Abstract
Objective
This study assesses relative contributions of "midline defects" (widening of the vagina) and "paravaginal defects" (separation of the lateral vagina from the pelvic sidewall).
Methods
Ten women with anterior predominant prolapse and 10 with normal support underwent pelvic MR imaging. 3-D models of the anterior vaginal wall (AVW) were generated to determine locations of the lateral AVW margin, vaginal width, and apical position.
Results
The lateral AVW margin was farther from its normal position in cases than controls throughout most of the vaginal length, most pronounced midvagina (effect sizes 2.2–2.8). Vaginal widths differed in the midvagina with an effect size of 1.0. Strong correlations between apical and paravaginal support were evident in mid and upper vagina (r=0.77–0.93).
Conclusions
Changes in lateral AVW location were considerably greater than changes in vaginal width in cases vs controls, both in number of sites affected and effect sizes. These "paravaginal defects" are highly correlated with apical descent.
Keywords: cystocele, paravaginal defect, midline defect, anterior wall prolapse, pelvic organ prolapse, MR imaging
Introduction
The anterior vaginal wall is the most common site of pelvic organ prolapse and the most frequent site of operative failure [1–7]. The traditional discussion of causal factors involved in anterior compartment prolapse has centered on debate between midline and paravaginal defects. Apical descent has recently been strongly associated with cystocele size [8–10]. Although surgical decisions are often based on which defect is presumed present, data are lacking regarding the relative importance of these defects. Furthermore, a technique which quantifies the magnitude of these clinically important factors is not currently available.
Recently, 3D magnetic resonance (MR) studies have allowed us to visualize not just the midline deformation of the anterior vaginal wall with Valsalva, but also the entire vaginal wall including its lateral margin [11]. The present study quantifies changes in vaginal width in order to evaluate the effect of “midline defects” and changes in the position of the lateral vagina in order to evaluate the “paravaginal defect.” In addition, we determine the correlation between these “paravaginal defects” and vaginal apex location.
Materials and Methods
MRI scans from 20 women (10 cases and 10 controls) were selected from an on-going University of Michigan institutional review board-approved (IRB # 1999-0395), case-control study of pelvic organ prolapse. All women in the control group were asymptomatic based on Pelvic Floor Distress Inventory and Pelvic Floor Impact Questionnaires, had negative full bladder stress tests, and did not have prolapse beyond the hymen. All cases were symptomatic and had a Ba POP-Q (Pelvic Organ Prolapse – Quantification) value at least 1 cm beyond the hymenal ring on clinical examination. Selected subjects had cystocele-predominant prolapse; women in whom the cervix or posterior wall was the leading point of prolapse were excluded. None of the subjects had previously undergone hysterectomy or prior pelvic floor surgery. For our cases, we screened 21 of the most recent MR images to find 10 with adequate visualization of the vaginal margins. The images of the 10 selected cases also adequately demonstrated the full extent of their prolapse on dynamic MRI, allowing visualization of the changes in the anterior vaginal wall at Valsalva.
As described in our previous studies [11], each woman underwent supine MR imaging both at rest and during maximal Valsalva using a 3 Telsa Philips Achieva scanner (Philips Medical Systems, Best, The Netherlands) with a 6-channel, phased array coil. Ultrasound gel was placed in the vagina to outline its contour. For standard anatomical scans made at rest turbo spin echo (TSE) techniques were used to image the sagittal, coronal, and axial planes. At rest, 30 images were obtained in each plane (repetition time (TR) range 2300–3000 ms, echo time (TE) 30 ms, 4 mm slice thickness, 1 mm gap, number of signal averages (NSA) 2, 256 × 255 voxels). Subjects then performed a Valsalva maneuver which they held for approximately 20 seconds to obtain images of the pelvis with the prolapse protruding maximally. With the prolapse protruding, 14 images were serially obtained from one ischial spine to the other in sagittal plane (TR range 1249–1253 ms, TE 80 ms, 6 mm slice thickness, 1 mm gap, SENSE factor 4, NSA 2, 320 × 178 voxels). A research associate with the POP-Q data from each subject’s clinical examination was present during MR imaging to assure that the prolapse reached the same size that had been previously identified in the clinic. If the prolapse did not reach the same magnitude as had been observed on clinical examination, the MR study was repeated with additional coaching to obtain images with the prolapse at its fullest extent.
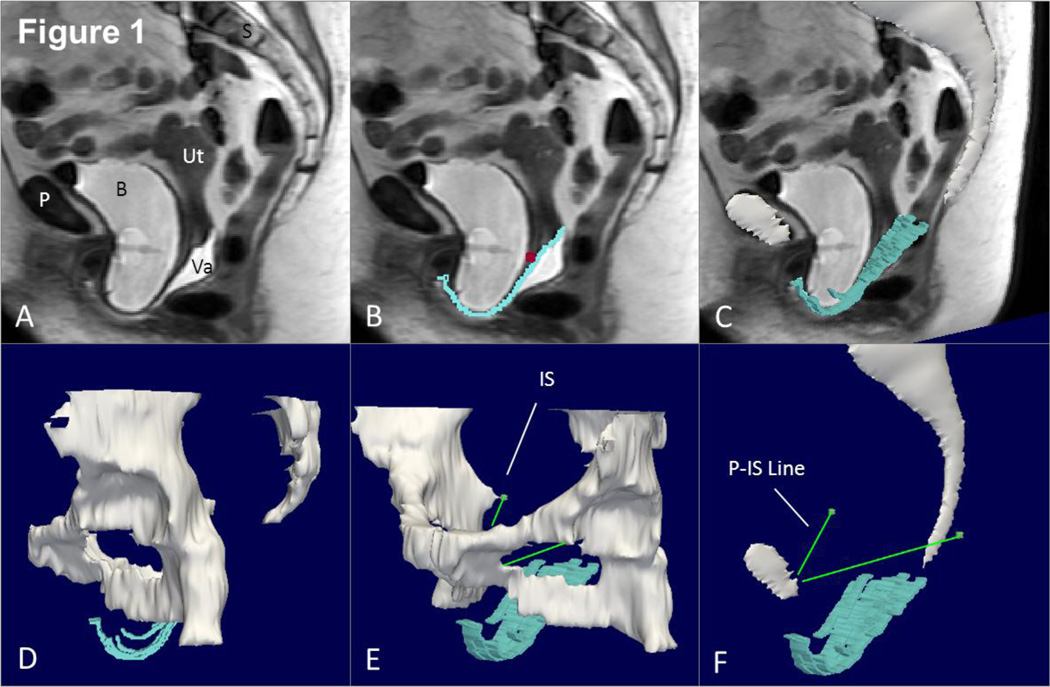
The original axial, sagittal and coronal Digital Imaging and Communications in Medicine (DICOM) static images were aligned with one another using rigid registration in the 3-D Slicer® software program (version 2.1b1, Brigham and Women’s Hospital, Boston, MA), ensuring that structures co-localized in all 3 axes by simultaneous review of 3-D scan planes in the viewer. Satisfactory alignment was possible in all 20 scans. Three-dimensional models were made of the following resting structures: bony pelvis and ischial spines using the axial images. Figure 1 panels A–F illustrate the modeling process and subsequent generation of a reference line as described below.
