Abstract
Mechanisms that control the differentiation and function of oligodendrocytes in the central nervous system are complex and involve multiple inputs from the surrounding environment, including localized concentrations of growth factors and the extracellular matrix. Dissection and analysis of these inputs are key to understanding the pathology of central nervous system demyelinating diseases such as multiple sclerosis, where the differentiation of myelinating oligodendrocytes from their precursors underlies the remission phase of the disease. In vitro co-culture models provide a mechanism for the study of factors that regulate differentiation of oligodendrocyte precursors but have been difficult to develop due to the complex nature of central nervous system myelination. This study describes development of an in vitro model that merges a defined medium with a chemically modified substrate to study aspects of myelination in the central nervous system. We demonstrate that oligodendrocyte precursors co-cultured with rat embryonic motoneurons on non-biological substrate (diethylenetriamine trimethoxy-silylpropyldiethylenetriamine), can be induced to differentiate into mature oligodendrocytes that express myelin basic protein, using a serum-free medium. This defined and reproducible model of in vitro myelination could be a valuable tool for the development of treatments for demyelinating diseases such as multiple sclerosis.
Keywords: Oligodendrocytes, MBP, Motoneurons, Co-Culture, In Vitro, DETA, Serum-Free
1. INTRODUCTION
Oligodendrocytes are the myelin-producing cells of the central nervous system (CNS).1,2 Their most prominent function is to produce the insulation for axons and thus facilitate saltatory conduction of action potentials. These cells arise from neuroepithelial cells during embryonic development of brain and ventral spinal cord. Oligodendrocyte precursor cells (OPCs) proliferate, migrate and undergo many developmental stages.3,4 OPCs, initially bipolar A2B5 expressing cells, differentiate, begin to extend multiple processes and express O4 sulfatide on their surface. Committed oligodendrocytes lose A2B5 reactivity after they begin to express O1 galactocerebroside. Differentiated oligodendrocytes are post-mitotic multipolar cells, expressing myelin basic protein (MBP) and upon maturation initiate the myelination of axons in the CNS. One oligodendrocyte can contact and wrap its processes around multiple axons to form compact myelin segments.1,5–10.
Understanding the mechanisms that regulate the differentiation of oligodendrocytes and their myelination of axons is crucial to understanding the pathology underlying demyelinating diseases such as multiple sclerosis. A characteristic of this disease is periods of remission associated with the differentiation of OPCs to mature oligodendrocytes and remyelination of demyelinated axons. Oligodendrocyte differentiation and myelination is regulated in response to a complex cascade of cues, including contacts with other cells and the extracellular matrix, and secreted factors such as growth factors.2,11,12 Studies of myelination in the peripheral nervous system have benefited greatly from the use of simplified co-culture systems featuring purified dorsal root ganglion (DRG) and Schwann cells. These studies have indicated an important role for axon derived cues in regulating myelination.13,14 However, it has been very difficult to establish similar systems to study CNS myelination due to its complexity. Common approaches include the use of DRG neurons as an axonal source,15–17 or the use of dissociated CNS tissue, explants or aggregates.18–21 However, these cultures have certain drawbacks that limit their application to the study of factors regulating OPC differentiation and myelination. These include the requirement for a high cellular density that limit optical approaches to studying myelination as well as the use of poorly defined substrates that exhibit problems with long-term cell attachment. In addition, the requirement for serum in many of these models undermines their usefulness in dissecting out the contribution of various growth factors to OPC differentiation and myelination.
In this study, we have established a CNS co-culture that consists of OPCs and embryonic motoneurons cultured on a self-assembling monolayer composeds of the silane diethylenetriamine trimethoxy-silylpropyldiethylenetriamine (DETA), in the presence of a defined serum free medium. This medium formulation has previously been shown to enhance the growth and long-term survival of motoneurons as well as promote their myelination by Schwann cells with accompanying node of Ranvier formation.22 Using this co-culture we were able to observe the differentiation of OPCs into mature MBP expressing oligodendrocytes. In addition, the use of DETA allowed us to control the topography of the co-culture by micropatterning using photolithography. This advance now allows for the acute optical visualization of the interaction between the oligodendrocyte and axon, which is not possible in denser organotypic-like cultures. This defined and reproducible system also provides precise control over individual cell behavior and allows other cell types to be added in a controlled manner. This system can be used to further improve understanding of CNS myelination and ultimately for development of therapies for demyelinating diseases such as multiple sclerosis.
2. MATERIAL AND METHODS
2.1. DETA Surface Modification
Glass coverslips (VWR 48366067, 22×422 mm2, No. 1) were first cleaned using 1:1 HCl–methanol followed by a concentrated H2SO4 soak for 2 h. The DETA (United Chemical Technologies Inc. T2910-KG) film was formed by the reaction of the cleaned surfaces with a 0.1% (v/v) mixture of the organosilane in freshly distilled toluene (VWR BDH1151). Coverslips were then boiled in deionized water and rinsed with acetone. The cleaned surfaces were heated to about 100 °C in the organosilane mixture, rinsed with toluene, reheated to about 100 °C in toluene, and then dried in the oven overnight (100 °C). Surfaces were characterized by static water contact angle measurements using a Rame-Hart Model 250 goniometer, and by X-ray photoelectron spectroscopy (XPS) using an Escalab 200i spectrometer (VG Scientific) by monitoring the N 1s peak. The values are reported as the mean±SEM.
