Abstract
The purpose of this investigation was to examine the associations between circulating markers of inflammation and arterial elasticity in healthy older women. Participants were 50 women older than 60 years of age, body mass index 27±4, and physically untrained. Large artery elasticity, small artery elasticity, systemic vascular resistance, total vascular impedance, estimated cardiac output, and estimated cardiac index were determined using pulse contour analysis. Serum concentrations of tumor necrosis factor-α, C-reactive protein (CRP), and interleukin 6 were assessed. Results from Pearson’s correlation revealed that tumor necrosis factor-α was inversely associated with large artery elasticity (−.426, p < .01) and estimated cardiac index (−.324, p < .05) and positively associated with systemic vascular resistance (.386, p < .01) and total vascular impedance (.416, p < .01). Additionally, C-reactive protein was inversely associated with large artery elasticity (−.308, p < .01). The overall implication was that tumor necrosis factor-α and C-reactive protein appear to be critical inflammatory cytokines associated with reductions in arterial elasticity in older women.
Key Words: Arterial elasticity, TNF-α, CRP, Hemodynamics.
CARDIOVASCULAR disease (CVD) remains the leading cause of morbidity and mortality in modern societies (1), and aging is considered one of the major risk factors (2). Furthermore, endothelial dysfunction and reduced arterial elasticity are common and early signs that contribute to the age-related increase in CVD risk (3). Although it is well known that hypertension in older adults (65 years of age and older) increases the risk for CVD three- to fourfold as compared with younger individuals (4), it is important to note that both endothelial dysfunction and reduced arterial elasticity often precedes clinically diagnosed hypertension (blood pressure [BP] threshold ≥140/90 mmHg [5]). Given that hypertension is the most common modifiable risk factor to reduce mortality in developing countries (6), it is important to understand and identify early pathophysiology and associations with hypertension so that potential targets for intervention can be identified.
Although the mechanisms underlying the etiology of hypertension are incompletely understood, it has become increasingly clear that low-grade chronic inflammation is often a critical initiating step in the progression of vascular disease (7–10). Indeed, tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and C-reactive protein (CRP) have all been shown to be associated with various forms of CVD (11–13). However, there is now convincing evidence in animal models that implicates TNF-α as the primary proinflammatory cytokine associated with arterial inflammation and endothelial dysfunction, likely occurring via activation of the nuclear factor kappa B pathway (14,15). Furthermore, although it has been well documented that circulating proinflammatory cytokines are elevated in older humans and are associated with an increased risk of atherosclerosis, the majority of studies performed to date have been in adults who already have several risk factors for atherosclerosis and other cardiovascular-related disease (13,16,17). Thus, it is difficult to determine whether an elevated inflammatory profile reflects the degree of CVD or if low-grade chronic inflammation is an age-related causative factor in the development of vascular dysfunction.
In order to better understand the relationships between low-grade chronic inflammation, arterial elasticity, and endothelial dysfunction, the objective of this study was to identify associations between circulating proinflammatory cytokines (IL-6, TNF-α, and CRP) with multiple hemodynamic and arterial elasticity measures in a carefully selected cohort of healthy nonsmoking postmenopausal women (>60 years of age) free of overt cardiovascular risk factors. Given that the observed age-related decrease in arterial elasticity has been shown to be absent or attenuated in endurance-trained adults and that endurance training can restore reduced arterial elasticity in previously sedentary individuals (18,19), a secondary objective was to examine the associations of cardiovascular fitness with arterial elasticity and hemodynamic measures.
Methods
Participants
This cross-sectional study consisted of 50 healthy postmenopausal women aged 60 years and older with no history of heart disease or type 2 diabetes, body mass index less than 30kg/m2, nonhypertensive (systolic blood pressure [SBP] <140 or diastolic blood pressure [DBP] <90 mmHg), normal resting electrocardiography (EKG) response at rest and during exercise, nonsmokers, and no use of medications known to affect cardiovascular or metabolic function. Preliminary screening for study inclusion included a physical examination, dual-energy X-ray absorptiometry, and a 12-lead EKG. Participants were excluded from the study if they were hypertensive, displayed any abnormal EKG responses at rest or during exercise, or dual-energy X-ray absorptiometry assessment revealed osteoporosis. Participants had no history of heart disease or diabetes mellitus (review of medical records). The study was approved by the Institutional Review Board for Human Use at the University of Alabama at Birmingham (UAB). All women provided informed consent prior to participating in the study.
