Abstract
Towards the middle of the twentieth century, neuroanatomy was on the decline. It was revived by the development of two new methods. One was the Nauta-Gygax method, which selectively stained nerve fibers that had been caused to degenerate by experimental lesions. This allowed connections between various parts of the nervous system to be better determined. The second was electron microscopy, which allowed the structure of neurons and the synapses between them to be examined in detail, and eventually this led to a revival of the Golgi impregnation methods. This occurred in the 1970s because of the desire of electron microscopists to determine the origins of the neuronal profiles they encountered in electron micrographs of various parts of the central nervous system. Eventually this led to the development of Golgi/EM techniques, whereby individual impregnated neurons could first be characterized by light microscopy and then thin sectioned for detailed analyses. Examining the axon terminals of such impregnated neurons, especially those in the cerebral cortex, for the first time revealed details of intercellular connections and allowed neuronal circuits to be postulated. However, Golgi/EM had only a brief, but fruitful existence. It was soon superceded by intracellular filling techniques, which allowed the added dimension that the physiological properties of identified neurons could also be determined.
Keywords: Electron microscopy, Golgi impregnations, synaptic connections
Towards the middle of the twentieth century, anatomy, and especially neuroanatomy, was in the doldrums. In neuroanatomy a few basic stains were available for examining the central nervous system and most parts of it had been studied extensively using these stains. Some of the stains showed neurons in their entirety, while others only showed certain parts of neurons. To examine individual nerve cells in their entirety a number of variations on the Golgi impregnation methods were available, but the uncertainty about the quality and extent of the staining produced after tissue had been impregnated led to the Golgi methods being surrounded by mystique. In addition many thought that there was not much point in using the Golgi stain since all of the cell types had already been examined by Cajal and described in his 1911 volumes. Consequently only a handful of investigators used the Golgi stain. It was also possible to show entire cells using methylene blue staining on live tissue, but this never became a popular stain.
Most investigators preferred to use sectioned material for their studies and a common technique to apply to them was silver staining. Again there were several silver staining methods, and each of them revealed all the nerve cells and processes contained within a section, so that the shapes of the nerve cell bodies could be readily visualized, as could the number and sizes of processes emerging from them. But unfortunately the silver stained processes emerging from the neurons soon entered the neuropil, interlaced with other processes and went out of the plane of section, so that they could rarely be followed for any distance. For revealing the sizes, shapes and packing densities of nerve cells, Nissl stains were popular, and myelin stains, such as the Heidenhain methods and the more routine luxol fast blue method, were available to follow the trajectories of nerve fibers that were organized into tracts. However, it was rarely possible to determine where the nerve fibers really ended, since myelinated nerve fibers axons usually lose their sheaths some distance before they terminate. Even the use of lesions to bring about chromatolysis could only be used when large neurons were affected, and the consequence was that while the available staining methods provided information about the kinds of neurons and their cytology in various parts of the nervous system, the limitations of the stains frustrated attempts to gain information about the neuronal circuits. A new era began after the end of the Second World War, when two quite new, and different, methods allowed more detailed information about connections and cytology of neurons to be obtained. These methods were the Nauta-Gygax stain and electron microscopy.
The Nauta-Gygax Stain
The Nauta-Gygax method selectively stains nerve fibers that have degenerated following experimental lesions (Nauta and Gygax, 1954), leaving most of the unaffected nerve fibers unstained. Using this method, degenerating nerve fibers can be traced quite close to their sites of termination, and it was believed that even some degenerating axons terminals were stained (see Guillery, 1970). Some time later Fink and Heimer (1967) introduced a modification of the Nauta-Gygax stain that did allow degenerating nerve boutons to be selectively stained, so that the actual sites of termination of nerve fibers could be determined (Heimer and Peters, 1968). Of course these degeneration techniques are only applicable to nerve fiber tracts in which the site of the lesion is some distance away from the site of termination and interpretation of the results depends upon the size and exact location of the lesions being used to bring about the degeneration of fibers. Nevertheless, these degeneration techniques opened up an era of tract tracing, because for the first time it became possible to determine the specifics about connections between parts of the central nervous system. Of course, the more recent tracts tracing methods using radioactive labels or dyes have now superceded even these degeneration methods and have allowed finer details of connections to be determined, but these latter methods did not start to become available until the 1970s.
Electron Microscopy
The other method that revived neuroanatomy was electron microscopy. This approach opened up a new era of understanding the cytology of neurons and neuroglial cells. It also made it possible to determine the kinds of structures that are postsynaptic to projection neurons, and for the first time made it feasible to determine the local connections between neurons of the central nervous system.
The development of the first transmission electron microscope with a useful resolving power is generally attributed to Ernst Ruska and Max Knoll, in Germany, in 1933, and Ruska’s efforts were recognized by him being awarded the Nobel Prize in Physics in 1986. In about 1935 Ruska joined the Seimens and Halske Corporation and helped to design the first commercially produced electron microscope, which came available in 1939, just as the Second World War began. But because of the imminence of war Germany decided to make these electron microscopes only available to its allies. However, Germany was not the only nation involved in the development of electron microscopes and the first useful prototype of a transmission electron microscope appears to be the one developed in 1935 in Britain by Metropolitan Vickers, while in North America, the first useful electron microscope seems to be the one developed by James Hillier and Albert Prebus in Toronto in 1939. Initially, the United States lagged behind in the development of electron microscopes, but by 1941 RCA had developed its own commercial transmission electron microscope, the RCA EM-B. Several of these instruments were produced. Most were used in the United States although others were sent to abroad to allies.