2.2. Patterns Preparation
DETA/Silane (Si) Polyethyleneglycol (PEG) SAMs patterns were utilized in order to determine interaction on single cell level, in which the patterns consisted of a co-planar monolayer of non-permissive (SiPEG) and permissive regions (DETA). The SiPEG monolayer was patterned using a deep UV (193 nm) excimer laser (Lambda Physik) at a pulse power of about 200 mJ and a frequency of 10 Hz for 45 seconds through a quartz photomask (Bandwidth Foundry, Eveleigh, Australia). A negative photomask consisting of transparent patterns on chromium background was used to fabricate the DETA/SiPEG SAM patterns. The energy dosage (8–15 J/cm2) was sufficient to ablate the regions of the SiPEG monolayer exposed to the UV light to yield reactive hydroxyl groups, and the irradiated surfaces were subsequently rederivatized with DETA. The rederivatization with DETA was done as described above, but at a lower temperature, 60 °C.
2.3. Contact Angle Measurements
Water contact angle measurements were measured with a Ramé-hart goniometer (Mountain Lakes, NJ). The contact angle of a static sessile drop (5 µl) of water was measured three times and averaged. Water contact angle on DETA was 48±2. Water contact angle on SiPEG was 37±2.
2.4. X-Ray Photoelectron Spectroscopy
The XPS characterization of DETA and SiPEG surfaces was performed utilizing a Thermo ESCALAB 220i-XL X-Ray photoelectron spectrometer equipped with an aluminium anode and a quartz monochromator. The surface charge compensation was achieved by using a low-energy electron flood gun. Survey scans were recorded in order to determine the relevant elements (pass energy of 50 eV, step size = 1 eV). High resolution spectra were recorded for Si 2p, C 1s, N 1s, and O 1s (pass energy of 20 eV, step size = 0 = 1 eV). The spectrometer was calibrated against the reference binding energies of clean Cu, Ag and Au samples. In addition, the calibration of the binding energy (BE) scale was made by setting the C 1s BE of carbon in a hydrocarbon environment at 285 eV. N 1s and Si 2p peak deconvolution was performed with Avantage version 3.25 software, provided by Thermo Electron Corporation.
2.5. Animals
Dated pregnant Sprague–Dawley rats were housed in an animal facility at the University of Central Florida. All research was approved by the Institutional Animal Care and Use Committee at the University of Central Florida and conformed to NIH guidelines. For the oligodendroglial cultures, neonatal rat pups were anesthetized and sacrificed at day 1 after the birth and cortical tissue was removed and dissected. Pregnant rats were anesthetized and sacrificed at embryonic day 15 for the motoneuron cultures. Embryos were removed by caesarean section and fetuses dissected under a stereomicroscope (Carl Zeiss, Stemi, 2000).
2.6. Purified Embryonic Motoneuron Culture
Rat spinal cord motoneurons were purified from the ventral horn cords from embryonic day 15 (E15) embryos as described previously.23,24 Briefly, pregnant rats were anaesthetized and terminated by inhalation of excess CO2. Spinal cords were removed from the E15 pups and the ventral horn tissue was dissected out and digested in 0.05% trypsin–EDTA for 15 min in a 37 °C water bath (Gibco 25300-120). Following incubation, the trypsin–EDTA was aspirated and the cells suspended in dissection media, containing 10% FBS, and the tissue gently triturated. The dissociated cell suspension was then centrifuged at 500 g for 10 min at 4 °C to pellet the cells. Next, the tissue was layered on a density gradient of Opti-prep (Sigma D1556) solution and centrifuged at 500 g for 15 min at 4 °C. After centrifugation, the resulting top two bands were collected in a 15 ml tube and the pellet discarded. The ventral horn cells were then applied to an immuopanning dish coated with goat affinity purified antibody to rat IgG and the low affinity nerve growth factor receptor p75 (Chemicon MAB365) in dissection medium for 45 min. This positive selection process provides attachment for the motoneurons while the other cells remained in suspension. After immuopanning the non-adherent cells were aspirated and the adherent motoneurons were removed from the dish in dissection medium to a 15 ml tube. Lastly, the neurons were pelleted by centrifugation at 500 g for 10 min and then resuspended in culture medium and plated at a cell density of 300 cells/mm2.
2.7. Preparation of Glial Culture and Isolation of OPCs
Mixed glial cell cultures were prepared following the previously published protocol.25 The cerebral cortexes were obtained from 1 day old rat pups and dissociated using 0.01% trypsin. Tissue was cultured in poly-d lysine (PDL) coated tissue culture flasks (T75) and maintained in a growth medium composed of Dulbecco’s Modified Eagle Media–high glucose (DMEM, Gibco BRL, Rockville, MD) with the addition of 15% fetal bovine serum and 1% Antibiotic Antimycotic (Gibco BRL) at 37 °C in a humidified atmosphere of 5% CO2. The medium was changed every 3–4 days. The length of the culture period was 9 days. After 7 days, the culture was rinsed three times with culture medium to remove floating microglia. After 9 days, the medium was replaced with 10 ml of fresh growth medium and flasks were secured on the surface of an orbital shaker and shaken for 15–18 h (37 °C, 250 rpm, stroke diameter of 1.5 in). OPCs were then harvested from the media by centrifugation and plated on the top of 3 day old EMN cultures, at a plating cell density of 300 cells/mm2.
2.8. Morphological Analysis
Phase-contrast images were taken with a commercial Nikon Coolpix 990 camera using the Zeiss Axiovert S100 microscope. Pictures were analyzed using Scion Image Software (Scion Corp., Frederick, MD).