Fig 1. Making a 3D model with P-IS line.
(A) Mid-sagittal MR image of subject with prolapse. (B) Outline of anterior vaginal wall in blue with cervicovaginal junction marked with a purple square. (C) 3D model of anterior vaginal wall shown in slightly skewed sagittal image. Mid-sagittal pelvic bones also in this image. (D) Illustrates more complete view of pelvic bones and rotating this slightly in (E) we can see the ischial spine (IS). A line from the insertion of the arcus tendineus fascia pelvis on the pubic bone to the ipsilateral ischial spine is then constructed (P-IS line in E). This serves as the reference line to generate the sidewall measurements. P – pubic symphysis, S – sacrum, B- bladder, Va – vagina, Ut – uterus, IS – ischial spine. © DeLancey 2010
To analyze the deformation of the anterior vaginal wall under load, 3-D models of the midsagittal pelvic bones (the pubic symphysis and sacrum) were constructed from the sagittal maximal Valsalva images and then aligned with the pelvic bones of the resting model. This identified the transformation (both rotation and translation) for the sagittal maximal Valsalva images such that subsequently constructed 3-D anterior vaginal wall models could be aligned with previously created resting models of the ischial spines. This step was necessary to allow creation a reference line established on each side of the pelvis representing the normal location of the arcus tendineus fascia pelvis (ATFP) from its Pubic attachments to the ipsilateral Ischial Spine (P-IS line).
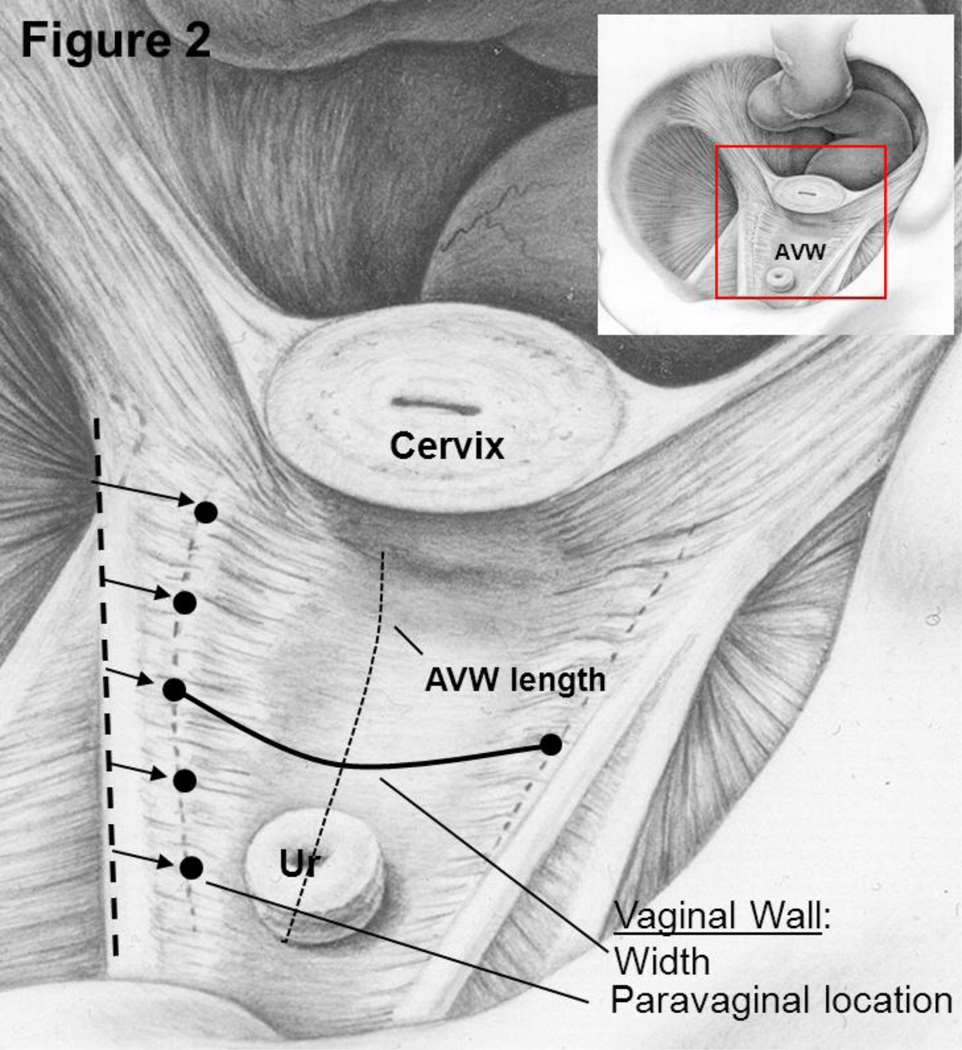
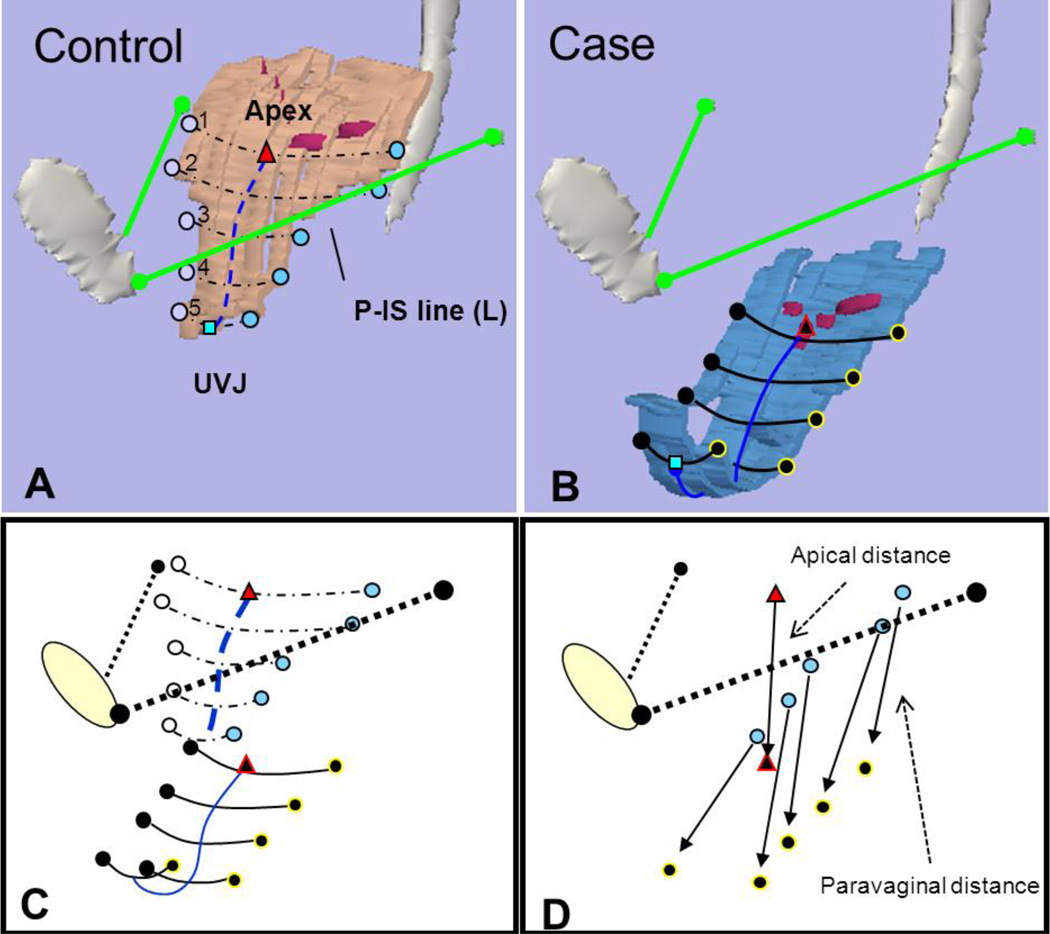
The maximal Valsalva models were imported into Imageware® (version 12, UGS Corporation, 2005) for measurement of vaginal width and determination of the distance of the lateral margin of the vagina from normal. Figure 2 illustrates how anterior vaginal wall width at maximal Valsalva was measured at 5 equidistant locations between the cervicovaginal and urethrovaginal junctions for both cases and controls. This