Because of the war few electron microscopes were available for biological research. However, Keith Porter and Albert Claude at the Rockefeller Institute in New York gained access to an RCA EM-B microscope, which was operated by Ernest Fullam at Interchemical Corporation, and they were able to examine a cultured fibroblast from a chick embryo that had been grown by Porter on a polyvinyl film. The fibroblasts were peeled off the film and transferred to a wire specimen grid, after which they were treated with osmium tetroxide, washed and then dried to prevent evaporation in the vacuum of the electron microscope. It is generally agreed that the resulting pictures were the first electron micrographs of a cell (Porter et al., 1945) and they revealed the Golgi apparatus, and a lace-like reticulum that Porter later called the endoplasmic reticulum.
Although this showed that it was feasible to examine cells by electron microscopy, it was obvious that to obtain more detailed images it would be much better to use sections of tissue. Attempts to produce such sections led to a flurry of activity and the rapid development of new technology, of which the following are the highlights. Producing thin sections for electron microscopy required a hard and stable embedding medium. The paraffin wax and celloidin that were routinely used to embed tissue for sectioning for light microscopy were too soft to produce thin sections and paraffin wax evaporated in the electron beam. A major step towards the production of a useful embedding medium occurred in 1949, when the plastics division at the US Bureau of Standard introduced methacrylates. This was soon followed by Latta and Hartmann (1950) showing that glass knives could be used to cut thin sections and that they were much sharper than the steel knives that had been used until that time. Then in 1952 Palade showed that preservation of tissue by immersion could be much improved if small pieces of fresh tissue were fixed using buffered osmic acid solutions, and in 1953 Porter and Blum introduced the first version of the Porter-Blum ultramicrotome that served for many years as the primary microtome in most electron microscope laboratories. The next important step took place in 1959, when Glauert et al. introduced epoxy resins for embedding tissue. This made thin sectioning easier and avoided the disintegration of blocks of tissue that frequently occurred when methacrylates polymerized. However, epoxy resins were more electron dense than methacrylates and so tissue in which osmium was the only electron dense material had little contrast in the electron beam. The increased contrast was provided by the use of lead stains such as lead hydroxide (Watson, 1958), lead tartrate (Millonig, 1961), and lead citrate (Reynolds, 1963), which are routinely used today.
While small pieces of tissue fixed by immersion in buffered osmic acid solutions and embedded in epoxy resins produced acceptable fixation of most tissues, the same was not true of tissue from the central nervous system, in which astrocytes and their processes swelled to produce unpleasant images. Initially this led to difficulties in the identification of the various types of neuroglial cells and there were also problems with the preservation of myelin. In an effort to solve this problem Palay and his colleagues (1962) turned to the intravascular perfusion of rats with buffered osmic acid solutions. This resulted in fixed brains that were black and hard and gave off fumes that fixed the olfactory epithelium and corneas of the investigators, but brains in which the fixation of the tissue was much superior to that produced by immersion fixation of small pieces. Perhaps fortunately, in 1963 Sabatini, Bensch and Barnett introduced glutaraldehyde as a less volatile fixative. And this led to brains being perfused with buffered glutaraldehyde solutions that often contained paraformadehyde (Karnovsky, 1965), after which pieces were removed, osmicated and embedded in epoxy resins for thin sectioning. This is the method of preparation now used routinely in most laboratories doing transmission electron microscopic studies of the nervous system. The design of ultramicrotomes has also improved and it is common to use diamond knives to produce the thin sections. As a point of interest, it might be added that Fernández-Moran in Venezuela first advocated the use of cleaved diamonds to produce thin sections as early as 1953, but diamond knives made by polishing cleaved surfaces did not become commercially available and within a reasonable price range until the 1970s.
Analysis of Electron Micrographs of Nervous Tissue
Although some improvements in both techniques and preservation of tissue were yet to be made, by the beginning of the 1950s investigators were examining thin sections of nervous tissue. Attention was first paid to the peripheral nervous system that had fewer components than the central nervous system. The peripheral nervous system also had the added advantage that when pieces of it were fixed by immersion reasonably good preservation was obtained. This was not true when pieces of the central nervous system were fixed by immersion, because such treatment usually led to a great deal of swelling and distortion of the components. Thus, as early as 1950 Fernández-Moran was able to demonstrate that peripheral myelin has a lamellar structure, and quite soon afterwards it was shown that the lamellae form a spiral and that the membrane forming the spiraled lamellae is in continuity with the plasma membrane of the Schwann cell (Geren 1954; Robertson, 1955). However, it was not until 1960 that the myelin sheaths of nerve fibers in the central nervous system were demonstrated to have a similar spiraled structure (Peters, 1960; Maturana, 1960). Also, because of the problems in obtaining adequate fixation the first article on the fine structure of a neuron in the central nervous system was not published until 1955 (Palay and Palade, 1955), when it was shown that the Nissl substance consists of arrays of granular endoplasmic reticulum and that neurons contained filaments, while the first description of a synapse in the vertebrate central nervous system was not published until 1956 (Palay, 1956).
As the preservation of central nervous tissue improved and better images were obtained the main problem facing those studying the central nervous system by electron microscopy was how to identify the various kinds of profiles seen in electron micrographs. It must be remembered that the stains used in light microscopic studies were designed to show only single nerve cells, or specific parts of nerve cells, but in electron micrographs investigators were presented with images in which profiles of all parts of neurons and neuroglial cells were represented. However, within a few years the criteria for identifying profiles of dendrites and axons were established and it was shown that there were at least two kinds of synapses present in the cerebral cortex (Gray, 1959), and it soon became apparent that there were even other morphological types of synapses in other parts of the central nervous system.