2.9. Immunocytochemistry
To characterize cells by immunocytochemistry, they were briefly washed with Hanks’ balanced salts solution and fixed in 4% paraformaldehyde for 18 min. Fixed cells were rinsed in PBS, permeabilized with 0.5% Triton 100× in PBS for 7 min, and then blocked with 5% donkey serum for 1 hr. Incubation with the primary antibody took place overnight at 4 °C. Primary antibodies used were mouse monoclonal A2B5 (MAB312, 1:250), O4 (MAB345, 1:100), O1 (MAB344, 1:200), MBP (1:25), and anti-neurofilament heavy chain (1:1000) (AB5539), all from Chemicon (Temecula, CA). Following rinsing in PBS, cells were incubated with an Alexa Fluor 488- conjugated anti-mouse IgG or Alexa Fluor 594-conjugated anti-chicken IgG for 2 hours at room temperature, rinsed with PBS again and mounted on glass slides under Vectashield mounting medium (H1000, Vector Laboratories, Burlingame, CA). For general visualization of cellular nuclei, the specimens were counterstained with DAPI. Immunoreactivity was observed and analyzed by using an Ultra VIEW™ LCI confocal imaging system (Perkin Elmer).
2.10. Quantification
Morphological and immunocytochemical quantification was performed on cells during various differentiation stages. For each coverslip, at least 10 pictures were taken from randomly chosen views under 200× magnification. All the marker-positive cells were counted, as well as the total number of cells in these views. At least three cover slips in each group were quantified and data were expressed as an average±standard deviation (SD).
3. RESULTS
3.1. Co-Cultures of OPCs with EMNs in Defined In Vitro System
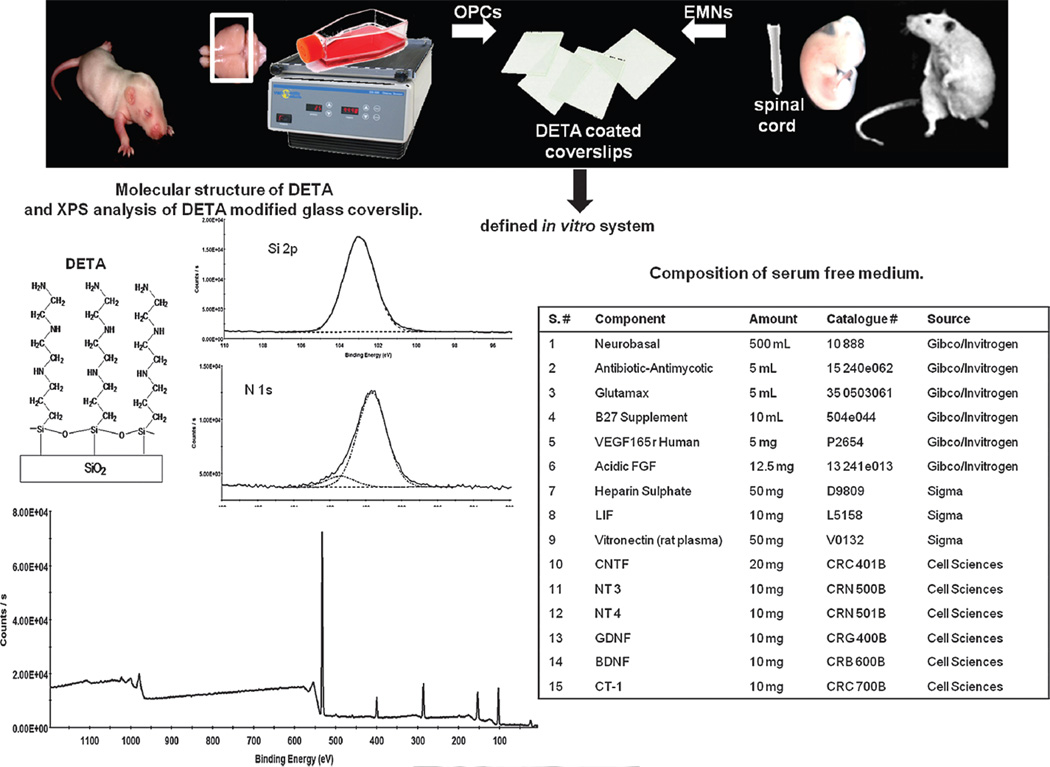
The main experimental steps in the OPCs–EMNs co-culture are illustrated in Figure 1. Essentially, DETA coated cover slips were prepared as described.26,27 DETA is an aminosilane that forms a covalently bound, uniform, self-assembled monolayer on the glass surface. The structure of DETA, containing multiple amines in its terminal group, is shown in Figure 1. Characterization of the DETA monolayer was carried out by determining the nitrogen/ silicon content of the coverslip surface using XPS (Fig. 1). We have previously identified optimum values for this determinant that support neuronal growth and have adapted our chemistry appropriately.22,27–29 Similarly, the hydrophilicity of the coverslip surface was determined using a static contact angle measurement (Fig. 1). Cover slips with optimal surface characteristics were seeded with motoneurons isolated from the spinal cords of embryonic day 15 rat embryos, by a process of density gradient centrifugation and immunopanning. These motoneurons were cultured in the presence of the medium described in Figure 1, which had previously been shown to support the growth and maintenance of both motoneurons and Schwann cells,22 in co-culture. After three days in culture neurons were seeded with OPCs isolated from the cerebral cortex of a 1 day old rat pup. Following the enrichment, EMNs were plated on the modified glass coverslips and allowed to grow for three days before the addition of OPCs.
Fig. 1.
Schematic representation of co-cultures of OPCs with EMNs in defined in vitro system. Diagram illustrates the main steps in the OPCs–EMNs co-culture and development of a defined in vitro system. This system involves modification of a glass coverslip with a monolayer of the synthetic, growth promoting substrate DETA. Cells attached to DETA were cultured in the serum free medium shown, containing factors that are optimal for cell survival and the differentiation of OPCs.