measurement did take into account the nonlinear configuration of the anterior vaginal wall. This measurement, therefore, was not a straight line distance between the lateral margins but followed the curve of the vagina so that the distance, in some cases, might be larger than the transverse diameter of the pelvis. In addition, we measured vaginal length between the cervicovaginal and urethrovaginal junctions. Secondly, we developed a technique to quantify the “paravaginal defect.” Using the P-IS line as our reference X-axis, the normal location of the lateral margin of the vagina was determined by calculating the mean three-dimensional coordinates (x,y,z) of women in the control group at each of these 5 equidistant points (see five points as illustrated on Figure 2, with their relationship to the P-IS line). Subsequently, for each subject at these five points, we calculated how far the lateral margin of the vagina was from the normal mean determine if there was a difference in paravaginal distance in women with prolapse compared to those with normal support (Figure 3). A similar process was done to assess the distance of the apex from the normal mean position at the cervicovaginal junction. From this point forward these two measurements will be referred to as the paravaginal distance and apical distance.
Fig 2. Measurement concept.
Inset orients one to the view into the pelvis looking over the pubic symphysis towards the rectum. Five black dots are shown along the lateral portion of the anterior vaginal wall (AVW) on the patient’s right side illustrating the division of the vagina in 5 equidistant locations between the cervicovaginal junction and the urethrovaginal junction. Vaginal wall length between these two is indicated by the dotted line. The solid line represents an example of the vaginal width measurement. The arrows indicate paravaginal location relative to a reference line along the arcus tendineus fascia pelvis (ATFP) on the sidewall. © DeLancey 2010
Fig 3. Determining distance from normal.
