Abstract
Introduction and Hypothesis
Two-dimensional magnetic resonance imaging (MRI) of posterior vaginal prolapse has been studied. However, the three-dimensional (3-D) mechanisms causing such prolapse remain poorly understood. This discovery project was undertaken to identify the different 3-D characteristics of models of rectocele-type posterior vaginal prolapse (PVPR) in women.
Methods
Ten women with (cases) and 10 without (controls) PVPR were selected from an ongoing case-control study. Supine, multi-planar MR imaging was performed at rest and maximal Valsalva. 3-D reconstructions of the posterior vaginal wall and pelvic bones were created using 3D Slicer v. 3.4.1. In each slice the posterior vaginal wall and perineal skin were outlined to the anterior margin of the external anal sphincter to include the area of the perineal body. Women with predominant enteroceles or anterior vaginal prolapse were excluded.
Results
The case and control groups had similar demographics. In women with PVPR two characteristics were consistently visible (10 of 10): 1) the posterior vaginal wall displayed a folding phenomenon similar to a person beginning to kneel (“Kneeling” shape); and 2) a downward displacement in the upper 2/3 of the vagina. Also seen in some, but not all of the scans were: 3) forward protrusion of the distal vagina (6 of 10); 4) perineal descent (5 of 10); and 5) distal widening in lower third of the vagina (3 of 10).
Conclusions
Increased folding (“Kneeling”) of the vagina and an overall downward displacement are consistently present in rectocele. Forward protrusion, perineal descent and distal widening are sometimes seen as well.
Keywords: posterior vaginal wall prolapse, pelvic organ prolapse, rectocele, 3D model, magnetic resonance imaging, anatomy
Introduction
Pelvic floor dysfunction results in 11% of women undergoing surgery [1] in the USA during their lifespan. Over 200,000 operations are performed for prolapse [2] with repair of posterior vaginal prolapse (PVP) included in 87% [3]. The annual estimated cost for these operations exceeds US $1 billion [4].
The structural deformations seen in women with anterior vaginal prolapse (cystocele) have received considerable attention and study [5–7]. The pathomechanics of PVP has received less attention and so is not yet well understood. Current imaging studies concerning rectocele have focused on the contour of the rectum seen in midline sagittal projection [8,9]. Changes in the overall contour of the posterior vaginal wall are less well documented and there is no consensus on what to measure. Although there is obvious deformation of the posterior vaginal wall in PVP, the exact nature of this 3-D deformation has not been clarified. The potential changes present in posterior vaginal wall width and the relationships involving the lateral margins of the posterior vaginal wall are also not clear [10,11].
This study was therefore undertaken by conducting a secondary analysis of data from a larger on-going case-control study of pelvic organ prolapse. We tested the hypothesis that it is possible to identify characteristic shapes of PVP visible on MR scans at rest and maximum Valsalva.
Materials and Methods
MRI scans of 10 women with rectocele type posterior vaginal prolapse (PVPR) and 10 with normal support (controls) were selected from an ongoing University of Michigan institutional review board-approved (IRB # 1999-0395) case-control study of pelvic organ prolapse. Women in the control group were recruited by newspaper and radio advertisement for healthy volunteers and had to be asymptomatic and had normal vaginal support with all pelvic organ prolapse quantification (POP-Q) points < −1 cm. All PVPR cases had posterior vaginal prolapse with posterior vaginal wall (PVW) extending at least 1 cm below the hymen based on POP-Q and had symptoms of bulging or protrusion. In order to be included the rectocele had to be the predominant aspect of the prolapse and extend at least one centimeter lower than the most dependent part of anterior wall or the uterus/apex. Women with predominant enteroceles or anterior vaginal prolapse were excluded. None of the subjects had previously undergone hysterectomy or prior pelvic floor surgery. The 26 scans of women with rectocele were further evaluated for inclusion according to the following criteria: prolapse size consistent with clinical examination (POP-Q), ability to hold Valsalva for the entire 17 seconds of scan acquisition, freedom from significant motion artifact, inclusion of all necessary structures, evenly distributed intravaginal ultrasound gel and sufficient definition of vaginal walls to allow models to be made. Ten of the 26 scans were selected based on above criteria. Similarly, the matched controls (who had an age difference within ±2 years, number of vaginal deliveries within ±1, and were of similar race) had to meet the above criteria with the exception of not having prolapse.