Resurrection of the Golgi techniques
The next problem was to determine where the dendrites, axons and axon terminals that were represented by profiles in the thin sections originated; and for the axon terminals what were the postsynaptic structures with which they were synapsing. Interestingly, this led to a renewed interest in impregnating tissue with the Golgi stain, because this was the principal technique for displaying the morphological features of individual neurons. One of the leaders in advocating Golgi images as a basis for interpreting electron micrographs was Sanford Palay, who together with Victoria Chan-Palay effectively used this combined approach to interpret the organization, cytological features and synaptic relationships of neurons in the cerebellar cortex (Palay and Chan-Palay, 1974). Because the cerebellar cortex contained only a few different kinds of neurons and has a regularly repeating morphology, interpretation of the fine structural images was relatively straightforward compared to the problems associated with interpretation of fine structural images obtained from the cerebral cortex and other brain regions.
In the cerebral cortex, the characteristics of pyramidal cells were well known from Golgi preparations, but there was little information about the other kinds of neurons present, beyond those shown in the drawings made by Cajal. As pointed out by DeFelipe and Jones (see DeFelipe and Jones, 1988) “With the exception of Lorente de Nó’s understandably superficial account of 1938, virtually no Golgi study of significance was published on the neocortex for nearly fifty years after 1922, the year in which Lorente de Nó, Cajal’s illustrious pupil, published his first - and the master himself published his last- paper on the cortex of rodents.” One important exception to this statement that should not be overlooked is the study carried out by O’Leary (1941) on the visual cortex of the cat.
For the cerebral cortex, among the first to return to analyses using Golgi staining were Szentágothai (1969) and Valverde (1970) and as an example of the state of knowledge of cortical neurons at that time, it is noteworthy that in a 1969 publication Szentágothai divides neurons of the cerebral cortex into two basic types, pyramidal cells and stellate cells. He shows both Golgi and electron microscope images and while he was clear about the distinguishing features of pyramidal cells, including the fact that their cell bodies and proximal dendrites receive axon terminals with flattened vesicles which were believed even then to be inhibitory (Colonnier, 1968), the description of the stellate cell type is very vague and in retrospect appears to apply to a pyknotic neuron. In the previous year, 1968, Colonnier had carried out an in depth survey of the types of synapses present in the visual cortex of the cat using formaldehyde perfused material and reinforced Gray’s (1959) conclusion that there are two basic types of synapses in cerebral cortex. However, Colonnier advocated a different nomenclature. He suggested that Gray’s type 1 synapses, at which the axon terminals have round vesicles and synaptic junctions with prominent postsynaptic densities, should be referred to as asymmetric synapses, and that Gray’s type 2 synapses, should be referred to as symmetric synapses, because of the symmetrically disposed densities at the synaptic junction. It is important to note that the use of aldehyde fixation resulted in the synaptic vesicles at symmetric synapses being pleomorphic, so that while some synaptic vesicles had round profiles others had oval, or elongated profiles. Moreover, Colonnier was able to show that pyramidal cells have only symmetric synapses on their cell bodies, mostly asymmetric synapses on their dendritic spines and few synapses on their dendritic shafts. Other neurons, which Colonnier believed to be stellate cells, had both symmetric and asymmetric synapses on their cell bodies and both types of synapses also occurred on smooth dendritic shafts, which Colonnier believed to belong to the stellate cells. Because lesioning pathways such as the corpus callosum led to the degeneration of axon terminals forming asymmetric synapses, it was generally concluded that the axon terminals forming asymmetric synapse originated from the plexuses of pyramidal cells, and this was in concert with the belief that asymmetric synapses are excitatory and symmetric ones are inhibitory.
Although it was known from Golgi studies that there are several different types of stellate, or non-pyramidal cells, in the cerebral cortex, when a cell body of one of these neurons was encountered in thin sections prepared for routine electron microscopy, it was not possible to determine to what type of non-pyramidal cell it belonged. Nor was it possible to be certain where the axon terminals forming symmetric synapses originated, although it was generally believed on the basis of Golgi preparations that they originated from intracortical plexuses of non-pyramidal cells. This view was supported by the studies of Szentágothai (1968) who showed that when slabs of cerebral cortex are isolated and examined two months later, many of the Gray type 1 axodendritic synapses had been lost, but the Gray type 2 synapses on the cell bodies of pyramidal cells persisted, suggesting that they originate from intrinsic neurons.
Another problem associated with studies of the cerebral cortex concerned the identity of the neurons postsynaptic to cortical inputs. For example although it was known from degeneration studies that thalamocortical axons terminate in layer 4 of cortex and form asymmetric synapses, the identity of the postsynaptic elements could not be determined by routine electron microscopy. To answer these and other questions the obvious solution seemed to be to examine Golgi impregnated neurons by electron microscopy.
Golgi Electron Microscopy
Among the early proponents of the Golgi electron microscope technique were Blackstad (1965) and Stell (1965) and an account of the early attempts to examine Golgi impregnated material by electron microscopy has been given by Blackstad (1970). The general approach was to fix tissue in aldehydes and then to carry out a Golgi impregnation en bloc, after which the block of impregnated tissue was sectioned, examined by light microscopy, and suitably impregnated neurons embedded for electron microscopy. The precipitate produced by the Golgi reaction is silver chromate, which fills an impregnated neuron and can make thin sectioning very difficult. Even if the solid precipitate of silver chromate does not fall out of the thin section, it completely obscures the cytoplasm of the impregnated neurons and when the thin section is hit by the beam of the electron microscope the deposit usually heats up and melts the surrounding plastic, leading to the formation of holes. Nevertheless, study of such preparations produced some useful data, but the difficulties inherent in using this procedure led to various attempts to reduce or alter the silver chromate precipitate. Accounts of some of these attempts can be found in articles by Blackstad (1965), Peters (1981) and Waterlood (1992). The most successful modification was that developed by Fairén et al. (1977), in which gold was substituted for the silver chromate. Essentially the impregnated neurons were gold toned by immersing sections in gold chloride, after which they were transferred to oxalic acid to reduce the gold, and finally deimpregnated in sodium thiosulfate that removes the silver salts. The result of the gold toning is that small gold particles now label the impregnated neurons and these particles do not impede thin sectioning or obscure the cytological details. An example of this approach is shown in Fig. 1.
