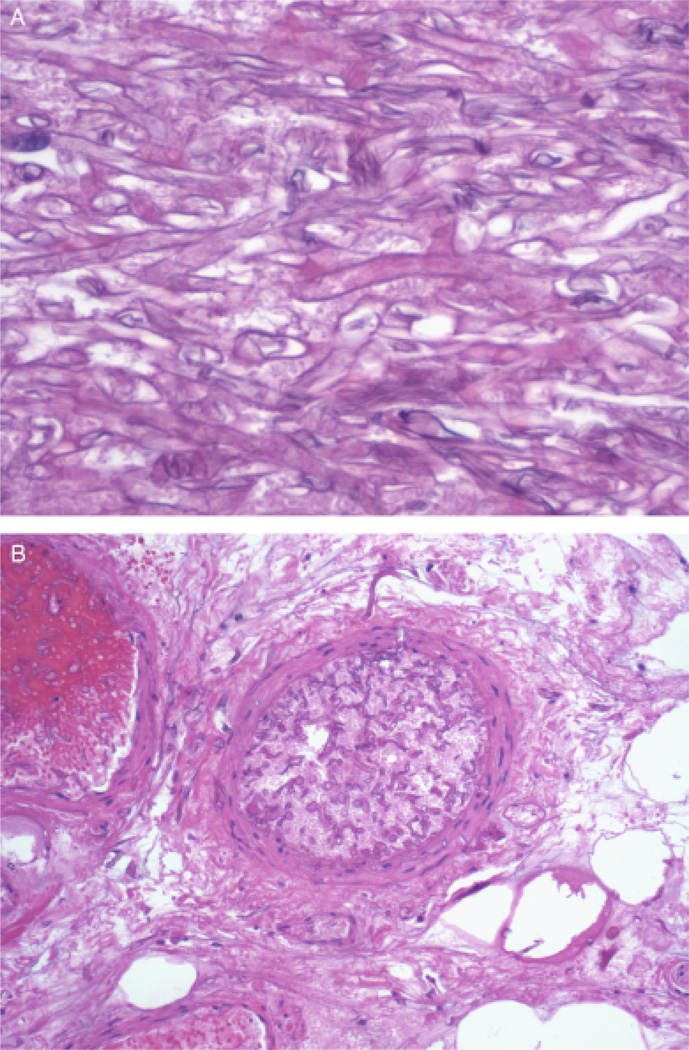
Within 48 h, tissue cultures from the penis and deep tissue grew a presumptive Zygomycete, based on macroscopic and microscopic morphology, growth characteristics, and temperature studies. The organism was further speciated as Rhizopus microsporus by nucleic acid sequencing using two targets, the Internal Transcribed Spacer region and the D1/D2 region of the large-subunit of the 28S rRNA gene. Histopathologic examination of surgical specimens from skin, adipose tissue, penis, and lymph nodes revealed broad, branching, non-septated fungal elements (Fig. 1A). Extensive tissue vascular infiltration and necrosis were noted (Fig. 1B).
Fig. 1.
(A) Methenamine silver stain of the necrotic tissue at × 400 magnification within the surgical specimen revealed broad, branching, non-septated fungal elements characteristic of Rhizopus growth at 37°C. (B) Methenamine silver stain of the necrotic tissue at × 100 magnification shows a thrombosed arteriole infiltrated with non-septated fungal elements, establishing the diagnosis of angioinvasive Rhizopus.