(A) Vaginal width and lateral wall locations at 5 equidistant points from apex at cervicovaginal junction to urethrovaginal junction (UVJ) in normal support. Pubis-ischial spine (P-IS line) in green from insertion of ATFP on pubic symphysis to ipsilateral ischial spine. (B) Similar markings in anterior compartment prolapse. Note, cervicovaginal junction modeled as purple dots along vaginal wall. (C) Alignment of control and prolapse data using P-IS reference line. (D) The distance that lateral locations and apex lie from normal is shown with arrows extending from normal location to location in prolapse. Triangle – apex, square – UVJ, circles – lateral vaginal wall, blue lines – vaginal length. © DeLancey 2010
Standard statistical analysis was applied using t-tests to compare means of vaginal length and distance of apex from normal. PROC MIXED in SAS® (SAS statistical software version 9.1, SAS Institute, Inc., Cary, NC) was used to carry out a repeated measures analysis of variance for vaginal width and paravaginal distances. Spearman’s correlation coefficients were determined to assess the relationships between apical and paravaginal distances. Lastly, Cohen’s d effect sizes were determined for statistically significant relationships to determine the strength of their relationship with their case/control status. There was no previous work in this area to provide data for an a priori power analysis; post hoc analysis were performed to determine if the study was adequately powered to conclude that there truly is no difference in non-statistically, but clinically, significant findings.
Results
Subject characteristics and mean POP-Q values are shown in Table 1. The case and control groups were matched for age, parity and BMI. No statistically significant differences existed between the two groups with the exception of the POP-Q, as was the study design.
Table 1.
Demographics
| Characteristics |
Cases (n=10) |
Controls (n=10) |
P-value |
|---|---|---|---|
| Age (yrs)a | 56.3(6.7) | 62.9(13.1) | 0.17 |
| BMI (kg/m2)a | 27.2(4.4) | 25.19(4.5) | 0.32 |
| Median parity (range) | 2 (1–5) | 2.5 (2–6) | |
| Raceb | 0.47 | ||
| Caucasian | 8 (80%) | 10 (100%) | |
| African American | 2 (20%) | 0 (0%) | |
| POP-Qa | |||
| Aa | 1.5(1.0) | −1.7(0.9) | 0.0001 |
| Ba | 2.2(1.6) | −1.6(1.0) | 0.0001 |
| C | −3.2(1.6) | −6.0(1.1) | 0.0002 |
| D | −6.5(1.1) | −8.9(1.1) | 0.0001 |
Data are mean (SD) unless otherwise specified
Fisher’s exact used to calculate P
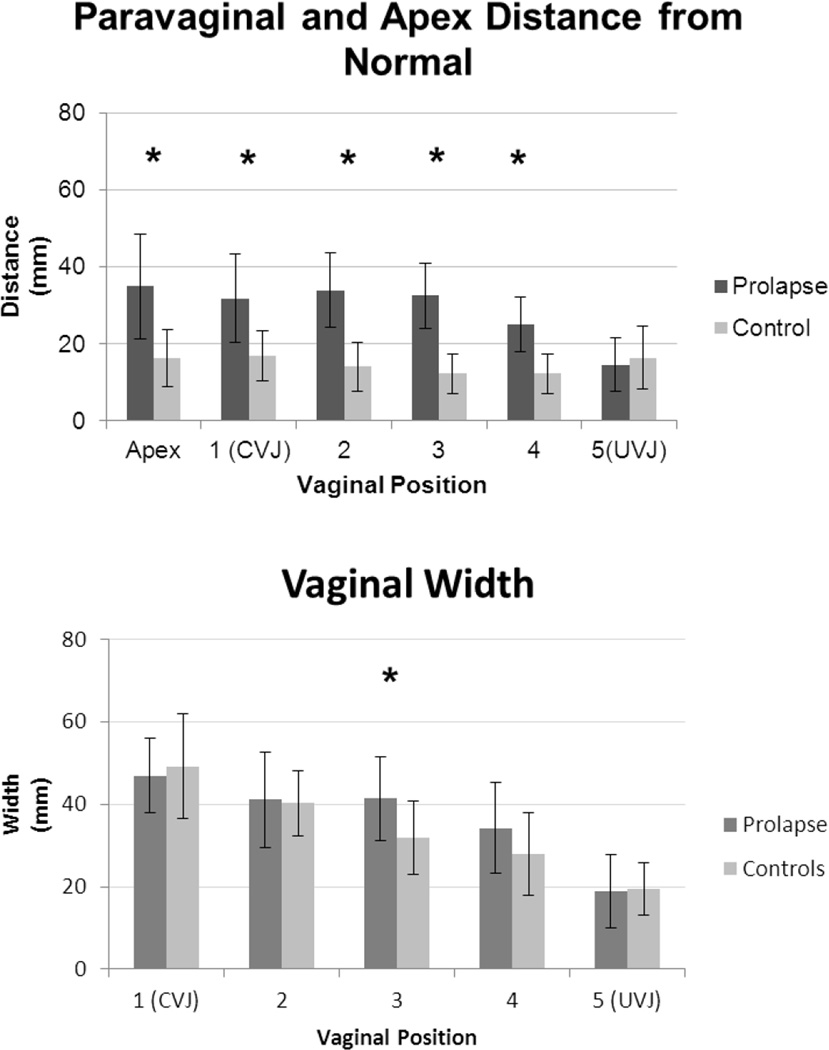
Group comparisons for paravaginal distances, vaginal width and apical distance are shown graphically in Figure 4. Table 2 details the magnitude of these differences. At maximal Valsalva, the lateral margin of the vagina lies significantly farther from its normal position in women with prolapse than in women with normal support. As can be seen in Figure 4A, these phenomenon occurred along the length of the vagina, locations “1 to 4”, with the largest difference at location “3” in the mid vagina, the area corresponding to the exposed area that slides beyond the support of the levator muscles and perineal body. At this point, the prolapse group mean (combining right and left sides) was 1.8 cm farther from the normal position than the control group mean (3.2 ± 0.8 cm vs. 1.4 ± 0.6 cm; p=0.0001).
Fig 4. Comparing apical and paravaginal distances from normal (A) and vaginal width (B).