Fig. 1.
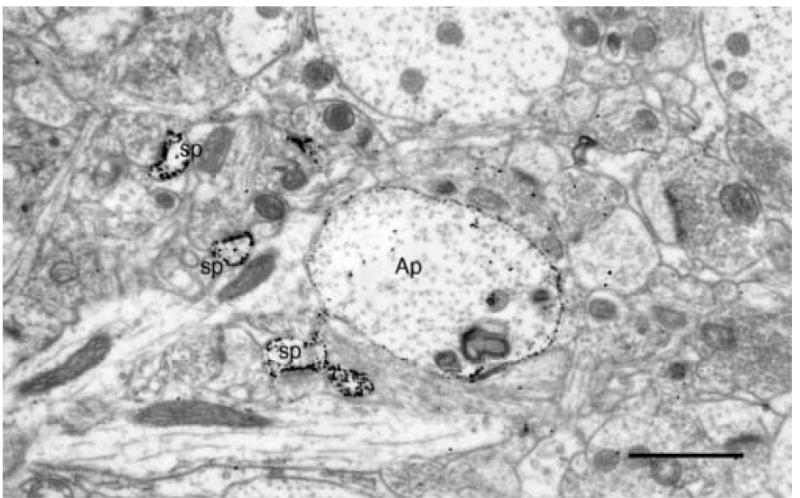
A transversely sectioned, gold toned, Golgi impregnated apical dendrite (Ap) of a layer 5 pyramidal cell. The small gold particles mark both the main shaft of the apical dendrite as well as the spines (sp) emanating from it. Scale bar = 1μm.
The strength of the Golgi/electron microscope (Golgi/EM) approach is that neurons can be first examined in the light microscope to determine their overall features, after which they can be thin sectioned and specific parts of the impregnated neurons examined in the electron microscope. Using this technique it was shown that in the cerebral cortex the axons of both pyramidal and spiny stellate cells have terminals that form asymmetric synapses, while the axons of non-pyramidal cells form symmetric synapses (e.g. LeVay, 1973; Parnavelas et al., 1977; Peters and Fairén, 1978). An example of a gold toned axon terminal from a smooth stellate cell forming a symmetric synapse with the cell body of a pyramidal cell is shown in Fig. 2. Also, for the first time it was possible to examine the synaptic relationships between two impregnated and specifically identified neurons, namely a stellate cell that formed synapses with a pyramidal cell (Peters and Proskauer, 1980). It also was shown, as only previously suspected, that all of the axonal boutons formed by an individual neuron are of the same morphological type. The weakness of the Golgi /EM approach is that the kinds of neurons that can be examined depends upon the vagaries of the Golgi impregnation. There is also the added problem that myelinated axons do not impregnate, which is the reason that some investigators using the Golgi methods prefer to use young animals in which the axons have not yet fully myelinated.
Fig.2.
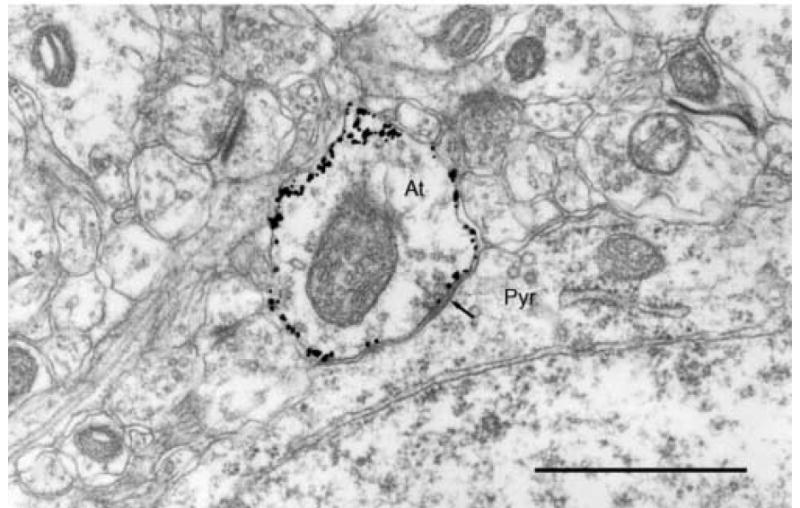
A gold toned axon terminal (At) from a smooth stellate cell in rat visual cortex forming a symmetric synapse (arrow) with the perikaryon of a pyramidal cell (pyr). Scale bar = 1μm.