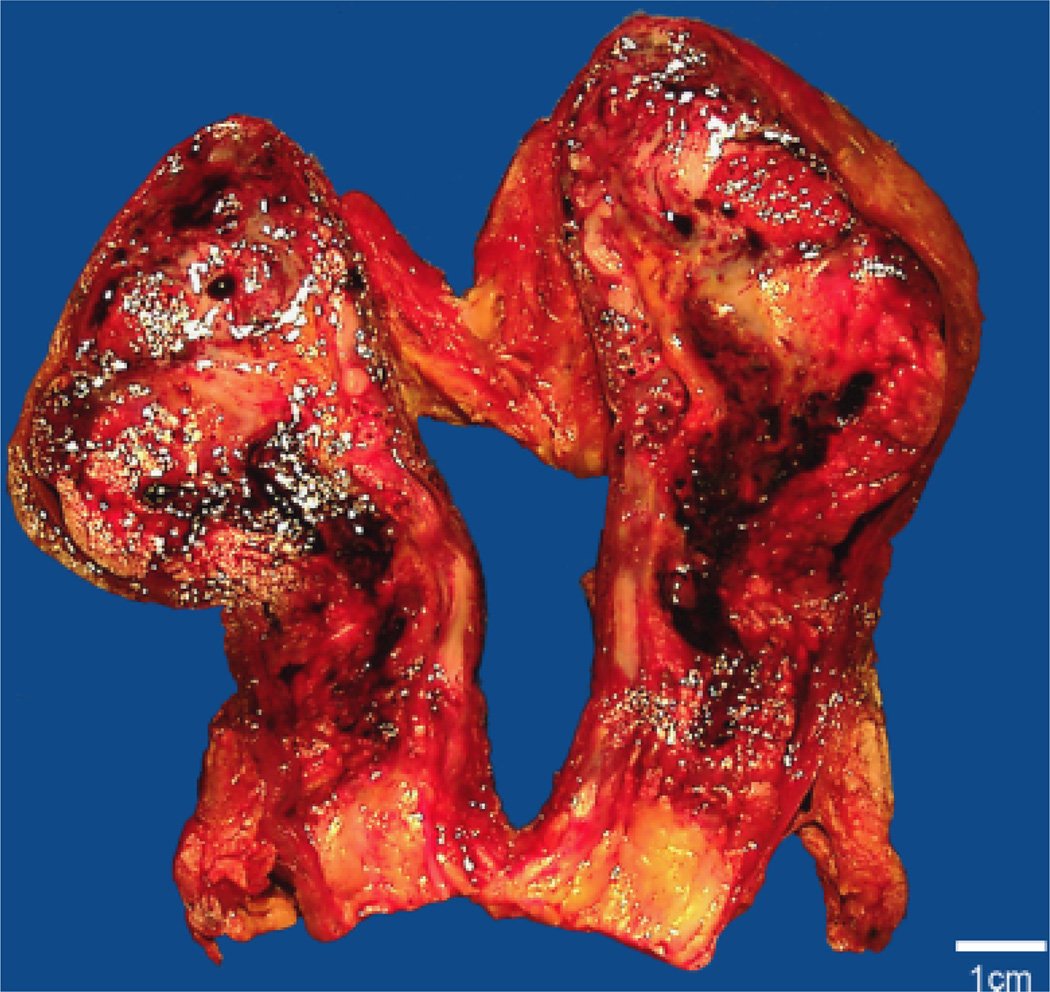
Further expansion of the skin necrosis was observed and the patient was taken back to the operating room for penectomy, scrotectomy, and bilateral orchiectomy (Fig. 2). Liposomal AmB was increased to 8 mg/kg IV daily and micafungin (100 mg IV daily) and posaconazole (400 mg by mouth twice daily) were added. The patient remained neutropenic (total white blood cell count 60 cells/mm3) and granulocyte infusion was attempted to restore immune function.
Fig. 2.
Photograph of the bivalved testes shows multiple dusky, necrotic, and hemorrhagic foci of tissue, with prominent involvement of the deep tissues of the spermatic cord and testes.
Despite 3 sequential extensive surgical debridements, the patient developed a non-contiguous necrotic skin lesion in the right gluteal area, potentially indicative of new hematogenous dissemination. Further surgical intervention was declined by the patient and his family. He died in hospice 2 weeks after his last debridement.
Discussion
This dramatic case of rapidly progressive and fatal cutaneous Rhizopus infection of the penis illustrates the diagnostic and therapeutic challenges of cutaneous Zygomycosis in the immunocompromised host.
From a clinical perspective, the differential diagnosis of cutaneous genital lesions in a leukemic patient is broad. On physical examination, the ulcerative lesions of early zygomycosis have a non-specific appearance and may be mistaken for mechanical trauma, viral eruptions (herpes simplex, varicella zoster), echythema gangrenosum, or tumor infiltration (3). In addition to Zygomycetes, other fungi such as Fusarium and Aspergillus can mimic the clinical presentation of cutaneous Rhizopus. In the case of our patient, the purpuric bullous lesions were initially thought to be the result of pressure necrosis due to a condom catheter. As the lesion progressed and developed a visible eschar, Fournier’s gangrene due to a classical bacterial pathogen was suspected.
In retrospect, several characteristics of the penis and perineum may have predisposed this patient to an invasive fungal infection. Ideal growth conditions for fungi include acidity, a temperature at or above 37°C, high moisture levels, and a glucose source. His blood glucose had been markedly elevated at times, presumably as a result of exogenous steroid administration. The use of barrier cream and local heat pad application may have further altered the penile/perineal milieu in favor of mycotic seeding and growth. Finally, the patient had significant general risk factors for Rhizopus including hematologic malignancy, chemotherapy-related neutropenia, and renal failure.
Cutaneous zygomycosis can be primary, due to inoculation at sites of local trauma, or less commonly, due to hematogenous spread from a primary pulmonary, rhinocerebral, or gastrointestinal source. In our patient, the most likely portal of entry was the breakdown of the normal epidermal barrier related to urinary catheter placement. Although prior institutional outbreaks of Rhizopus have been linked to ostomy bags, adhesive bandages, wooden tongue depressors, and contaminated air ventilation systems (7–11), no definitive link between a contaminated source and our patient could be established.