Note that “1” represents the apex at the cervicovaginal junction (CVJ) and position “5,” the urethrovaginal junction (UVJ) at the distal end. Asterisks mark statistically significant differences. Note that right and left paravaginal distance means have been combined to one overall mean. Standard deviation shown. © DeLancey 2010
Table 2.
Effect sizes and Correlations
| Measurement | Case Mean* | Control Mean * |
Difference | Pd | Effect size | Spearman Correlation with apex |
Pe |
|---|---|---|---|---|---|---|---|
| Paravaginal Righta | |||||||
| 1 | 31.9(12.5) | 15.5(8.2) | 16.4 | <0.001 | 1.6 | 0.92 | <0.001 |
| 2 | 36.0(9.3) | 16.5(6.5) | 19.5 | <0.001 | 2.5 | 0.93 | <0.001 |
| 3 | 34.4(8.9) | 14.0(5.9) | 20.4 | <0.001 | 2.8 | 0.85 | <0.001 |
| 4 | 25.1(6.7) | 12.1(5.0) | 13.0 | 0.001 | 2.2 | 0.69 | <0.001 |
| 5 | 15.3(8.4) | 12.2(4.7) | 3.1 | 0.419 | 0.5 | 0.42 | 0.06 |
| Paravaginal Lefta | |||||||
| 1 | 31.8(11.1) | 17.3(7.0) | 14.5 | <0.001 | 1.6 | 0.85 | <0.001 |
| 2 | 32.0(9.9) | 17.3(7.0) | 14.7 | <0.001 | 1.7 | 0.90 | <0.001 |
| 3 | 30.7(7.9) | 14.1(7.0) | 16.6 | <0.001 | 2.2 | 0.77 | <0.001 |
| 4 | 25.0(8.1) | 12.4(7.0) | 12.6 | 0.0004 | 1.8 | 0.55 | <0.001 |
| 5 | 13.7(5.6) | 12.4(5.8) | 1.3 | 0.689 | 0.2 | 0.32 | 0.17 |
| Vaginal widtha | |||||||
| 1 | 46.9 (9.0) | 49.2 (12.7) | −2.2 | 0.616 | |||
| 2 | 41.1 (11.5) | 40.3 (7.9) | 0.8 | 0.851 | |||
| 3 | 41.3 (10.2) | 31.9 (8.9) | 9.5 | 0.035 | 1.0 | ||
| 4 | 34.2 (11.0) | 27.9 (10.1) | 6.4 | 0.154 | |||
| 5 | 18.9 (8.9) | 19.5 (6.4) | −0.6 | 0.89 | |||
| Apexc | 35.0 (13.6) | 16.4 (8.0) | 18.6 | 0.0016 | 1.7 | ||
| Vaginal lengthb | 72.4 (15.3) | 58.3 (7.4) | 14.1 | 0.0216 | 1.2 |
data presented as mean (SD)
PROC MIXED
unequal variance t-test
equal variance t-test
p-value for test of equal means
p-value for test of correlations
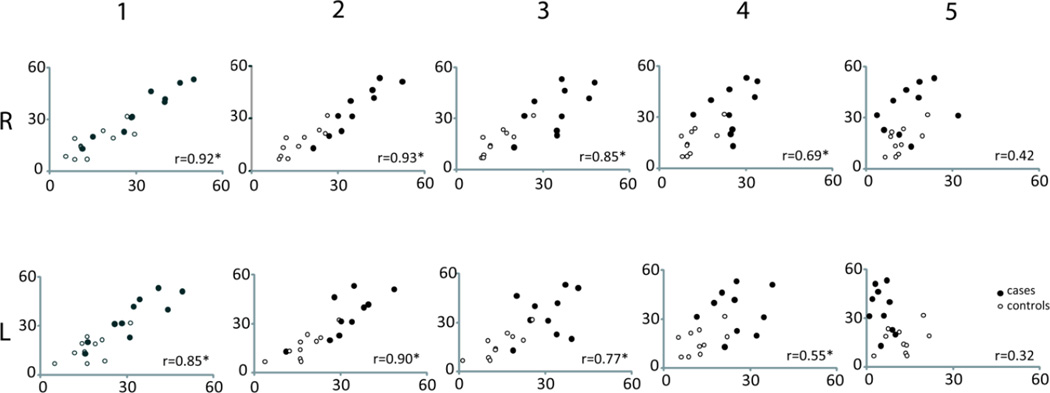
In addition, the apex was significantly farther from the normal mean location in women with prolapse (3.5 ± 1.4 cm vs. 1.6 ± 0.8 cm in controls; p=0.002). The apical distance strongly correlated with paravaginal distances for locations 1–3 with Spearman correlation coefficients ranging from 0.77 to 0.93. This relationship was slightly weaker for position “4” and did not hold at position “5” near the urethrovaginal junction. Figure 5 contains scatter plots of these relationships at positions “1–5.” Please note that while Figure 4A combines right and left sides when comparing cases and controls to simplify the comparison, these scatter plots keep right and left sides separate to better visualize the raw data. Statistical analysis using PROC MIXED did not show any statistically significant difference between sides.
Fig 5. Scatter plots of apical distance (y-axis) as a function of paravaginal distance (x-axis).
Note that “1” represents the apex at the cervicovaginal junction (CVJ) and position “5,” the urethrovaginal junction (UVJ) at the distal end. Closed circles represent cases, while open circles represent controls. All distance measurements are in mm. R and L are shown separately. Correlation coefficients are shown for each position on right and left (r value). Statistically significant correlations have (*). © DeLancey 2010
The mid vagina was wider in women with prolapse compared to their normal counterparts. At location “3,” the mean vaginal width was 4.1 ± 1.0 cm in women with prolapse as opposed to 3.2 ± 0.9 cm in those with normal support (p=0.04). There was a similar trend in position “4”; however, at the study sample size, this finding was not statistically significant (Figure 4B). We considered 20% a clinically significant difference in vaginal width, and at both positions “3” and “4,” the difference exceeded this. Post-hoc power analysis for the non-statistically significant position “4” revealed that the current study was not powered adequately to detect this difference at position “4,” and would require approximately 55 individuals in each group to reach 80% power with an alpha of 0.05. In addition, mean anterior vaginal length was different in cases and controls (7.2 ± 1.5 cm and 5.8 ± 0.7 cm, respectively; p = 0.02).