For the cerebral cortex, most of the focus of Golgi/EM was on the nonpyramidal neurons, of which very little was known, especially about where their axons terminate. In general terms, these studies showed that some nonpyramidal neurons, such as the multipolar and bitufted cells, form symmetric synapses with the cell bodies and dendritic shafts of both pyramidal and nonpyramidal neurons (e.g., LeVay, 1973; Peters and Fairén, 1978; Peters and Proskauer, 1980; Fairén and Smith-Fernández. 1992), while the basket cells prefer to synapse on the cell bodies and proximal dendrites of pyramidal and non-pyramidal cells (e.g. DeFelipe and Fairén, 1982; Somogyi et al., 1983). On the other hand a type of double bouquet cell examined by Somogyi and Cowey (1981; 1984) preferred to synapse with the dendritic shafts that appeared to belong to other non-pyramidal cells. But the nonpyramidal cell type with the most specific terminations was shown to be the chandelier cell (see Figs. 3 and 4), which synapses almost exclusively with the initial axon segments of groups of pyramidal cells (e.g. Somogyi, 1977; Fairén and Valverde, 1980; Peters et al., 1982; Somogyi et al., 1982). The outcome of these studies was that for the first time some of the intrinsic connectivity of the cerebral cortex was revealed, so that neuronal circuits could be proposed.
Fig. 3.
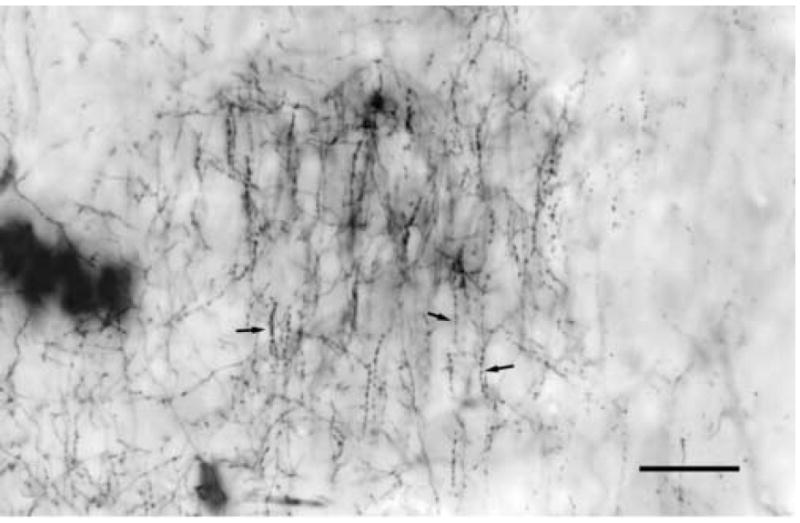
A gold toned, Golgi impregnated plexus of a chandelier cell in layer 3 of rat visual cortex. The plexus consists of vertical strings of axonal bouton (arrows) that extend along the initial axon segments of pyramidal cells. Scale bar = 50μm.
Fig.4.
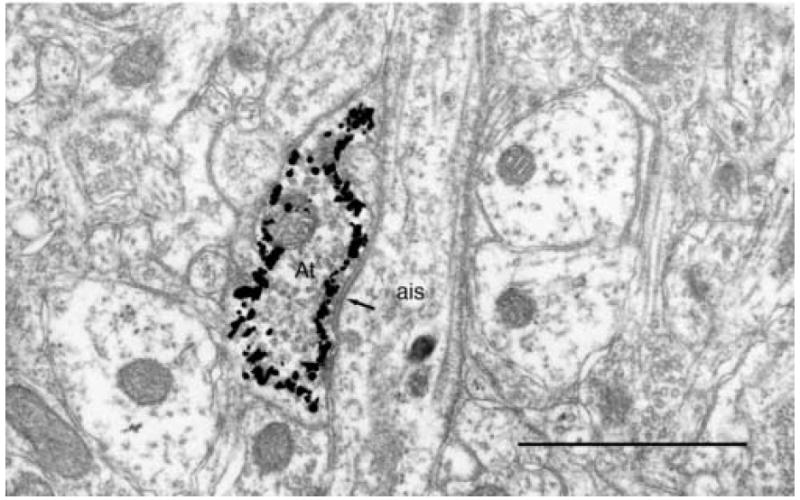
Light microscopic image of the axon terminal (At) from the axon plexus of a chandelier cell forming a symmetric synapse (arrow) with the initial axon segment (ais) of a layer 3 pyramidal cell. Scale bar = 1μm.
In another set of studies, Golgi/EM was combined with axon terminal degeneration (Peters et al., 1977). Thus after lesions had been made in the lateral geniculate nucleus of the rat, and the visual cortex Golgi impregnated, it was shown that the degenerating thalamic afferents form synapses with all neuronal elements contained in layer IV of visual cortex that are capable of forming asymmetric synapses. This includes the spines of basal dendrites of layer III pyramidal cells, the spines of the apical dendrites of layer V pyramidal cells (Fig. 5), the spines of spiny stellate cells, and the perikarya and dendrites of smooth multipolar and bipolar cells (Peters et al., 1979; Peters and Kimerer, 1981). A similar result was obtained when this approach was used to examine the thalamic afferents to mouse somatosensory cortex (White, 1978; Hersch and White, 1981) and to cat visual cortex (Hornung and Garey, 1981), thus showing that the thalamic afferents to cerebral cortex do not terminate on specific cortical cell types.
Fig. 5.