As described in our previous work [6,12], each subject underwent supine multi-planar, two-dimensional, fast spin, proton density MR imaging both at rest and during maximal Valsalva using a 3 T superconducting magnet (Philips Medical Systems Inc, Bothell, WA) with version 2.5.1.0 software. At rest, each 30 images were serially obtained at the axial, sagittal, and coronal, with 20×20 cm fields of view, 4 mm slice thickness, and a 1 mm gap between slices. During maximal Valsalva, each 14 images were serially obtained at the same three serial planes, with 36×36 cm fields of view, 6 mm slice thickness, and 1 mm gap. In order for the images to be considered adequate, they had to allow visualization of vaginal margins.
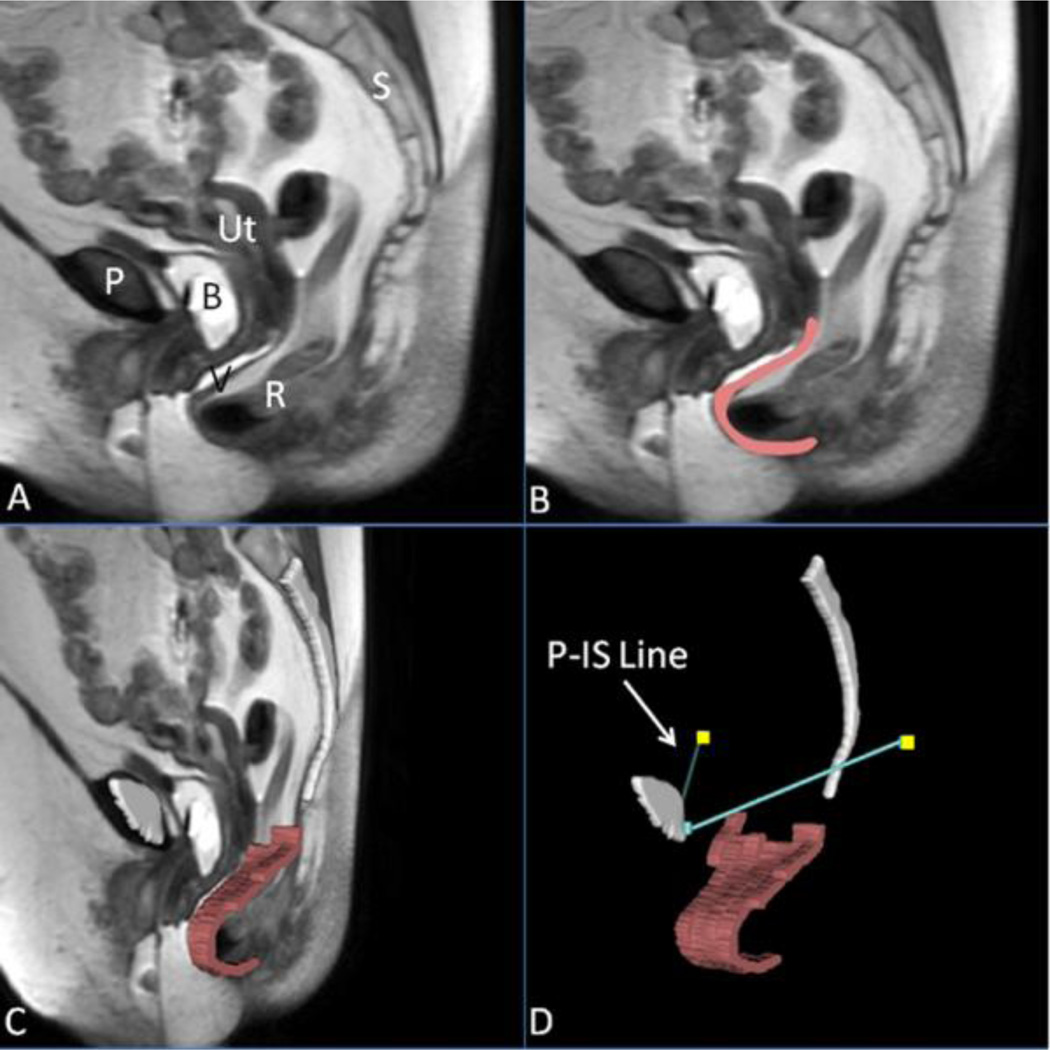
The MR images from axial, sagittal and coronal planes were imported into 3D Slicer 3.4.2009-10-15 (Brigham and Women’s Hospital, Boston, MA). The resting axial and sagittal images were aligned first, with manual registration and fixed landmarks such as pubic bone and sacrum. 3-D models were made of the following resting structures: bony pelvis and ischial spines using axial images, and posterior vaginal wall using sagittal images based on our previous anatomic work [10,13,14]. Figure 1 illustrates the 3-D model generation process and subsequent reference line as described below. The vaginal wall was modeled using sagittal strips to avoid artifacts from smoothing. The models were compared to the original MR images to confirm their fidelity.