Effect sizes for statistically significant findings show that case-control paravaginal distances are more than twice as large as vaginal width changes. (Table 2)
Discussion
It has been possible to quantify apical descent, cystocele size and vaginal length in midline sagittal straining images. However it has not previously been possible to quantify the size of paravaginal defects and the degree of vaginal widening, both of which are relevant to the two major hypotheses of cystocele causation. This study uses a recently developed 3D MR imaging strategy [11] to visualize and quantify anterior vaginal wall displacement and deformation at maximal Valsalva so that women with prolapse can be compared to women with normal support. It objectively measures the deformation, determining whether the vaginal wall is wider, suggesting a midline defect, or separates from the sidewall, suggesting a paravaginal defect. The outcomes of our study include the following findings: (1) the largest differences are in paravaginal distance in women with prolapse; (2) this distance is strongly correlated with apical distance; (3) in women with prolapse, the vaginal wall widens somewhat in the area at mid vagina, that region that extends below, the support of the levator muscles and perineal body, and (4) this effect size of vagina width between cases and controls was smaller than that associated with paravaginal changes.
In essence, what this study shows is that the lateral vagina is displaced from its normal position in women with anterior wall prolapse proving the hypothesis that paravaginal defects exist. It also confirms anterior vaginal wall widening, albeit at a much lesser extent than the paravaginal changes. It is of special interest that paravaginal distance is highly correlated with apical distance. This confirms the mechanistic observation that these two phenomenon are related [12] and the recent predictions of biomechanical models [13]. While the 3D models appear consistent with paravaginal defects, whether this fits with the classical description of the defect is another issue. We are not suggesting, at present, that MRI is required before selecting surgical management in treating anterior vaginal wall prolapse. However, it is possible to see a day when the ability to quantify paravaginal defect, apical descent and vaginal widening could have great clinical utility and help in selecting which woman might need an apical suspension and which may need steps to reduce vaginal widening.
This study both corroborates and extends existing literature and our current understanding of anterior vaginal wall support. As in other studies, our subjects with prolapse had increased apical distance and increased vaginal length when compared to women with normal support [8–10, 14]. Our findings expand upon our earlier published study [11] establishing the appearance of the anterior vaginal wall at maximal Valsalva in which we describe the morphological changes seen in 3D models to include downward translation (or downward movement along the length of the vagina) and vaginal cupping. This study quantifies these changes by defining the extent of lateral movement from normal and vaginal widening in response to Valsalva.
The concept of a paravaginal defect has been described since the early 1900s. The first description of separation of the lateral vagina from the arcus tendineus fascia pelvis along the pelvic sidewall is attributed to White [15]. This observation went largely unnoticed until the landmark contributions of Richardson in the late 1970’s/early 1980’s. Richardson et al. described the paravaginal defect and reported on the results of a novel repair in a large series of surgical patients [16,17]. Despite the fact that this separation had been described a century ago and actively discussed for almost 30 years, there remains significant controversy on the subject, ranging from its prevalence in population studies of women with anterior wall prolapse (ranging from 37%–88%) to the ability to diagnose these defects both pre-operatively and intra-operatively [4,12,16–18–20]. Barber et al. reported good sensitivity and negative predictive values, but poor specificity and positive predictive values for preoperative identification of these defects on physical examination [18]. Segal et al. found lower sensitivities and higher specificities for clinical evaluation of these defects and found that the classic teaching of presence or absence of rugae did not correlate with defect status [20]. Intraoperative identification of these defects is plagued with concerns of missing defects with vaginal assessment or creating iatrogenic defects when trying to assess the integrity of these tissues. In his comparison of clinical exam with ultrasound imaging of paravaginal defects, Dietz et al. found poor correlations between the two and suggested the paravaginal defect “be regarded as an unproven concept.” [19] To date, we are not aware of other studies that have measured the degree of a paravaginal defect and widening of the vagina so that they could be compared between symptomatic and asymptomatic women.
Our observations concern the distance that the lateral margin of the prolapsed vagina lies from its position in women with normal support. Technology currently limits our ability to see and model the thin arcus tendineus fascia pelvis (ATFP) accurately in women at maximal Valsalva, even though we can model it at rest. Therefore, although we know there is the significant difference in the paravaginal distance compared to women with normal support, we do not know the relationship of the ATFP with the lateral vagina in these women. Even without knowing the position of the ATFP, the displacement described in our study clearly is what is clinically recognized in women as a paravaginal defect. One of the classic tests for diagnosing a paravaginal defect is to correct the presumed defect with support during Valsalva (using either a ring forceps, vaginal analyzer, or tongue depressor along the lateral vagina at the ischial spine to see if it reduces the prolapse, and if so, this is deemed a paravaginal defect). This “test” would also be minimizing the apical movement and may be reducing the cystocele for this reason. We propose that it is at least plausible that these are manifestations of the same phenomenon.
The observation that apical and paravaginal distances are part of the same phenomena is in agreement with surgical results. For example, abdominal sacrocolpopexy eliminates cystocele in the vast majority of cases. Shippey et al. recently reported anatomic outcomes of sacrocolpopexy in women with and without paravaginal repair. Sixty-two patients had concomitant paravaginal repair, while 108 subjects did not. Although the data trended towards higher anterior wall failure rates in the group without a paravaginal repair, the study wasn’t powered to detect this difference. In addition, once they controlled for concomitant Burch, the presence/absence of a paravaginal repair did not seem as influential [21]. Although this may solely have been an inadequate power issue, it does raise the thought that perhaps restoration of the apical position in these women was adequate to restore normal anterior vaginal wall support.