Body Composition Measurements
Body composition was measured by dual-energy X-ray absorptiometry (Prodigy; Lunar Radiation, Madison, WI). The scans were analyzed with the use of ADULT software, version 1.33 (Lunar Radiation).
Aerobic Capacity Testing
Maximum oxygen uptake (VO2max) was measured by indirect calorimetry on a treadmill using a modified Bruce protocol. Participants warmed up for 4 minutes at a speed of 3 mph on a 2.5% grade, grade was increased 2.5% each minute until volitional exhaustion. Volume of O2 and CO2 were measured continuously by open-circuit spirometry and analyzed using a Sensormedics metabolic measurement cart (model 2900, Yorba Linda, CA). Heart rate was monitored by a Polar Vantage XL heart rate monitor (Polar Beat, Port Washington, NY). The highest VO2 achieved within the last 2 minutes of exercise was recorded as VO2max.
Laboratory Analyses
Inflammatory markers were assessed using ELISAs. All samples were analyzed in duplicate. TNF-α was analyzed using the high-sensitivity ELISA kit (Quantikine HSTA00C, R&D Systems, Minneapolis, MN). IL-6 was assayed using the high-sensitivity ELISA kit (Quantikine HS600B, R&D Systems). CRP was assayed with the high-sensitivity ELISA kit (030–9710s, ALPCO, Windham, NH). HDL, LDL, and triglycerides were measured with the Ektachem DT II system.
Arterial Elasticity Evaluation
Hemodynamic and arterial elasticity variables such as SBP, DBP, and mean arterial blood pressures (MAP), pulse rate, large artery elasticity (LAE) and small artery elasticity (SAE), total vascular impedance , systemic vascular resistance (SVR), estimated cardiac output (ECO), and estimated cardiac index were measured using noninvasive pulse wave analysis (HDI/Pulse Wave TM CR-2000, Hypertension Diagnostics, Eagan, MN). The arterial pulse wave analysis of the radial artery is based on a modified Windkessel model that allows evaluation of the large conduit arteries and the small microcirculatory arteries (20). Briefly, with participants in the seated position, a solid-state pressure transducer array (tonometer) was placed over the radial artery of the dominant arm to record the pulse contour. The waveform was calibrated by the oscillometric method. Once a stable measurement was achieved, a 30 second analog tracing of the radial waveform was digitized at 200 samples per second. Before, during, and after the waveform assessment, an automated oscillatory BP measurement was taken on the contralateral arm. The first maximum waveform observed represents the action of the arteries following cardiac ejection and reflects the large arteries, whereas the second rebound wave reflects compliance of the smaller arteries. SVR was calculated as the MAP divided by the ECO. Estimated cardiac index was calculated as the ECO divided by the body surface area. Total vascular impedance was determined from the modified Windkessel model evaluated at the frequency of the measured heart rate (21).
Statistical Analyses
Descriptive characteristics are reported as means and standard deviations. All variables were evaluated for residual normality and logarithmic transformations were performed when appropriate. Simple Pearson correlations were used to examine associations of SBP, DBP, MAP, pulse rate, LAE, small artery elasticity, total vascular impedance, SVR, ECO, and estimated cardiac index with TNF-α, IL-6, CRP, and VO2max. Multiple linear regression analyses for LAE and SVR were done to make adjustments for potential confounders in understanding the relationships between vascular measures and inflammatory markers. Due to limitations of observation to variable ratio, two separate models were used: one with adjustments made for TNF-α, HDL, LDL, and triglycerides and the other with adjustments for TNF-α, age, %fat, and SBP. Collinearity between variables was within acceptable limits for all variables in all models with all variable inflation factors less than 1.7. A partial correlation analysis, adjusted for age, was used to identify associations between VO2max and arterial elasticity measures. For all analyses, a p value less than .05 was deemed statistically significant. All data were analyzed using the Statistical Package for the Social Sciences (SPSS, version 19.0, Chicago, IL).