3.2. Characterization of OPCs Alone Following the Isolation
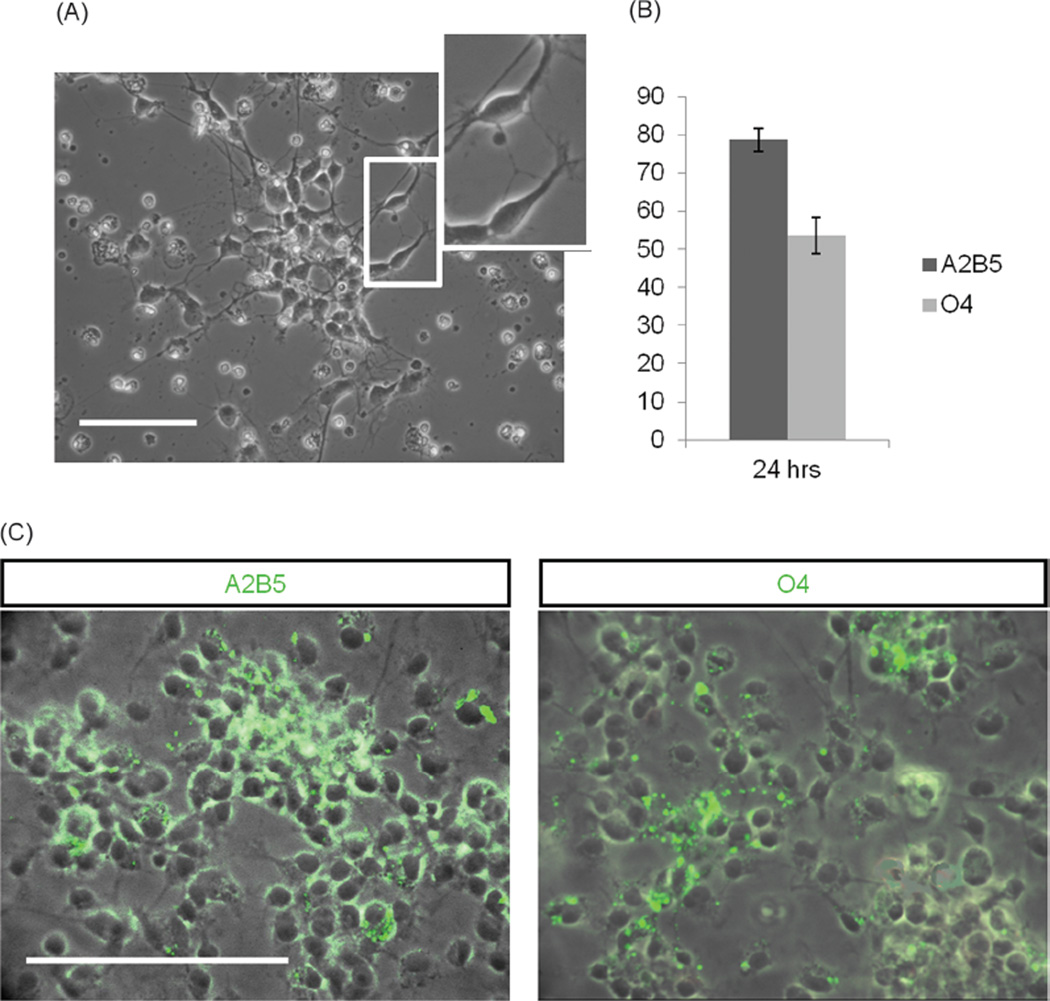
To characterize the OPCs in the defined system immediately following the shake-off isolation, cells were plated separately from EMNs on DETA coated coverslips and allowed to attach. Following the attachment, the cellular morphology was carefully monitored. Within 24 hrs after the plating OPCs exhibited bi-polar morphology, typical of early OPCs, as illustrated in Figure 2(A). After 24 hrs, cells were fixed and analyzed for expression of markers of oligodendroglial lineage using immunocytochemistry. Quantification of the results revealed that 78.6 ± 3.1% of cells purified in this fashion expressed A2B5 and 53.6±4.7% of cells expressed O4, suggesting that the cells plated onto DETA initially exhibited an early OPC phenotype (Figs. 2(B and C)). In support of this idea, cells were negative for O1 and MBP, markers of differentiated oligodendrocytes.
Fig. 2.
Characterization of newly isolated OPCs from the rat pup cortex. (A) A typical bipolar morphology of OPCs 24 hrs after “shake-off” isolation. (B) Results of immunocytochemical analysis revealed that 78.6±3.1% of cells expressed A2B5 and 53.6±4.7% of cells expressed O4. (C) Immunocytochemistry for A2B5 and O4 expression 24 hrs after shake off. Scale bars 100 µm.