Although this “jibes” with why addressing apical support at the time of cystocele repair is crucial, it then begs the question as to why surgical approaches such as an anterior repair alone are successful for cystocele repair. In his discussion on the clinical relevance of a paravaginal defect, Karram raises a similar question concerning anterior compartment recurrence rates of 5–15% which are independent of whether or not a paravaginal repair is performed [22]. Our study did reveal widening at one position in this area of exposed vagina, but it seems that this minor difference is not sufficient to explain why narrowing the vagina would correct the entire cystocele. A second area within this region (position 4) also appeared to trend towards having a significant (22%) widening, although was not statistically significant at this smaller sample size. Perhaps anterior colporrhaphy, by reducing the surface area of the exposed vagina, brings the anterior wall back into contact with the posterior vaginal wall, reducing the tension on the support ligaments. In a sample size of ten women with anterior wall predominant prolapse we cannot capture the full spectrum of defects: clinically we see women with good apical support and isolated anterior wall defects. Perhaps this latter population is the one that has a successful outcome with an isolated anterior repair. It is not possible to conclude this from our study; however, the present measurement technique allows quantification of the degree of defects present so that future outcome studies could determine which factors are correlated with treatment success or failure.
So is there a midline defect? The widening visible in the mid to distal region of the vagina indicates a change from normal. It does not indicate whether this is the cause or effect of cystocele, nor whether it is a change in the material properties of the vaginal wall or the result of a break in the “anteroposterior separation of the fascia [that] occurs between the vagina and overlying bladder and/or urethra” as described by Richardson et al. [15]. The observed stretching may also be the result of the increased pressure differential across the exposed portion of the vagina that no longer has the posterior vaginal wall to counteract increases in intra-abdominal pressure. This study cannot differentiate between stretching of the anterior vaginal wall and a break in the fascia (if these exist) because we are unable to visualize the presence of a break, or separation of the fascia with the current MRI technology. Our results do indicate, however, that regardless of the underlying mechanism, this widening has a much smaller effect size than the paravaginal distances.
Several factors must be considered when interpreting the results of this study. While we are able to identify large and significant changes in the location of the lateral vagina in women with prolapse and assess the relative size of the differences in paravaginal distance and vaginal width, our study sample size did not have the power to detect more subtle changes in vaginal width. These changes are of lesser magnitude as reflected in their small effect sizes and are not as great as the paravaginal distance changes. In addition, we recognize that a larger sample size and further studies are indicated to capture the full spectrum of different cystoceles. While mentioning this, it is important to recognize that we had to screen 21 cases to find 10 adequate image sets in women with prolapse. Certainly, as imaging techniques improve, this ratio will also improve. As in our previous studies, supine MR images were obtained which may limit the descent of pelvic floor, although earlier studies do not document differences when compared with images obtained in the seated position in open scanners [23,24]. Clinicians do make the majority of their clinical decisions based on examinations performed in women who are supine, and we feel that with adequate Valsalva, the maximal extent of the prolapse can be achieved. The ability to sustain Valsalva is difficult in some women and is a likely contributor to the large number excluded in the initial subject selection process. Because the sulci are difficult to differentiate on MR images, vaginal gel was used to illuminate the lateral extent of the vagina. Although it significantly improved visualization, this gel does result in some degree distortion of the vaginal shape by filling the vagina. We suspect this effect is minimal as the gel’s viscosity was such that Valsalva efforts seemed to result in expulsion of gel, rather than a redistribution which could distort the vaginal wall.
So is it a midline or paravaginal defect? Our results indicate that there are changes in both regions of the vagina, but show that the changes at the lateral vagina and the altered relationship with the pelvic sidewall are much larger than the widening of the mid-distal region of the vaginal wall. In addition, the strong correlation between the “paravaginal defect” and apical distance cause us to theorize that these may be manifestations of the same phenomenon. Which came first – the apical displacement or the pulling away from the pelvic sidewall? Although an answer is not possible with this study, the present method not only provides the means to explore this in the future, but also provides the ability to quantify paravaginal defects, apical descent and vaginal widening to aid in analyzing the outcomes of specific interventions and, ultimately, clinically impact treatment selection.
Acknowledgements
We gratefully acknowledge support from the National Institute of Child Health and Human Development Grants R01 HD 38665 with additional investigator support from the Office for Research on Women’s Health SCOR on Sex and Gender Factors Affecting Women’s Health 1 P50 HD044406
Footnotes
Podium presentation at the 31st American Urogynecologic Society meeting in Long Beach, Ca.
Dr. John OL DeLancey and Dr. James Ashton-Miller are consultants to American Medical Systems and Johnson and Johnson Personal Products. Jiajia Luo’s PhD is partly funded by American Medical Systems. The other authors have no disclosures to report.