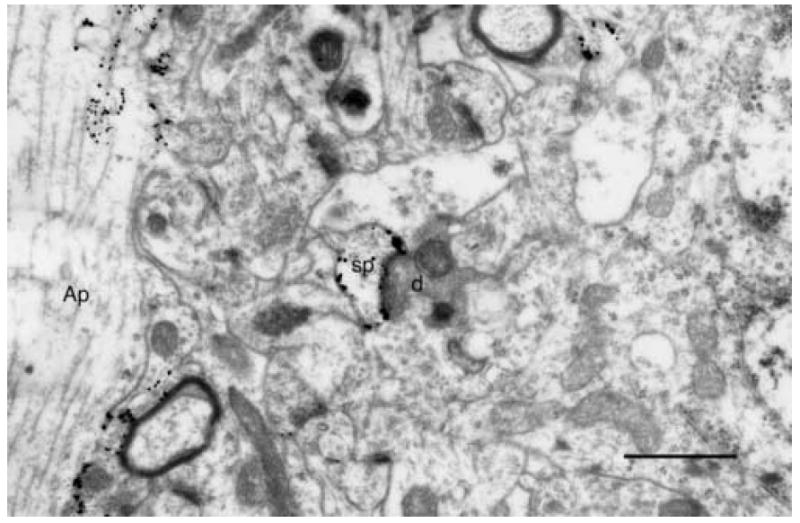
The spine (sp) of a Golgi impregnated and gold toned apical dendrite (Ap) in rat visual cortex forming an asymmetric synapse with a degenerating geniculo-cortical axon terminal (d). Scale bar = 1μm.
The outcome of these studies was that for the first time the sites of termination of afferents to the cerebral cortex were revealed, and some of the intrinsic connectivity of the cerebral cortex was understood. Consequently it became possible to speculate meaningfully about cortical neuronal circuits. However, although the Golgi/EM studies made important contributions to our understanding on neuronal circuits, the technique had only a short life. It was soon superseded by the technique of intracellular filling, which was first introduced in 1976 (e.g. Cullheim and Kellerth, 1976; Kitai et al, 1976; Light and Durvic, 1976).
The technique of recording from neurons that are subsequently filled to reveal their morphology has rapidly expanded and this approach has advanced our knowledge of cortical circuitry immensely (e.g., Somogyi et al. 1998). And in retrospect it has become evident that Golgi impregnations do not impregnate neurons randomly, as had been believed since the time of Cajal. For example, prior to intracellular filling and the introduction of antibody labeling, which showed double bouquet cells to be quite common (e.g., Peters and Sethares, 1997), only a few double bouquet cells had been visualized (see Somogyi and Cowey, 1984). Furthermore it has also become evident that the Golgi method does not show the complete axonal plexuses of most neurons, because the axonal plexuses revealed by intracellular filling are generally much richer than those impregnated by the Golgi method (e.g., Martin, 1988).
What is the future of the Golgi technique? Because of increased ease of use and the greater amount of information that can be obtained from intracellular filling, one can suspect that few investigators will be using the Golgi method in the future. And interestingly electron microscopy now finds itself in the doldrums and it has become little more than a handmaiden to other approaches. Furthermore, while the electron microscopists of the 1970s and 1980s strove to produce high quality images, this is no longer a worthwhile goal, since most editors of Journals believe that words speak louder than pictures and reduce images to such an extent that no details can be seen in the electron microscopic and other images presented in many scientific Journals. It is also true that the fine structure of most parts of the normal central nervous system of normal animals has been examined, so there is little new to be seen. The future of electron microscopy lies in detailing the binding sites of antibodies, and in examining the brains and other tissues of animals that have been affected by such events as disease, increasing age, and alterations in their genetics, because electron microscopy is still the best means to determine subtle alterations in morphology.
Acknowledgments
I wish to thank Claire Folger for her help with preparing the illustrations and Mike Bowley for his comments on an early version of this article.
Grant support: National Institute on Aging Program Project Grant 1PO AG 00001.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blackstad TW. Mapping of experimental axon degeneration by electron microscopy of Golgi preparations. Z Zellforsch. 1965;67:819–834. doi: 10.1007/BF00339303. [DOI] [PubMed] [Google Scholar]
- Blackstad TW. Electron microscopy of Golgi preparations for the study of neuronal relations. In: Nauta WJH, Ebbesson SOE, editors. Contemporary Research Methods in Neuroanatomy. Springer-Verlag; New York: 1970. pp. 162–172. [Google Scholar]
- Cajal S, Ramon y. In: Histologie du Système Nerveux del’Homme et des Vertebres. Azoulay L, translator. Paris: Maloine; 1911. [Google Scholar]
- Colonnier M. Synaptic patterns on different cell types in the different laminae of the cat visual cortex: an electron microscope study. Brain Res. 1968;9:268–287. doi: 10.1016/0006-8993(68)90234-5. [DOI] [PubMed] [Google Scholar]
- Culheim S, Kellerth JO. Combined light and electron microscopic tracing of neurons including axons and synaptic terminals after intracellular injection of horseradish peroxidase. Neurosci Lettr. 1976;2:307–313. doi: 10.1016/0304-3940(76)90165-8. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Fairén A. A type of basket cell in superficial layers of cat visual cortex. A Golgi-electron microscope study. Brain Res. 1982;244:9–16. doi: 10.1016/0006-8993(82)90898-8. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Jones EG. Cajal on the Cerebral Cortex. An Annotated Translation of the Complete Writings. Oxford University Press; New York: 1988. [Google Scholar]
- Fairén A, Peters A, Saldanha J. A new procedure for examining Golgi impregnated neurons by light and electron microscopy. J Neurocytol. 1977;6:311–338. doi: 10.1007/BF01175194. [DOI] [PubMed] [Google Scholar]
- Fairén A, Smith-Fernández A. Electron microscopy of Golgi-impregnated interneurons: notes on the intrinsic connectivity of the cerebral cortex. Microscop Res Tech. 1992;23:289–305. doi: 10.1002/jemt.1070230405. [DOI] [PubMed] [Google Scholar]