3.3. Characterization of OPCs in Co-Culture with EMNs
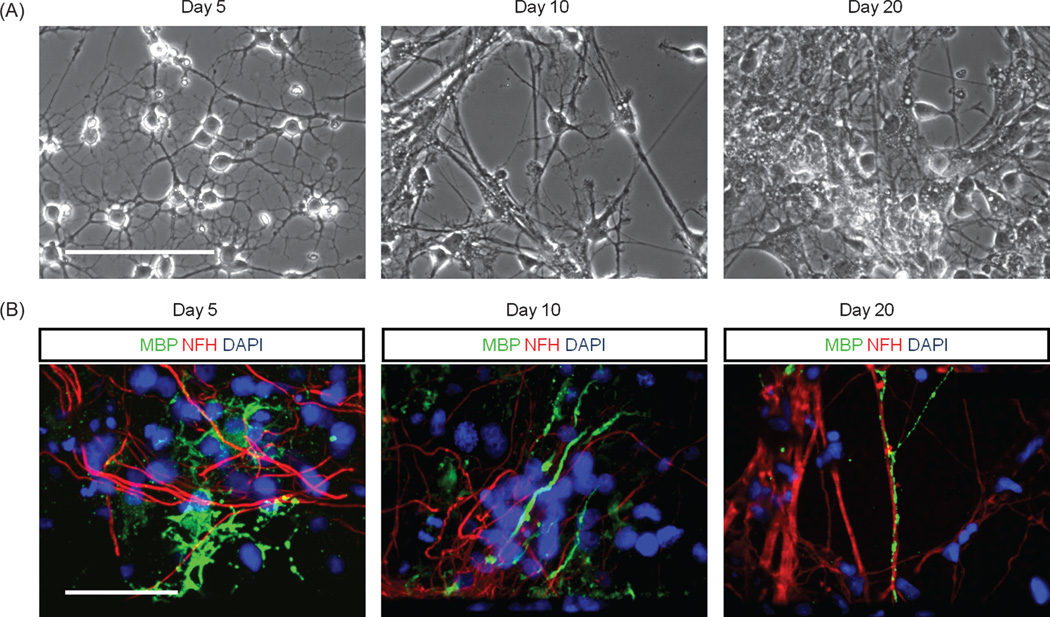
A number of factors play a role in the maturation of OPCs including axonal interactions.2,12,19 and substrate contact.30,31 Therefore OPCs were examined to determine if they could differentiate into mature oligodendrocytes during co-culture with EMNs in the defined system using DETA as a substrate. Within five days of co-culture, OPCs started to exhibit a mature phenotype indicated by the elaboration of an extensive network of processes (Fig. 3(A)) and the expression of MBP, a characteristic marker of mature oligodendrocytes and a major constituent of the myelin sheath. EMNs were detected by employing neuron specific neurofilament heavy chain antibody, identifying the neuro–axonal compartments. The results indicated that at day 5, 23.6±5.7% of cells, at day 10, 42.94±3.2% of cells and at day 20, 49.1 ± 2.0% of cells expressed MBP. At day 5 in co-culture, MBP positive oligodendro-cytes exhibited elaborate processes and by day 10 their processes started to align with NF–H positive axons. Yet, oligodendrocytes did not extend multiple distinctive MBP positive segments suggestive of fully mature compact myelin. The clustering of voltage-gated sodium channels at nodes of Ranvier was also not detected, suggesting that the oligodendrocytes in these cultures were restricted in their ability to form myelin.
Fig. 3.
Expression of markers of oligodendroglial lineage in co-culture. (A) Light microscopy images indicating the morphology of OPCs at day 5, 10 and 20 in co-culture. (B) Immunocytochemical analysis detecting MBP, NF–H and DAPI at day 5, 10 and 20 of co-culture. Images illustrate details of mature ramified oligodendrocytes at day 5 and oligodendrocytes wrapping their processes around axons at day 10 and 20. Scale bars 100 µm.
3.4. Interaction Between OPCs and EMNs on the Single Cell Level
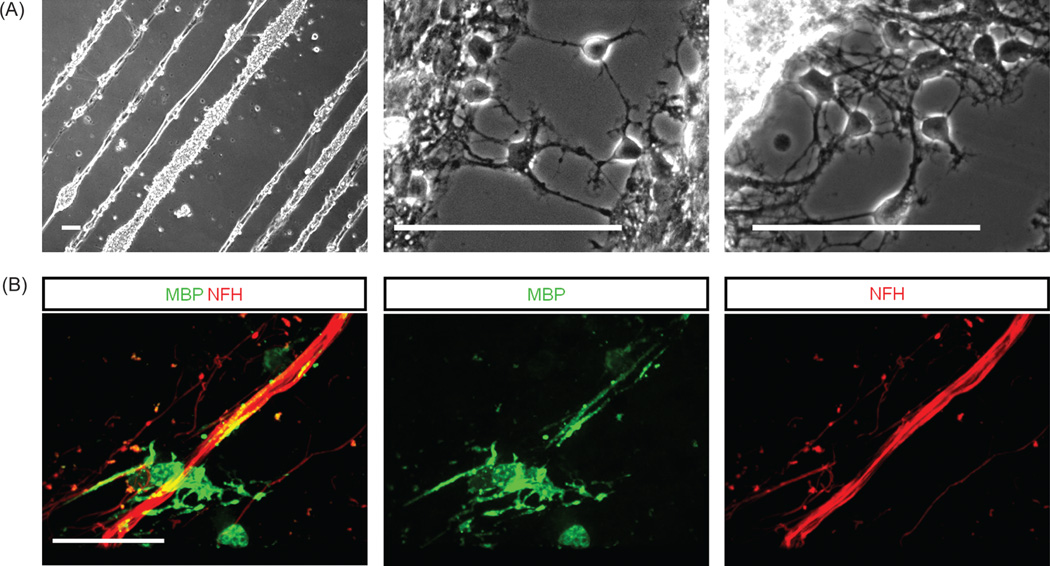
In order to investigate the interaction between OPCs and EMNs on the single cell level, cells were co-cultured on glass coverslips modified with photolithography to form line patterns composed of DETA. Line thicknesses were 10, 20, 50 and 100 µm. Areas in between the lines contained polyethyleneglycol (PEG), to form a hydrophilic surface that was largely resistant to cell attachment. Distances between lines were 40, 70 and 100 µm. Cells attached and survived well in this highly restrictive micropatterned environment. Figure 4(A) illustrates the morphology of cells at 20 days after plating on these line patterns. At day 20, cells were fixed and analyzed for the expression of MBP and NF–H. Results indicated that oligodendrocytes fully differentiated (as determined by MBP expression) on the DETA lines. Furthermore, they extended their MBP positive processes and wrapped them around multiple, precisely oriented axons as shown in Figure 4(B). The survival and interaction between both types of cells in this controllable and highly restricted environment represents an important step in the development of a completely defined in vitro myelination model.