Fig 1. Making a 3D prolapse model including the P-IS line.
(A) Mid-sagittal MR image of subject with posterior prolapse; (B) Outline of posterior vaginal wall in pink; (C) Addition of midsagittal pelvic bones (white) and 3D model of posterior vaginal wall shown in slightly skewed sagittal image; (D) Straining posterior vaginal wall model and its relationship to the normalized ATFP, shown here as the turquoise lines extending from the public symphysis to the ischial spines (yellow squares), or the P-IS line. P, pubic symphysis; S, sacrum; B, bladder; R, rectum; V, vagina; Ut, uterus; IS, ischial spine. (© DeLancey 2011)
To analyze deformation of posterior vaginal wall under load, 3-D models of midsagittal pubic symphysis and sacrum were reconstructed using the MR images from maximal Valsalva and aligned with the pelvic bones of the resting images. This registration information was then applied to the soft tissue images making it possible to align the subsequently constructed 3-D posterior vaginal wall with the previously created resting models using the pubic symphysis and sacrum. A reference line was constructed on each side of the pelvis representing the normal location of the arcus tendineus fascia pelvis (ATFP) from its pubic attachments to the ipsilateral ischial spine (“P-IS” line) for visual reference and consideration for future measurement purposes [6,12].
To compare the 3-D reconstruction models among case and control groups, the resting and maximal Valsalva 3-D models were imported into Microsoft PowerPoint®. All models were aligned by the position of the pubis and with a dotted line indicating the usual location of perineal body. Then the above models were compared visually among the case and control groups, with morphological changes identified using descriptive terminology. Two physician co-authors scored the frequency for the characteristic in both case and control groups. A descriptive statistical analysis was performed of case and control demographics. Fisher’s exact tests were used to determine the statistical significance (p<.01) of the proportions of women in each group who manifest each phenomenon.
Results
Subjects’ characteristics and POP-Q values are shown in Table 1. The case and control groups were matched by race, age, and vaginal parity. No subjects in either group had undergone a hysterectomy and all cases were rectocele type prolapse predominantly. Statistically significance differences were found at points C, Ap, and Bp during clinical POP-Q examination for the two groups.
Table 1.
Demographics
| Cases | Controls | |||
|---|---|---|---|---|
| Characteristics | (n=10) | (n=10) | P-value | |
| Age (yrs)i | 54.9(8.7) | 54.2(8.9) | .860 | |
| BMI (kg/m2)i | 28.9(5.5) | 28.4(8.6) | .825 | |
| Parityi | 2.6(0.7) | 2.8(1.0) | .678 | |
| Raceii | ||||
| Caucasian | 9 (90%) | 10 (100%) | 1.00 | |
| POP-Q (cm)i | ||||
| Aa | −1.2(0.9) | −1.9(1.0) | .111 | |
| Ba | −1.0(0.9) | −1.9(1.0) | .081 | |
| C | −5.2(1.4) | −6.8(1.2) | <.001 | |
| D | −7.5(1.6) | −8.9(1.4) | .003 | |
| Ap | 1.7(0.8) | −1.7(0.7) | <.001 | |
| Bp | 1.7(0.8) | −1.7(0.7) | <.001 | |
| GHrest | 3.9(1.3) | 3.3(1.0) | .240 | |
| LHrest | 7.5(1.7) | 6.4(0.8) | .066 | |
| TVL | 10.3(1.3) | 10.2(1.2) | .790 | |
Data are mean(SD); GHrest, genital hiatus at rest; LHrest, levator hiatus at rest; TVL, total vaginal length.