Once local infection is established, it is not uncommon for cutaneous lesions to progress to disseminated disease. The largest review of zygomycosis in the literature of 929 cases includes 176 cutaneous infections. Of these, 56% of cases remained localized, 24% progressed to involve deeper structures, and 20% spread hematogenously to involvement of a remote site. Conversely, hematogenous spread leading to secondary skin lesions was exceedingly rare with only 6/176 cases in this review (12).
With genital lesions in particular, the proximity of the inguinal lymph nodes makes the perineum a dangerous site of infection. The lymphatic drainage of the penis is particularly complex, as the more superficial tissues drain to the deep and superficial inguinal nodes, whereas the deeper tissues drain to the external iliac chain nodes within the pelvis. Invasion of the deep soft tissues of the penis, therefore, provides an anatomic gateway for pelvic invasion.
In our patient, surgical pathology from the initial debridement demonstrated lymphovascular invasion, although deeper lymphadenectomy was never attempted. Despite systemic antifungal therapy and multiple surgeries, the subsequent development of a non-contiguous necrotic lesion on the patient’s buttocks area suggested spread through the pelvic lymphatic system and/or hematogenous dissemination. A plausible source for these secondary lesions was the penile site, as both the physical exam and imaging revealed no other typical primary source, such as lungs or sinuses.
Treatment of zygomycosis requires surgical debridement in addition to systemic antifungal therapy with the deoxycholate or lipid formulation of AmB (13), as voriconazole has little or no activity against Rhizopus species. While in vitro data suggest that posaconazole may also be effective (14, 15), breakthrough has been described in leukemic patients who are already receiving posaconazole for antifungal prophylaxis (16, 17). Drugs of the echinocandin class have been demonstrated to enhance the activity of AmB in animal models (18). Clinical data to demonstrate benefit of combination therapy are currently limited to small retrospective studies (19). Immune function is critical in controlling the infection (20, 21). Innovative therapies such as granulocyte-colony stimulating factor and donor lymphocyte infusions have been used. Other therapeutic strategies, such as hyperbaric oxygen and iron chelation, have been tried based on in vitro evidence of efficacy, despite a lack of clinical evidence (22). Despite aggressive surgical debridement; systemic antifungal therapy with liposomal AmB, micafungin, and posaconazole; and adjuvant therapy with granulocyte infusions, our patient developed progressive cutaneous lesions. Mortality rates of 10% have been reported for localized cutaneous zygomycosis, 26% with extension to deeper structures, and 94% with disseminated disease (12). Given his extremely poor prognosis, our patient chose hospice care.
This case illustrates the development of a rapidly progressive, angioinvasive, and ultimately fatal cutaneous Rhizopus infection of the penis in a patient with AML. A high index of suspicion for invasive mycoses is critical in any immunosuppressed patient with necrotizing cutaneous lesions. Practitioners should be aware that cutaneous mold infections may manifest as Fournier’s gangrene. Penile lesions, in particular, may have a unique propensity to disseminate rapidly because of the lymphatic drainage of the perineum, at which point they become nearly uniformly fatal. Prompt diagnosis and aggressive therapy combining early skin biopsy, surgical management, systemic antifungals, immune therapy, and other innovative approaches are critical.
Acknowledgements
K.A.M. has served on the advisory board of Astellas, Merck, and Pfizer, and received grants from Astellas and Merck.
Footnotes
Possible conflicts of interest: All other authors report no potential conflicts of interest.