Results
Descriptive statistics are shown in Table 1. The partial correlations of inflammatory markers with arterial elasticity and hemodynamic variables are reported in Table 2. Simple correlation analyses revealed a significant positive association between TNF-α and SBP, DBP, MAP, total vascular impedance, and SVR and a significant inverse association with LAE and estimated cardiac index (p < .05). Additionally, there was a significant inverse association between CRP and LAE (p < .05). No significant associations were observed between IL-6 and arterial elasticity and hemodynamic measurements.
Table 1.
Descriptive Statistics of Participants.
| Participant characteristics | Mean ± SD |
|---|---|
| Age (y) | 64.9±3.9 |
| Body weight (kg) | 73.6±11.4 |
| Body mass index (kg/m2) | 27.1±4.3 |
| Body fat (%) | 44.1±6.4 |
| VO2max (mL/kg/min) | 22.8±4.5 |
| TNF-α (pg/mL) | 6.6±2.2 |
| IL-6 (pg/mL) | 1.9±1.6 |
| CRP (mg/L) | 2.9±3.1 |
| HDL (mg/dL) | 63.3±18.6 |
| LDL (mg/dL) | 128.1±29 |
| TG (mg/dL) | 111.4±46 |
| SBP (mmHg) | 127.7±15.2 |
| DBP (mmHg) | 69.9±9.6 |
| MAP (mmHg) | 92.2±11.3 |
| Pulse rate (BPM) | 63.9±7.6 |
| LAE (mL/mmHg × 10) | 13.1±4.7 |
| SAE (mL/mmHg × 100) | 3.9±1.8 |
| TVI (dyne/s/cm−5) | 166.7±44.9 |
| SVR(dyne/s/cm−5) | 1580±269.7 |
| ECO (L/min) | 4.8±0.6 |
| ECE (ms) | 329.6±26.1 |
Note: CRP = C-reactive protein; DBP = diastolic blood pressure; ECE = estimate cardiac ejection time; ECO = estimated cardiac output; IL-6 = interleukin-6; LAE = large artery elasticity; MAP = mean arterial pressure; SAE = small artery elasticity; SBP = systolic blood pressure; SVR = systemic vascular resistance; TG = triglycerides; TNF-α = tumor necrosis factor; TVI = total vascular impedance.
Table 2.
Pearson Correlation Matrix for Inflammatory Markers and Vascular Functional Measurements
| SBP | DBP | MAP | Pulse rate | LAE | SAE | TVI | SVR | ECO | ECI | |
|---|---|---|---|---|---|---|---|---|---|---|
| TNF-α | 0.54 | 0.32 | 0.48 | 0.183 | −0.43 | −0.18 | 0.42 | 0.38 | −0.14 | −0.32 |
| IL-6 | −0.18 | −0.29 | −0.13 | 0.10 | −0.03 | −0.08 | −0.04 | −0.23 | 0.08 | 0.09 |
| CRP | 0.21 | −0.05 | 0.08 | 0.21 | −0.31 | −0.15 | 0.26 | 0.02 | 0.09 | −0.04 |
Notes: CRP = C-reative protein; DBP = diastolic blood pressure; ECI = estimated cardiac index; ECO = estimated cardiac output; IL-6 = interleukin-6; LAE = large artery elasticity; MAP = mean arterial pressure; SAE = small artery elasticity; SBP = systolic blood pressure; SVR = systemic vascular resistance; TNF-α = tumor necrosis factor; TVI = total vascular impedance.
Bold values indicate p < .05.