Data are n(%); P is from Fisher’s exact test.
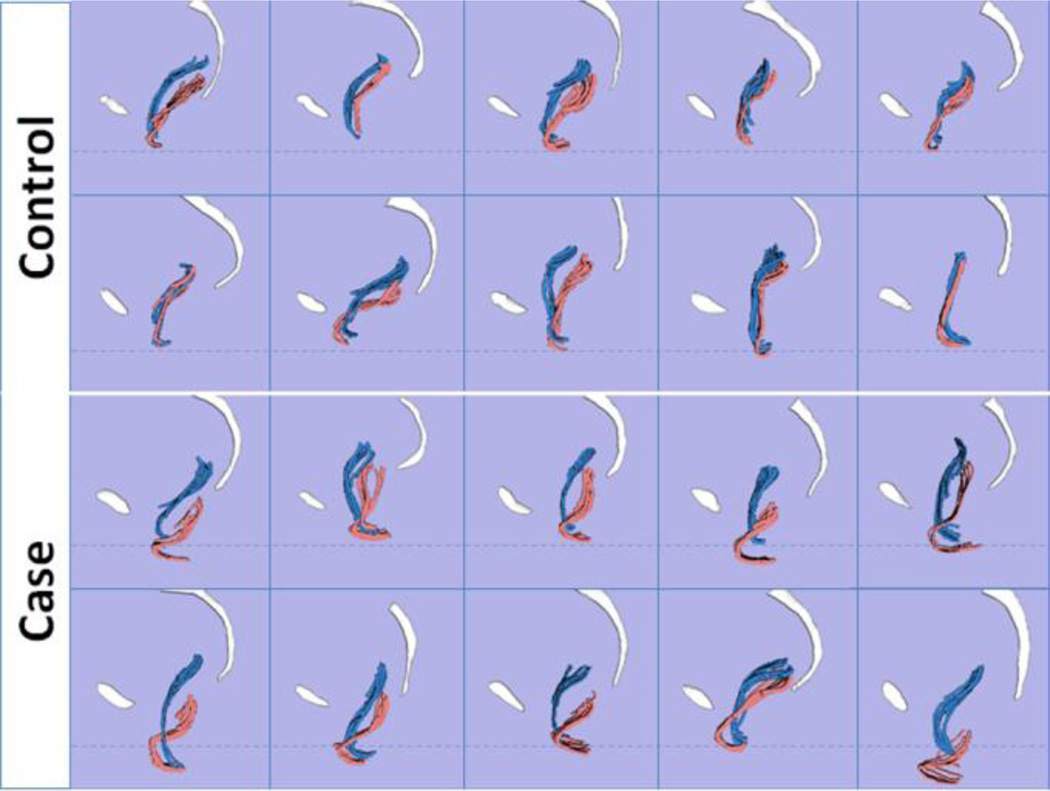
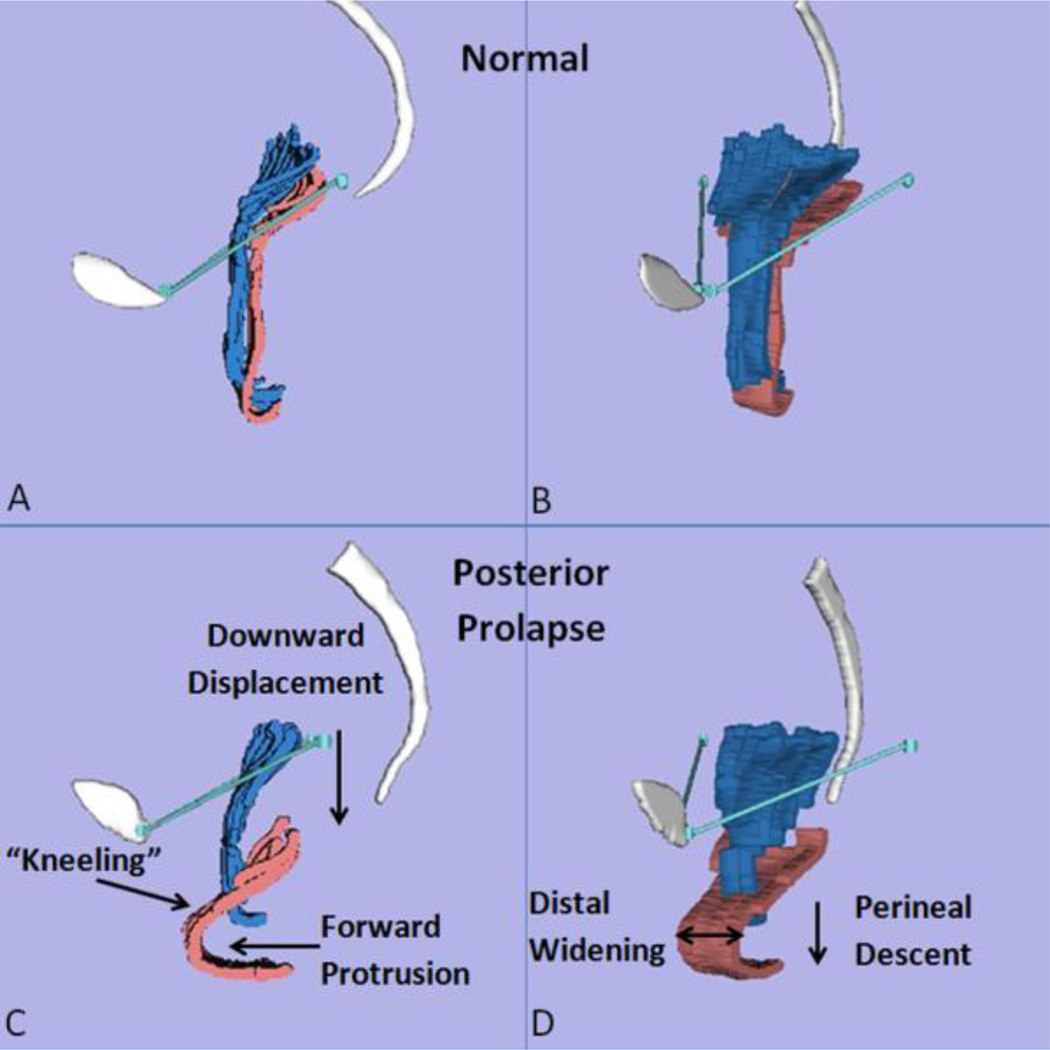
The lateral views of rest and strain models in all 20 subjects are shown in Fig. 2. With Valsalva, two characteristics were consistently visible in women with PVPR (10/10): 1) the posterior vaginal wall displayed a folding phenomenon similar to a person beginning to kneel (“Kneeling”) (Fig. 3C); and 2) downward displacement in the upper 2/3 part of the vagina (Fig. 3C). In addition to the “Kneeling” and downward displacement characteristics, in women with PVPR the posterior vaginal wall underwent other morphologic changes. For example, forward protrusion of the distal vagina (6/10) can be seen in some subjects (Fig. 3C). About half subjects (5/10) had perineal descent. To be considered as forward protrusion and perineal descent phenomena, the amount of forward or downward movement have to be significant enough (e.g., lower 1/3 of vaginal wall lose contact compared to control group). In addition, distal widening in lower third of the vagina was seen in a few (3/10) subjects. The complete comparison of the frequency of the above morphologic findings is shown in Table 2 for the case and control groups.
Fig 2. 3D case and control models comparison.
Lateral view of posterior vaginal walls of 10 controls and 10 cases during rest (blue) and Valsalva (pink). The vaginal wall was modeled using sagittal strips to avoid artifacts from smoothing. Pubis and sacrum are shown in white. Dotted lines indicate the average level of the perineal body for visual reference. (© DeLancey 2011)
Fig 3. Characteristics of posterior prolapse.
Comparison of control (A,B) and case (C,D) in lateral view (A,C) and oblique view (B,D) showing five characteristic features (C,D) during rest (blue) and Valsalva (pink): 1) Increased folding (“Kneeling”); 2) Downward displacement in the upper 2/3 part of the vagina; 3) Forward protrusion; 4) Perineal descent; 5) Distal widening in the lower third part of the vagina. Pubis and sacrum are shown in white. The P-IS line is shown in turquoise. (© DeLancey 2011)
Table 2.