Fig. 4.
Co-culture of OPCs with EMNs and MBP expression on a line pattern. (A) Light microscopy images of co-cultures grown on line patterns composed of DETA/SiPEG SAMs, for 20 days. (B) Immunocytochemical analysis of mature MBP positive oligodendrocyte interacting with multiple NF-positive axons. Scale bars 100 µm.
4. DISCUSSION
The results of this study have demonstrated that cortical OPCs can be co-cultured with EMNs in a defined, serum free system, on an artificial substrate which can be patterned by photolithography. OPCs from the rat cortex initially isolated as immature A2B5 expressing bipolar cells were able to differentiate on the DETA substrate such that greater than 50% of them exhibited a mature morphology and expressed MBP. These OPCs/oligodendrocytes interacted with EMNs, differentiated, expressed MBP and wrapped their processes around axons, although they did not appear to ultimately form a compact myelin sheath. The use of DETA allowed for micropatterning of the co-culture using photolithograpy. This now enables the detailed optical analysis of the interaction between the myelinating glia cell and the axon, which is not possible in the more complex organotypic-like cultures currently being utilized.
In this defined model, an artificial substrate consisting of a self-assembling monolayer of DETA was utilized.27,32–34 The triamine moiety of DETA resembles the structure of spermidine, a well known growth factor.35,36 DETA is an aminosilane that forms a covalently bound, uniform, self-assembled monolayer on glass surfaces. At physiologic pH, the amines carry partial positive charges, providing a hydrophilic surface that promotes cellular attachment. DETA also promotes long-term cell survival because it is non-digestible by matrix proteases secreted by cells.29,32 Prior to its use, DETA surfaces were characterized by XPS analysis and hydrophilicity was determined using static contact angle measurements. This allowed for tight quality control and ensured reproducibility between different cultures. Similarly, the use of a well-defined media composition eliminated the vagaries associated with the use of serum due to inter-batch variability.
An interesting question raised by this series of experiments is why we were not able to detect myelinated axonal segments in our co-cultures, despite the fact that cultures were allowed to develop out to beyond 20 days, which is easily enough time for myelin formation to occur. We do not believe that this reflects problems with the basics of our co-culture system for the following reasons:
We have used this same media and the DETA substrate to establish co-cultures of EMN’s with Schwann cells and have successfully observed myelination,22 and
We have used the same substrate and media for a co-culture of EMNs with OPCs derived from the E15 spinal cord and purified by immunopanning with OPC markers, and have observed myelin segments with accompanying node of Ranvier formation (Davis et al., manuscript in preparation).
We therefore assume that our inability to form myelin segments in this system results from our source of OPCs. Recent studies have shown that maturation of an oligodendrocyte into an MBP expressing cell does not necessarily reflect its ability to myelinate and that maturing oligodendrocytes can exhibit a narrow window of myelin competency.19 The development of the defined co-culture system described here will allow us to study this characteristic in more detail.
Many of the previous models that have studied oligodendrocyte-axon interactions in vitro have co-cultured oligodendrocytes with DRG neurons.12,15,37 However the cell bodies of DRG neurons are located outside of the CNS with their axons being limited in their exposure to the CNS environment. As it is known that similar signals can differentially regulate CNS and PNS myelination then the combination of oligodendrocytes with DRG axons does not represent a useful recapitulation of CNS myelination.38 In order to represent a more accurate representation of the CNS environment, several studies have reported oligodendrocyte-CNS neuron co-cultures.18,20,21,39,40 These co-cultures have involved mostly explants or cell aggregates grown on PDL or matrigel coated coverslips.18–20,41 These reported methods have significant disadvantages and technical challenges, involving difficulties with the attachment of the explant or aggregates to the substrate and an inability to optically track or distinguish individual cells within the culture.42
This study has documented the development of an improved CNS model for oligodendrocyte-axonal interactions. Both type of cells, OPCs and EMNs, originate from different sources, which overcomes difficulties that require specialized cell labeling to determine cellular origins. In addition, cells were plated as dissociated cultures, overcoming the challenge of poor attachment of aggregates to biological tissue culture substrates and allowing greater optical resolution of culture cellular components. In co-cultures, OPCs were observed to interact with EMNs, and after 5 days start to become differentiated and exhibit the ramified phenotype of a mature, MBP positive oligodendrocyte. Immunocytochemical analysis revealed that at this stage 23.6±5.7% of cells expressed MBP and by day 20 of co-culture almost 50% of the cells were MBP positive and wrapped their processes around NF–H positive axons.
Another advantage to using a defined substrate is the ability to micropattern the surface to allow the study of oligodendrocyte-axon interactions on the single cell level. Lines, composed of DETA, were separated by a cell repellent surface represented by PEG. Co-cultures attached to the lines, differentiated and survived for over a month in this highly restrictive environment. Line patterns allowed for the analysis of single oligodendrocytes aligning and wrapping their processes around multiple axons. Further studies and development of this novel, defined in vitro model will allow for a more precise assessment of factors and environment in which CNS myelination occurs. The results could be used to elucidate underlying causes of demyelinating diseases such as multiple sclerosis, central pontine myelinolysis, transverse myelitis, Devic’s disease and others.
5. CONCLUSIONS
This work has documented the establishment of an in vitro CNS myelination model. The model utilizes a serum-free defined medium and a chemically defined synthetic substrate. The defined conditions promote cell attachment, growth and survival. Furthermore, they allow for precise control and evaluation of factors influencing OPC-axon interactions and further the understanding of CNS myelination in vivo.