- Fairén A, Valverde F. A specialized type of neuron in the visual cortex of cat. A Golgi and electron microscope study of chandelier cells. J Comp Neurol. 1980;194:761–779. doi: 10.1002/cne.901940405. [DOI] [PubMed] [Google Scholar]
- Fernández-Moran M. Electron microscope observations on the fine structure of the myelinated nerve fiber sheath. Exp Cell Res. 1950;1:143–149. [Google Scholar]
- Fink RP, Heimer L. Two methods for selective silver impregnation of degenerating axons and their synaptic endings in the central nervous system. Brain Res. 1967;4:369–374. doi: 10.1016/0006-8993(67)90166-7. [DOI] [PubMed] [Google Scholar]
- Geren BB. The formation from the Schwann cell surface of myelin in the peripheral nerves of chick embryos. Exp Cell Res. 1954;7:558–562. doi: 10.1016/s0014-4827(54)80098-x. [DOI] [PubMed] [Google Scholar]
- Glauert AM, Glauert RH. Araldite as as embedding medium for electron microscopy. J Biophys Biochem Cytol. 1958;4:191–194. doi: 10.1083/jcb.4.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauert AM, Rogers GE, Glauert RH. A new embedding medium for electron microscopy. Nature. 1956;178:803. doi: 10.1038/178803a0. [DOI] [PubMed] [Google Scholar]
- Guillery RW. Light-and electron-microscopical studies of normal and degenerating axons. In: Nauta WJH, Ebbsson SOE, editors. Contemporary Research Methods in Neuroanatomy. Springer-Verlag; New York: 1970. pp. 77–105. [Google Scholar]
- Gray EG. Axo-somatic and axo-dendritic synapses of the cerebral cortex. An electron microscope study. J Anat. 1959;93:420–433. [PMC free article] [PubMed] [Google Scholar]
- Heimer L, Peters A. An electron microscope study of a silver stain for degenerating boutons. Brain Res. 1968;8:337–346. doi: 10.1016/0006-8993(68)90053-x. [DOI] [PubMed] [Google Scholar]
- Hersch SM, White EL. Thalamocortical synapses involving identified neurons in mouse primary somatosensory cortex: A terminal degenerataion and Golgi/EM study. J Comp Neurol. 1981;195:253–263. doi: 10.1002/cne.901950206. [DOI] [PubMed] [Google Scholar]
- Hornung JP, Garey LJ. The thalamic projection to cat visual cortex. Ultrastructure of neurons identified by Golgi impregnation or retrograde horseradish peroxide transport. Neuroscience. 1981;6:1053–1068. doi: 10.1016/0306-4522(81)90070-1. [DOI] [PubMed] [Google Scholar]
- Karnovsky MJ. A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J Cell Biol. 1965;27:137A–138A. [Google Scholar]
- Kitai ST, Kosis JD, Preston RJ, Sugimori M. Monosynaptic input to caudate neurons identified by intracellular injection of horseradish peroxidaase. Brain Res. 1976;109:601–606. doi: 10.1016/0006-8993(76)90039-1. [DOI] [PubMed] [Google Scholar]
- Latta H, Hartmann JF. Use of a glass edge in thin sectioning for electron microscopy. Proc Soc Biol Med. 1950;74:436–439. doi: 10.3181/00379727-74-17931. [DOI] [PubMed] [Google Scholar]
- LeVay S. Synaptic patterns in the visual cortex of the cat and monkey: Electron microscopy of Golgi preparations. J Comp Neurol. 1973;150:53–86. doi: 10.1002/cne.901500104. [DOI] [PubMed] [Google Scholar]
- Light AR, Durvic RG. Horseradish peroxidase. An improvement in intracellular staining of single electrophysiologically characterized neurons. Exptl Neurol. 1976;53:847–853. doi: 10.1016/0014-4886(76)90159-x. [DOI] [PubMed] [Google Scholar]
- Martin K. From single cells to simple circuits in the cerebral cortex. Quart J Exptl Physiol. 1988;73:637–702. doi: 10.1113/expphysiol.1988.sp003190. [DOI] [PubMed] [Google Scholar]
- Maturana HR. The fine structure of the optic nerve of Anurans- an electron microscope study. J Biophys Biochem Cytol. 1960;7:107–120. doi: 10.1083/jcb.7.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millonig G. A modified procedure for lead staining thin sections. J Biophys Biochem Cytol. 1961;11:736–739. doi: 10.1083/jcb.11.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta WJH, Gygax PA. Silver impregnation of degenerating axons in the central nervous system: A modified technique. Stain Technol. 1954;29:91–93. doi: 10.3109/10520295409115448. [DOI] [PubMed] [Google Scholar]
- O’Leary JL. Structure of the area striata of the cat. J Comp Neurol. 1941;75:131–164. [Google Scholar]
- Palade GE. A study of fixation for electron microscopy. J Exp Med. 1952;95:285–297. doi: 10.1084/jem.95.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay SL. Synapses in the central nervous system. J Biophys Biochem Cytol. 1956;2(Suppl):193–202. doi: 10.1083/jcb.2.4.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay SL, McGee-Russell SM, Gordon S, Grillo MA. Fixation of neural tissues for electron microscopy by perfusion with solutions of osmium tertroxide. J Cell Biol. 1962;12:385–410. doi: 10.1083/jcb.12.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay SL, Palade G. The fine structure of neurons. J Biophys Biochem Cytol. 1955;1:69–88. doi: 10.1083/jcb.1.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay SL, Chan-Palay V. Cerebellar Cortex. Cytology and Organization. Springer-Verlag; New York: 1974. [Google Scholar]
- Parnavelas JG, Sullivan K, Lieberman AR, Webster KE. Neurons and their synaptic organization in the visual cortex of the rat. Electron microscopy of Golgi preparations. Cell Tissue Res. 1977;183:499–517. doi: 10.1007/BF00225663. [DOI] [PubMed] [Google Scholar]