Frequency of morphologic characteristics within case and controls
| Cases | Controls | P-value | |
|---|---|---|---|
| Characteristics | (n=10) | (n=10) | (Fisher’s exact) |
| Kneelingi | 10(100%) | 2(20%) | <0.001 |
| Downward Displacementi | 10(100%) | 3(30%) | <0.01 |
| Forward Protrusioni | 6(60%) | 2(20%) | 0.170 |
| Perineal Descenti | 5(50%) | 3(30%) | 0.650 |
| Distal Wideningi | 3(30%) | 0(0%) | 0.211 |
Data are n(%); Downward displacement is mainly for upper 2/3 of vagina; Distal widening is mainly for the lower third of vagina.
Discussion
We examined the appearance of the posterior vaginal wall at maximal Valsalva in normal women and women with rectocele as a secondary analysis of an ongoing case-control study of pelvic organ prolapse. The hypothesis was supported in that we found consistent changes in the shape and position of the vagina in women with posterior vaginal prolapse. Increased folding (“Kneeling”) of the vagina and an overall downward displacement were consistently present in rectocele. Other less consistent phenomena included forward protrusion, perineal descent, and distal widening.
The “Kneeling” is not just a forward protrusion of the distal vaginal wall, but a more complex phenomenon as it can occur in the absence of forward protrusion. The addition of descent of the upper vagina in those with “Kneeling” seemed to ‘pinch off’ the bowel as the upper vagina moved toward the levator plate; this suggests that it may play a role in obstructive defecatory dysfunction. Further measurements will be needed to confirm or refute this hypothesis.
These findings are consistent with findings in 2-D sagittal imaging [10] but qualitatively are much richer in the information they provide. In addition, with this 3-D MR imaging-based modeling technique, we can better visualize the relationship between the lateral wall and reference lines such as the P-IS line so that a quantitative unified 3-D biomechanical model can be created to test different hypotheses related to mechanism of the PVPR. Furthermore, it allows us to evaluate the degree of vaginal widening that is seen in some subjects.
We were surprised that distal widening of the vagina was not seen universally in the population with PVPR. Certainly it is an expected finding, but on reflection this fits with clinical experience that not all rectoceles are the same. Now that it is possible to image vaginal width, researchers can pursue explanations for why one woman has this phenomenon and others do not, and determine if it is related to defecatory dysfunction seen in some women with PVP.
One prominent aspect of rectoceles was the downward displacement of the upper vagina. This is accompanied by a change in the relationship between the vagina and the P-IS line that identifies the normal location of the fascial arch. This raises the issue of whether or not there is a “posterior paravaginal defect”. In work on the anterior vaginal wall [15,16] we have seen that paravaginal defect and apical descent are essentially two aspects of the same phenomenon. Further work will be needed to clarify this issue.
There are significant differences of opinion among experts in the field regarding the anatomical factors responsible for rectoceles and the relationship between surgical approaches and outcome [17,18]. The differences of opinion start simply with how to define or quantify the rectocele. Urogynecologists often struggle with an adequate way of describing the rectocele [19,20]. Most imaging studies to date have focused on measurements of the rectal contour with contrast during defecation which is a different phenomenon than the movement of the posterior vaginal wall elicited during pelvic examination [9,21]. Both have their separate roles in understanding problems with posterior vaginal wall support. Obtaining accurate 3-D information about the structural changes present in individual women shows great promise as an investigative technique and may lead to specific surgical therapies.
A lack of objective reproducible preoperative tests that can identify the specific nature of each patient’s defect has prevented clinical trials that test the hypothesis that a specific approach may have better results in patients who have a specific type of rectocele. The current project is a start towards developing an assessment strategy that can identify different anatomical defects responsible for a woman’s anatomical problem. Next steps might include quantitative measures of posterior vaginal wall morphology to test specific hypotheses and then evaluation of these abnormalities to the status of surrounding structures such as the levator ani muscle and the pelvic fascia. We are not implying by this research that we believe MR imaging is, at present, necessary for the clinical management of a rectocele, but if this line of investigation is successful in capturing clinically important differences between different types of rectoceles that affect surgical outcome then it may become helpful. Of course symptoms are not always tied to anatomy and this line of investigation does not diminish the importance of assessing other causes of defecation difficulty [22].