Acknowledgments
This work was supported by NIH/NIBIB grant R01EB009429 and NIH/NINDS grant R01NS050452. “The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Biomedical Imaging and Bioengineering, the National Institute of Neurological Disorders and Stroke, or the National Institutes of Health.”
References and Notes
- 1.Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol. Rev. 2001;81:871. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- 2.Miller RH. Regulation of oligodendrocyte development in the vertebrate cns. Prog. Neurobiol. 2002;67:451. doi: 10.1016/s0301-0082(02)00058-8. [DOI] [PubMed] [Google Scholar]
- 3.Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- 4.Wolswijk G, Noble M. Identification of an adult-specific glial progenitor cell. Development. 1989;105:387. doi: 10.1242/dev.105.2.387. [DOI] [PubMed] [Google Scholar]
- 5.Bansal R, Pfeiffer SE. Novel stage in the oligodendrocyte lineage defined by reactivity of progenitors with r-mab prior to o1 anti-galactocerebroside. J. Neurosci. Res. 1992;32:309. doi: 10.1002/jnr.490320303. [DOI] [PubMed] [Google Scholar]
- 6.Behar TN. Analysis of fractal dimension of o2a glial cells differentiating in vitro. Methods. 2001;24:331. doi: 10.1006/meth.2001.1203. [DOI] [PubMed] [Google Scholar]
- 7.Curtis R, Cohen J, Fok-Seang J, Hanley MR, Gregson NA, Reynolds R, Wilkin GP. Development of macroglial cells in rat cerebellum. I. Use of antibodies to follow early in vivo development and migration of oligodendrocytes. J. Neurocytol. 1988;17:43. doi: 10.1007/BF01735376. [DOI] [PubMed] [Google Scholar]
- 8.Pfeiffer SE, Warrington AE, Bansal R. The oligodendrocyte and its many cellular processes. Trends Cell. Biol. 1993;3:191. doi: 10.1016/0962-8924(93)90213-k. [DOI] [PubMed] [Google Scholar]
- 9.Sternberger NH, del Cerro C, Kies MW, Herndon RM. Immunocytochemistry of myelin basic proteins in adult rat oligodendroglia. J. Neuroimmunol. 1985;7:355. [PubMed] [Google Scholar]
- 10.Volpe JJ. Neurology of the Newborn. 5th edn. Philadelphia: Elsevier Health Sciences; 2008. [Google Scholar]
- 11.Emery B. Regulation of oligodendrocyte differentiation and myelination. Science. 2010;330:779. doi: 10.1126/science.1190927. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg SS, Kelland EE, Tokar E, De la Torre AR, Chan JR. The geometric and spatial constraints of the microenvironment induce oligodendrocyte differentiation. Proc. Natl. Acad. Sci. USA. 2008;105:14662. doi: 10.1073/pnas.0805640105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lubetzki C, Demerens C, Anglade P, Villarroya H, Frankfurter A, Lee VM, Zalc B. Even in culture, oligodendrocytes myelinate solely axons. Proc. Natl. Acad. Sci. USA. 1993;90:6820. doi: 10.1073/pnas.90.14.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aguayo AJ, Charron L, Bray GM. Potential of schwann cells from unmyelinated nerves to produce myelin: A quantitative ultrastructural and radiographic study. J. Neurocytol. 1976;5:565. doi: 10.1007/BF01175570. [DOI] [PubMed] [Google Scholar]
- 15.Wood P, Okada E, Bunge R. The use of networks of dissociated rat dorsal root ganglion neurons to induce myelination by oligodencrocytes in culture. Brain Res. 1980;196:247. doi: 10.1016/0006-8993(80)90732-5. [DOI] [PubMed] [Google Scholar]
- 16.He M, Howe DG, McCarthy KD. Oligodendroglial signal transduction systems are regulated by neuronal contact. J. Neurochem. 1996;67:1491. doi: 10.1046/j.1471-4159.1996.67041491.x. [DOI] [PubMed] [Google Scholar]
- 17.Shaw MK, Kim C, Hao HW, Chen F, Tse HY. Induction of myelin basic protein-specific experimental autoimmune encephalomyelitis in c57bl/6 mice: Mapping of t cell epitopes and t cell receptor v beta gene segment usage. J. Neurosci. Res. 1996;45:690. doi: 10.1002/(SICI)1097-4547(19960915)45:6<690::AID-JNR5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Trapp BD, Webster HD, Johnson D, Quarles RH, Cohen SR, Murray MR. Myelin formation in rotation-mediated aggregating cell cultures: Immunocytochemical, electron microscopic, and biochemical observations. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1982;2:986. doi: 10.1523/JNEUROSCI.02-07-00986.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watkins TA, Emery B, Mulinyawe S, Barres BA. Distinct stages of myelination regulated by gamma-secretase and astrocytes in a rapidly myelinating cns coculture system. Neuron. 2008;60:555. doi: 10.1016/j.neuron.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z, Ma Z, Wang Y, Li Y, Lu H, Fu S, Hang Q, Lu PH. Oligodendrocyte-spinal cord explant co-culture: An in vitro model for the study of myelination. Brain Res. 2009;1309:9. doi: 10.1016/j.brainres.2009.10.060. [DOI] [PubMed] [Google Scholar]
- 21.DeLong GR. Histogenesis of fetal mouse isocortex and hippocampus in reaggregating cell cultures. Dev. Biol. 1970;22:563. doi: 10.1016/0012-1606(70)90169-7. [DOI] [PubMed] [Google Scholar]