- Peters A. The structure of myelin sheaths in the central nervous system of Xenopus Laevis (Daudin) J Biophys Biochem Cytol. 1960;7:121–126. doi: 10.1083/jcb.7.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A. The Golgi-electron microscope technique. In: Johnson JE, editor. Current Trends in Morphological Techniques. Vol. 1. CRC Press Inc.; Boca Raton, Florida: 1981. pp. 187–212. [Google Scholar]
- Peters A, Fairén A. Smooth and sparsely-spined stellate cells in the visual cortex of the rat: A study using a combined Golgi-electron microscope technique. J Comp Neurol. 1978;181:129–172. doi: 10.1002/cne.901810108. [DOI] [PubMed] [Google Scholar]
- Peters A, Kimerer LM. Bipolar neurons in rat visual cortex. A combined Golgi-electron microscopic study. J Neurocytol. 1981;10:921–946. doi: 10.1007/BF01258522. [DOI] [PubMed] [Google Scholar]
- Peters A, Proskauer CC. Synaptic relationship between a multipolar cell and a pyramidal cell in rat visual cortex. A combined Golgi-electron microscopic study. J Neurocytol. 1980;9:163–183. doi: 10.1007/BF01205156. [DOI] [PubMed] [Google Scholar]
- Peters A, Proskauer CC, Feldman ML, Kimerer L. The projection of the lateral geniculate nucleus to area 17 of the rat cerebral cortex. V. Degenerating axon terminals synapsing with Golgi-impregnated neurons. J Neurocytol. 1979;8:331–357. doi: 10.1007/BF01236125. [DOI] [PubMed] [Google Scholar]
- Peters A, Proskaue CC, Ribak CE. Chandelier cells in rat visual cortex. J Comp Neurol. 1982;206:397–416. doi: 10.1002/cne.902060408. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C. The organization of double bouquet cells in monkey striate cortex. J Neurocytol. 1997;26:779–797. doi: 10.1023/a:1018518515982. [DOI] [PubMed] [Google Scholar]
- Peters A, White EL, Fairén A. Synapses between identified neuronal elements. An electron microscopic demonstration of degenerating axon terminals synapsing with Golgi-imprengated neurons. Neurosci Lett. 1977;6:171–175. doi: 10.1016/0304-3940(77)90013-1. [DOI] [PubMed] [Google Scholar]
- Porter KR, Claude A, Fullam E. A study of tissue culture cells by electron microscopy. Methods and preliminary observations. J Exp Med. 1945;81:233–241. doi: 10.1084/jem.81.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JD. The ultrastructure of adult vertebrate peripheral myelinated nerve fibers in relation to myelinogenesis. J Biophys, Biochem Cytol. 1955;1:271–278. doi: 10.1083/jcb.1.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini DD, Bensch K, Barnett RJ. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzyme activity by aldehyde fixation. J Cell Biol. 1963;17:19–57. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P. A specific “ axo-axonal” interneuron in the visual cortex of the rat. Brain Res. 1977;136:345–350. doi: 10.1016/0006-8993(77)90808-3. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Cowey A. Combined Golgi and electron microscopic study of the synapses formed by double bouquet cells in the visual cortex of the cat and monkey. J Comp Neurol. 1981;195:457–566. doi: 10.1002/cne.901950402. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Cowey A. Double bouquet cells. In: Peters A, Jones EG, editors. Cerebral Cortex. Vol. 1. Plenum Press; New York: 1984. pp. 337–360. [Google Scholar]
- Somogyi P, Freund TF, Cowey A. The axo-axonic interneuron in the cerebral cortex of the rat, cat and monkey. Neuroscience. 1982;7:2577–2608. doi: 10.1016/0306-4522(82)90086-0. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Kisvarday ZF, Martin KAC, Whitteridge D. Synaptic connections of morphologically identified and physiologically chacterized large basket cells in the striate cortex of cat. Neuroscience. 1983;10:261–294. doi: 10.1016/0306-4522(83)90133-1. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Tamas G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Res Rev. 1998;26:113–135. doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- Stell WK. Correlation of retinal architecture and ultrastructure in Golgi preparations of goldfish retina. Anat Rec. 1965;153:389–397. doi: 10.1002/ar.1091530409. [DOI] [PubMed] [Google Scholar]
- Szentágothai J. The possible histological basis of inhbition. In: Asratyan EA, editor. Progress in Brain Research. Vol. 22. Amsterdam: Elsevier; 1968. pp. 148–160. [DOI] [PubMed] [Google Scholar]
- Szentágothai J. Architecture of the cerebral cortex. In: Jasper H, Ward AA, Pope A, editors. Basic Mechanisms of the Epilepsies. Boston: Little Brown; 1969. pp. 13–8. [Google Scholar]
- Valverde F. Short axon neuronal subsystems in the visual cortex of the monkey. Int J Neurosci. 1971;1:181–197. doi: 10.3109/00207457109146970. [DOI] [PubMed] [Google Scholar]
- Waterlood FG. Techniques for converting Golgi precipitate in CNS neurons into stable electron microscopic markers. Microscop Res Tech. 1992;23:275–288. doi: 10.1002/jemt.1070230404. [DOI] [PubMed] [Google Scholar]
- Watson ML. Staining of tissue sections for electron microscopy with heavy metals. II Application of solutions containing lead and barium. J Biophys Biochem Cytol. 4:727–729. doi: 10.1083/jcb.4.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]