Several factors must be kept in mind when interpreting the result of these studies. This is a small sample of specifically selected women with distal posterior vaginal wall prolapse. We specifically selected women with predominant rectoceles in order to have a more homogeneous sample to analyze. It will be necessary to study women who also have significant cystocele or uterine prolapse in association with rectocele to gain an understanding of more complex prolapses. In addition, the changes seen in women with enteroceles will also need to be studied. The MR images are obtained in the supine position and not during defecation. However, these studies are similar to supine pelvic examination with Valsalva that clinicians use to examine the prolapse and perform a POP-Q examination (except for somewhat less thigh abduction). It is a limitation that we used gel in the vagina to help with visualization and in some instances in normal women it fills the upper vagina thereby slightly changing the contour. The characteristic features were identified by consensus between two of the clinical authors (JOLD, KAL) based on morphological patterns in preparation for developing a quantitative system.
This study is a first step to analyze the structural 3-D deformations involved in rectocele. It includes qualitatively studying the characteristic changes of posterior vaginal wall both at rest and maximal Valsalva from MR images for case and control groups. Future quantification of the differences between women with and without prolapse should give insights into the mechanism of the posterior vaginal wall prolapse and potentially lead to better surgical treatment strategies.
Acknowledgements
We gratefully acknowledge support from the National Institutes of Health Office for Research on Women’s Health, Specialized Centers of Research on Sex and Gender Factors Affecting Women’s Health, Grant P50 HD 044406.
Dr. John O.L. DeLancey and Dr. James A. Ashton-Miller receive research support from American Medical Systems and Kimberly Clark Corporation. Dr. Ashton-Miller receives research support from Proctor & Gamble, Inc. Dr. DeLancey receives research support from Johnson and Johnson. Dr. Dee E. Fenner receives research support from American Medical Systems. Jiajia Luo’s doctoral studies are partially funded by American Medical Systems and Kimberly Clark Corporation.
Footnotes
Podium presentation at the 32nd Annual Scientific Meeting of the American Urogynecologic Society, Sept 14, 2011 to Sept 17, 2011, Providence, Rhode Island.
Disclosure of Interest:
Dr. Kindra A. Larson has no disclosure to report.
Author Participation:
JJ Luo: Protocol/project development, Data collection or management, Data analysis, Manuscript writing/editing
KA Larson: Protocol/project development, Data analysis, Manuscript editing
DE Fenner: Protocol/project development, Data analysis, Manuscript editing
JA Ashton-Miller: Protocol/project development, Data analysis, Manuscript editing
JOL DeLancey: Protocol/project development, Data collection or management, Data analysis, Manuscript editing
References
- 1.Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–506. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 2.Boyles SH, Weber AM, Meyn L. Procedures for pelvic organ prolapse in the United States, 1979–1997. Am J Obstet Gynecol. 2003;188(1):108–115. doi: 10.1067/mob.2003.101. [DOI] [PubMed] [Google Scholar]
- 3.Silva WA, Pauls RN, Segal JL, Rooney CM, Kleeman SD, Karram MM. Uterosacral ligament vault suspension: five-year outcomes. Obstet Gynecol. 2006;108(2):255–263. doi: 10.1097/01.AOG.0000224610.83158.23. [DOI] [PubMed] [Google Scholar]