References
- 1.Bethge WA, Schmalzing M, Stuhler G, et al. Mucormycoses inpatients with hematologic malignancies: an emerging fungal infection. Haematologica. 2005;90(Suppl ECR22):62–64. [PubMed] [Google Scholar]
- 2.Neofytos D, Horn D, Anaissie E, et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis. 2009;48(3):265–273. doi: 10.1086/595846. [DOI] [PubMed] [Google Scholar]
- 3.Karam A, Ianotto JC, Metges JP, et al. Ulceration of the penis due to Absidia corymbifera. Br J Dermatol. 2003;148(6):1286–1287. doi: 10.1046/j.1365-2133.2003.05371.x. [DOI] [PubMed] [Google Scholar]
- 4.Cohen-Ludmann C, Kerob D, Feuilhade M, et al. Zygomycosis of the penis due to Rhizopus oryzae successfully treated with surgical debridement and a combination of high-dose liposomal and topical amphotericin B. Arch Dermatol. 2006;142(12):1657–1658. doi: 10.1001/archderm.142.12.1657. [DOI] [PubMed] [Google Scholar]
- 5.Grossklaus DJ, Dutta SC, Shappel S, Kirchner FK. Cutaneous mucormycosis presenting as a penile lesion in a patient with acute myeloblastic leukemia. J Urol. 1999;161(6):1906–1907. [PubMed] [Google Scholar]
- 6.Williams JC, Schned AR, Richardson JR, et al. Fatal genitourinary mucormycosis in a patient with undiagnosed diabetes. Clin Infect Dis. 1995;21(3):682–684. doi: 10.1093/clinids/21.3.682. [DOI] [PubMed] [Google Scholar]
- 7.Antoniadou A. Outbreaks of zygomycosis in hospitals. Clin Microbiol Infect. 2009;15(Suppl 5):55–59. doi: 10.1111/j.1469-0691.2009.02982.x. [DOI] [PubMed] [Google Scholar]
- 8.LeMaile-Williams M, Burwell L, Salisbury D, et al. Outbreak of cutaneous Rhizopus arrhizus infection associated with Karaya ostomy bags. Clin Infect Dis. 2006;43(9):e83–e88. doi: 10.1086/508277. [DOI] [PubMed] [Google Scholar]
- 9.Maravi-Poma E, Rodriguez-Tudela JL, de Jalon JG, et al. Outbreak of gastric mucormycosis associated with the use of wooden tongue depressors in critically ill patients. Intensive Care Med. 2004;30(4):724–728. doi: 10.1007/s00134-003-2132-1. [DOI] [PubMed] [Google Scholar]
- 10.Gartenberg G, Bottone EJ, Keusch GT, Weitzman I. Hospital-acquired mucormycosis (Rhizopus rhizopodiformis) of skin and subcutaneous tissue: epidemiology, mycology and treatment. N Engl J Med. 1978;299(20):1115–1118. doi: 10.1056/NEJM197811162992007. [DOI] [PubMed] [Google Scholar]
- 11.Cheng VC, Chan JF, Ngan AH, et al. Outbreak of intestinal infection due to Rhizopus microsporus. J Clin Microbiol. 2009;47(9):2834–2843. doi: 10.1128/JCM.00908-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 13.Pagano L, Valentini CG, Caira M, Fianchi L. Zygomycosis: current approaches to management of patients with haematological malignancies. Br J Haematol. 2009;146(6):597–606. doi: 10.1111/j.1365-2141.2009.07738.x. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez MM, Pastor FJ, Calvo E, Salas V, Sutton DA, Guarro J. Correlation of in vitro activity, serum levels, and in vivo efficacy of posaconazole against Rhizopus microsporusin a murine disseminated infection. Antimicrob Agents Chemother. 2009;53(12):5022–5025. doi: 10.1128/AAC.01026-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arikan S, Sancak B, Alp S, Hascelik G, McNicholas P. Comparative in vitro activities of posaconazole, voriconazole, itraconazole, and amphotericin B against Aspergillus and Rhizopus and synergy testing for Rhizopus. Med Mycol. 2008;46(6):567–573. doi: 10.1080/13693780801975576. [DOI] [PubMed] [Google Scholar]
- 16.Mousset S, Bug G, Heinz WJ, Tintelnot K, Rickerts V. Breakthrough zygomycosis on posaconazole prophylaxis after allogeneic stem cell transplantation. Transpl Infect Dis. 2010;12(3):261–264. doi: 10.1111/j.1399-3062.2009.00479.x. [DOI] [PubMed] [Google Scholar]
- 17.Lekakis LJ, Lawson A, Prante J, et al. Fatal rhizopus pneumonia in allogeneic stem cell transplant patients despite posaconazole prophylaxis: two cases and review of the literature. Biol Blood Marrow Transplant. 2009;15(8):991–995. doi: 10.1016/j.bbmt.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Ibrahim AS, Gebremariam T, Fu Y, Edwards JE, Jr, Spellberg B. Combination echinocandin-polyene treatment of murine mucormycosis. Antimicrob Agents Chemother. 2008;52(4):1556–1558. doi: 10.1128/AAC.01458-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reed C, Bryant R, Ibrahim AS, et al. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis. 2008;47(3):364–371. doi: 10.1086/589857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pagano L, Offidani M, Fianchi L, et al. Mucormycosis in hematologic patients. Haematologica. 2004;89(2):207–214. [PubMed] [Google Scholar]
- 21.Kara IO, Tasova Y, Uguz A, Sahin B. Mucormycosis-associated fungal infections in patients with haematologic malignancies. Int J Clin Pract. 2009;63(1):134–139. doi: 10.1111/j.1742-1241.2006.01145.x. [DOI] [PubMed] [Google Scholar]
- 22.Tragiannidis A, Groll AH. Hyperbaric oxygen therapy and other adjunctive treatments for zygomycosis. Clin Microbiol Infect. 2009;15(Suppl 5):82–86. doi: 10.1111/j.1469-0691.2009.02986.x. [DOI] [PubMed] [Google Scholar]