Author Participation:
K.A. LARSON - Protocol/project development, Data collection or management, Data analysis, Manuscript writing/editing
J. LUO - Protocol/project development, Data analysis
K. GUIRE – Data analysis, Manuscript editing
L. CHEN - Protocol/project development, Data analysis
J.A. ASHTON-MILLER - Protocol/project development, Data analysis, Manuscript editing
J.O.L. DeLANCEY - Protocol/project development, Data collection or management, Data analysis, Manuscript editing
References
- 1.Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–506. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 2.Benson JT, Lucente V, McClellan E. Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: A prospective randomized study with long-term outcome evaluation. Am J Obstet Gynecol. 1996;175(6):1418–1421. doi: 10.1016/s0002-9378(96)70084-4. discussion 1421-2. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen JN, Burchette RJ. Outcome after anterior vaginal prolapse repair: A randomized controlled trial. Obstet Gynecol. 2008;111(4):891–898. doi: 10.1097/AOG.0b013e31816a2489. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen JK. Current concepts in the diagnosis and surgical repair of anterior vaginal prolapse due to paravaginal defects. Obstet Gynecol Surv. 2001;56(4):239–246. doi: 10.1097/00006254-200104000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Maher C, Baessler K. Surgical management of anterior vaginal wall prolapse: An evidencebased literature review. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(2):195–201. doi: 10.1007/s00192-005-1296-3. [DOI] [PubMed] [Google Scholar]
- 6.Maher CF, Murray CJ, Carey MP, Dwyer PL, Ugoni AM. Iliococcygeus or sacrospinous fixation for vaginal vault prolapse. Obstet Gynecol. 2001;98(1):40–44. doi: 10.1016/s0029-7844(01)01378-3. [DOI] [PubMed] [Google Scholar]
- 7.Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183(6):1365–1373. doi: 10.1067/mob.2000.110910. discussion 1373-4. [DOI] [PubMed] [Google Scholar]
- 8.Hsu Y, Chen L, Summers A, Ashton-Miller JA, DeLancey JO. Anterior vaginal wall length and degree of anterior compartment prolapse seen on dynamic MRI. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(1):137–142. doi: 10.1007/s00192-007-0405-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rooney K, Kenton K, Mueller ER, FitzGerald MP, Brubaker L. Advanced anterior vaginal wall prolapse is highly correlated with apical prolapse. Am J Obstet Gynecol. 2006;195(6):1837–1840. doi: 10.1016/j.ajog.2006.06.065. [DOI] [PubMed] [Google Scholar]
- 10.Summers A, Winkel LA, Hussain HK, DeLancey JO. The relationship between anterior and apical compartment support. Am J Obstet Gynecol. 2006;194(5):1438–1443. doi: 10.1016/j.ajog.2006.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larson KA, Hsu Y, Chen L, Ashton-Miller JA, DeLancey JO. Magnetic resonance imaging-based three-dimensional model of anterior vaginal wall position at rest and maximal strain in women with and without prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2010 doi: 10.1007/s00192-010-1161-x. Epub 2010 May 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeLancey JO. Fascial and muscular abnormalities in women with urethral hypermobility and anterior vaginal wall prolapse. Am J Obstet Gynecol. 2002;187:93–98. doi: 10.1067/mob.2002.125733. [DOI] [PubMed] [Google Scholar]
- 13.Chen L, Ashton-Miller JA, DeLancey JO. A 3D finite element model of anterior vaginal wall support to evaluate mechanisms underlying cystocele formation. J Biomech. 2009;42(10):1371–1377. doi: 10.1016/j.jbiomech.2009.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L, Ashton-Miller JA, Hsu Y, DeLancey JO. Interaction among apical support, levator ani impairment, and anterior vaginal wall prolapse. Obstet Gynecol. 2006;108(2):324–332. doi: 10.1097/01.AOG.0000227786.69257.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White GR. Cystocele: a radical cure by suturing lateral sulci of vagina to white line of pelvic fascia. J Am Med Assoc. 1909;21:1707–1711. doi: 10.1007/BF02765486. [DOI] [PubMed] [Google Scholar]
- 16.Richardson AC, Lyon JB, Williams NL. A new look at pelvic relaxation. Am J Obstet Gynecol. 1976;126:568–573. doi: 10.1016/0002-9378(76)90751-1. [DOI] [PubMed] [Google Scholar]
- 17.Richardson AC, Edmonds PB, Williams NL. Treatment of stress urinary incontinence due to paravaginal fascial defect. Obstet Gynecol. 1981;57:357–362. [PubMed] [Google Scholar]
- 18.Barber MD, Cundiff GW, Weidner AC, Coates KW, Bump RC, Addison WA. Accuracy of clinical assessment of paravaginal defects in women with anterior vaginal wall prolapse. Am J Obstet Gynecol. 1999;181:87–90. doi: 10.1016/s0002-9378(99)70440-0. [DOI] [PubMed] [Google Scholar]
- 19.Dietz HP, Pang S, Korda A, Benness C. Paravaginal defects: a comparison of clinical examination and 2D/3D ultrasound imaging. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2005;45:187–190. doi: 10.1111/j.1479-828X.2005.00377.x. [DOI] [PubMed] [Google Scholar]
- 20.Segal JL, Vassallo BJ, Kleeman SD, Silva WA, Karram MM. Paravaginal defects: prevalence and accuracy of preoperative detection. Int Urogynecol J. 2004;15:378–383. doi: 10.1007/s00192-004-1196-y. [DOI] [PubMed] [Google Scholar]
- 21.Shippey SH, Quiroz LH, Sanses TVD, Knoepp LR, Cundiff GW, Handa VL. Anatomic outcomes of abdominal sacrocolpopexy with or without paravaginal repair. Int Urogynecol J. 2010;21:279–283. doi: 10.1007/s00192-009-1013-8. [DOI] [PubMed] [Google Scholar]
- 22.Karram MM. What is the clinical relevance of a paravaginal defect? Int Urogynecol J. 2004;15:1–2. doi: 10.1007/s00192-004-1120-5. [DOI] [PubMed] [Google Scholar]
- 23.Bertschinger KM, Hetzer FH, Roos JE, Treiber K, Marincek B, Hilfiker PR. Dynamic MR imaging of the pelvic floor performed with patient sitting in an open-magnet unit versus with patient supine in a closed-magnet unit. Radiology. 2002;223(2):501–508. doi: 10.1148/radiol.2232010665. [DOI] [PubMed] [Google Scholar]
- 24.Fielding JR, Griffiths DJ, Versi E, Mulkern RV, Lee ML, Jolesz FA. MR imaging of pelvic floor continence mechanisms in the supine and sitting positions. AJR Am J Roentgenol. 1998;171(6):1607–1610. doi: 10.2214/ajr.171.6.9843296. [DOI] [PubMed] [Google Scholar]