- 4.Subak LL, Waetjen LE, van den Eeden S, Thom DH, Vittinghoff E, Brown JS. Cost of pelvic organ prolapse surgery in the United States. Obstet Gynecol. 2001;98(4):646–651. doi: 10.1016/s0029-7844(01)01472-7. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Ashton-Miller JA, DeLancey JO. A 3D finite element model of anterior vaginal wall support to evaluate mechanisms underlying cystocele formation. J Biomech. 2009;42(10):1371–1377. doi: 10.1016/j.jbiomech.2009.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larson KA, Hsu Y, Chen L, Ashton-Miller JA, DeLancey JO. Magnetic resonance imaging-based three-dimensional model of anterior vaginal wall position at rest and maximal strain in women with and without prolapse. Int Urogynecol J. 2010;21(9):1103–1109. doi: 10.1007/s00192-010-1161-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu Y, Chen LY, Summers A, Ashton-Miller JA, DeLancey JOL. Anterior vaginal wall length and degree of anterior compartment prolapse seen on dynamic MRI. Int Urogynecol J. 2008;19(1):137–142. doi: 10.1007/s00192-007-0405-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganeshan A, Anderson EM, Upponi S, Planner AC, Slater A, Moore N, D'Costa H, Bungay H. Imaging of obstructed defecation. Clin Radiol. 2008;63(1):18–26. doi: 10.1016/j.crad.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Kelvin FM, Maglinte DD, Hale DS, Benson JT. Female pelvic organ prolapse: a comparison of triphasic dynamic MR imaging and triphasic fluoroscopic cystocolpoproctography. AJR Am J Roentgenol. 2000;174(1):81–88. doi: 10.2214/ajr.174.1.1740081. [DOI] [PubMed] [Google Scholar]
- 10.Lewicky-Gaupp C, Yousuf A, Larson KA, Fenner DE, Delancey JO. Structural position of the posterior vagina and pelvic floor in women with and without posterior vaginal prolapse. Am J Obstet Gynecol. 2010;202(5):497, e491–e496. doi: 10.1016/j.ajog.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pannu HK, Scatarige JC, Eng J. Comparison of Supine Magnetic Resonance Imaging With and Without Rectal Contrast to Fluoroscopic Cystocolpoproctography for the Diagnosis of Pelvic Organ Prolapse. J Comput Assist Tomo. 2009;33(1):125–130. doi: 10.1097/RCT.0b013e318161d739. [DOI] [PubMed] [Google Scholar]
- 12.Larson KA, Luo J, Yousuf A, Ashton-Miller JA, Delancey JO. Measurement of the 3D geometry of the fascial arches in women with a unilateral levator defect and "architectural distortion". Int Urogynecol J. 2012;23(1):57–63. doi: 10.1007/s00192-011-1528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeLancey JO. Structural anatomy of the posterior pelvic compartment as it relates to rectocele. Am J Obstet Gynecol. 1999;180(4):815–823. doi: 10.1016/s0002-9378(99)70652-6. [DOI] [PubMed] [Google Scholar]
- 14.Hsu Y, Lewicky-Gaupp C, DeLancey JO. Posterior compartment anatomy as seen in magnetic resonance imaging and 3-dimensional reconstruction from asymptomatic nulliparas. Am J Obstet Gynecol. 2008;198(6):651, e651–e657. doi: 10.1016/j.ajog.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Summers A, Winkel LA, Hussain HK, DeLancey JO. The relationship between anterior and apical compartment support. Am J Obstet Gynecol. 2006;194(5):1438–1443. doi: 10.1016/j.ajog.2006.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larson KA, Luo J, Guire KE, Chen L, Ashton-Miller JA, Delancey JO. 3D analysis of cystoceles using magnetic resonance imaging assessing midline, paravaginal, and apical defects. Int Urogynecol J. 2012;23(3):285–293. doi: 10.1007/s00192-011-1586-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleeman SD, Karram M. Posterior pelvic floor prolapse and a review of the anatomy, preoperative testing and surgical management. Minerva Ginecol. 2008;60(2):165–182. [PubMed] [Google Scholar]
- 18.Paraiso MF, Barber MD, Muir TW, Walters MD. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006;195(6):1762–1771. doi: 10.1016/j.ajog.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 19.Altman D, Lopez A, Kierkegaard J, Zetterstrom J, Falconer C, Pollack J, Mellgren A. Assessment of posterior vaginal wall prolapse: comparison of physical findings to cystodefecoperitoneography. Int Urogynecol J. 2005;16(2):96–103. doi: 10.1007/s00192-004-1220-2. discussion 103. [DOI] [PubMed] [Google Scholar]
- 20.Kenton K, Shott S, Brubaker L. Vaginal topography does not correlate well with visceral position in women with pelvic organ prolapse. Int Urogynecol J. 1997;8(6):336–339. doi: 10.1007/BF02765592. [DOI] [PubMed] [Google Scholar]
- 21.Kenton K, Shott S, Brubaker L. The anatomic and functional variability of rectoceles in women. Int Urogynecol J. 1999;10(2):96–99. doi: 10.1007/pl00004019. [DOI] [PubMed] [Google Scholar]
- 22.Cundiff GW, Fenner D. Evaluation and treatment of women with rectocele: focus on associated defecatory and sexual dysfunction. Obstet Gynecol. 2004;104(6):1403–1421. doi: 10.1097/01.AOG.0000147598.50638.15. [DOI] [PubMed] [Google Scholar]