- 22.Rumsey JW, Das M, Stancescu M, Bott M, Fernandez-Valle C, Hickman JJ. Node of ranvier formation on motoneurons in vitro. Biomaterials. 2009;30:3567. doi: 10.1016/j.biomaterials.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camu W, Henderson CE. Rapid purification of embryonic rat motoneurons: An in vitro model for studying mnd/als pathogenesis. J. Neurol. Sci. 1994;124(Suppl):73. doi: 10.1016/0022-510x(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 24.Camu W, Henderson CE. Purification of embryonic rat motoneurons by panning on a monoclonal antibody to the low-affinity ngf receptor. J. Neurosci. Methods. 1992;44:59. doi: 10.1016/0165-0270(92)90114-s. [DOI] [PubMed] [Google Scholar]
- 25.McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell Biol. 1980;85:890. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murugan R, Molnar P, Rao KP, Hickman JJ. Biomaterial surface patterning of self assembled monolayers for controlling neuronal cell behavior. Int. J. Biomed. Eng. Technol. 2009;2:104. doi: 10.1504/ijbet.2009.022911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaffner AE, Barker JL, Stenger DA, Hickman JJ. Investigation of the factors necessary for growth of hippocampal neurons in a defined system. J. Neurosci. Methods. 1995;62:111. doi: 10.1016/0165-0270(95)00063-1. [DOI] [PubMed] [Google Scholar]
- 28.Stenger DA, Pike CJ, Hickman JJ, Cotman CW. Surface determinants of neuronal survival and growth on self-assembled monolayers in culture. Brain Res. 1993;630:136. doi: 10.1016/0006-8993(93)90651-3. [DOI] [PubMed] [Google Scholar]
- 29.Das M, Molnar P, Devaraj H, Poeta M, Hickman JJ. Electrophysiological and morphological characterization of rat embryonic motoneurons in a defined system. Biotechnol. Prog. 2003;19:1756. doi: 10.1021/bp034076l. [DOI] [PubMed] [Google Scholar]
- 30.Kotter MR, Li WW, Zhao C, Franklin RJ. Myelin impairs cns remyelination by inhibiting oligodendrocyte precursor cell differentiation. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2006;26:328. doi: 10.1523/JNEUROSCI.2615-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ingber DE. The architectural basis of cellular mechanotransduction. Annu. Rev. Physiol. 1997;59:575. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- 32.Das M, Molnar P, Gregory C, Riedel L, Jamshidi A, Hickman JJ. Long-term culture of embryonic rat cardiomyocytes on an organosilane surface in a serum-free medium. Biomaterials. 2004;25:5643. doi: 10.1016/j.biomaterials.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 33.Das M, Bhargava N, Gregory C, Riedel L, Molnar P, Hickman JJ. Adult rat spinal cord culture on an organosilane surface in a novel serum-free medium. In Vitro Cell. Dev. Biol. Anim. 2005;41:343. doi: 10.1007/s11626-005-0006-2. [DOI] [PubMed] [Google Scholar]
- 34.Das M, Rumsey JW, Gregory CA, Bhargava N, Kang JF, Molnar P, Riedel L, Guo X, Hickman JJ. Embryonic motoneuron-skeletal muscle co-culture in a defined system. Neuroscience. 2007;146:481. doi: 10.1016/j.neuroscience.2007.01.068. [DOI] [PubMed] [Google Scholar]
- 35.Eisenberg T, Knauer H, Schauer A, Buttner S, Ruckenstuhl C, Carmona-Gutierrez D, Ring J, Schroeder S, Magnes C, Antonacci L, Fussi H, Deszcz L, Hartl R, Schraml E, Criollo A, Megalou E, Weiskopf D, Laun P, Heeren G, Breitenbach M, Grubeck-Loebenstein B, Herker E, Fahrenkrog B, Frohlich KU, Sinner F, Tavernarakis N, Minois N, Kroemer G, Madeo F. Induction of autophagy by spermidine promotes longevity. Nat. Cell. Biol. 2009;11:1305. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- 36.Kaeberlein M. Spermidine surprise for a long life. Nat. Cell. Biol. 2009;11:1277. doi: 10.1038/ncb1109-1277. [DOI] [PubMed] [Google Scholar]
- 37.Bunge RP, Wood PM. Tissue culture studies of interactions between axons and myelinating cells of the central and peripheral nervous system. Prog. Brain Res. 1987;71:143. doi: 10.1016/s0079-6123(08)61820-8. [DOI] [PubMed] [Google Scholar]
- 38.Chan JR, Watkins TA, Cosgaya JM, Zhang C, Chen L, Reichardt LF, Shooter EM, Barres BA. Ngf controls axonal receptivity to myelination by schwann cells or oligodendrocytes. Neuron. 2004;43:183. doi: 10.1016/j.neuron.2004.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomson CE, Hunter AM, Griffiths IR, Edgar JM, McCulloch MC. Murine spinal cord explants: A model for evaluating axonal growth and myelination in vitro. J. Neurosci. Res. 2006;84:1703. doi: 10.1002/jnr.21084. [DOI] [PubMed] [Google Scholar]
- 40.Woodruff RH, Fruttiger M, Richardson WD, Franklin RJ. Platelet-derived growth factor regulates oligodendrocyte progenitor numbers in adult cns and their response following cns demyelination. Mol. Cell. Neurosci. 2004;25:252. doi: 10.1016/j.mcn.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 41.Almazan G, Honegger P, Matthieu JM. Triiodothyronine stimulation of oligodendroglial differentiation and myelination. A developmental study. Dev. Neurosci. 1985;7:45. doi: 10.1159/000112275. [DOI] [PubMed] [Google Scholar]