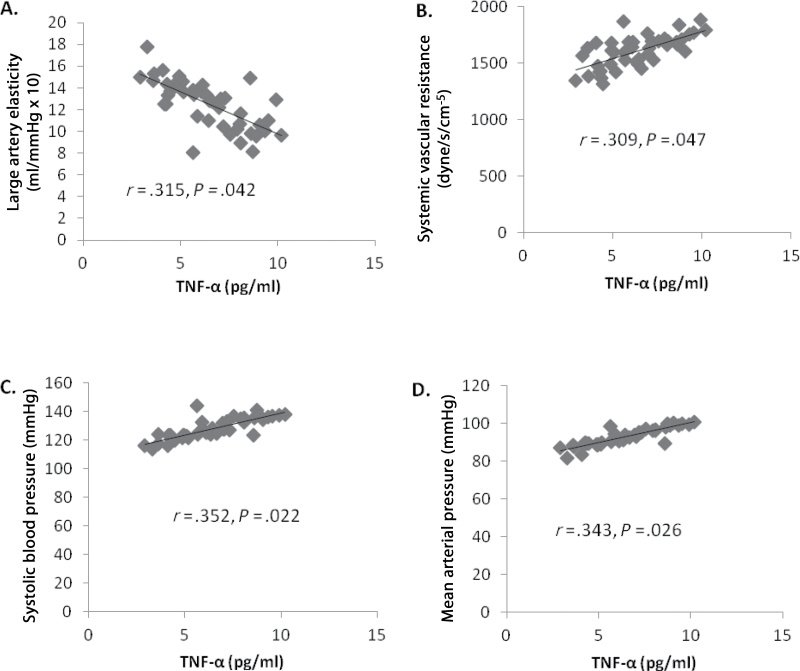
Multiple linear regression analyses revealed an independent relationship between TNF-α and LAE and SVR after adjusting for blood lipids (HDL, LDL, and triglycerides). However, these relationships no longer existed when adjusting for age, SBP, and %fat. Furthermore, an independent relationship was observed for SBP and %fat with SVR (Tables 3 and 4). The age and %fat adjusted mean associations between TNF-α and elasticity and hemodynamic measures are shown in Figure 1.
Table 3.
Multiple Linear Regression Model of LAE and SVR With TNF-α, HDL, LDL, and TG
| Dependent variables | Independent variables | Standardized β | p values |
|---|---|---|---|
| LAE Model R 2 = 0.192 | TNF-α | −0.430 | .006 |
| HDL | −0.070 | .697 | |
| LDL | 0.102 | .516 | |
| TG | −0.090 | .626 | |
| SVR Model R 2 = 0.280 | TNF-α | 0.355 | .015 |
| HDL | −0.001 | .995 | |
| LDL | 0.349 | .023 | |
| TG | −0.195 | .266 |
Notes: LAE = large artery elasticity; SVR = systemic vascular resistance; TG = triglycerides; TNF-α = tumor necrosis factor.
Bold values indicate p < .05.
Table 4.
Multiple Linear Regression Model of LAE and SVR With TNF-α, Age, SBP, and %fat.
| Dependent variables | Independent variables | Standardized β | p values |
|---|---|---|---|
| LAE Model R 2 = 0.366 | TNF-α | −0.133 | .392 |
| Age | −0.247 | .099 | |
| SBP | −0.273 | .089 | |
| % fat | −0.218 | .092 | |
| SVR Model R 2 = 0.572 | TNF-α | 0.048 | .976 |
| Age | 0.028 | .711 | |
| SBP | 0.696 | .001 | |
| %fat | −0.313 | .005 |
Notes: LAE = large artery elasticity; SVR = systemic vascular resistance; SBP = systolic blood pressure; TNF-α = tumor necrosis factor.
Bold values indicate p < .05.
Figure 1.
Multiple linear regression analyses, adjusting for age and %fat, between (A) tumor necrosis factor-α (TNF-α) and large artery elasticity, (B) TNF-α and systemic vascular resistance (SVR), (C) TNF-α and systolic blood pressure, and (D). TNF-α and mean arterial pressure.
These data suggest the relationship of TNF-α with hemodynamic and arterial elasticity measures in older women are independent of differences in blood lipids, however, the relationship between TNF-α and SVR appears to be in part associated with SBP and %fat.
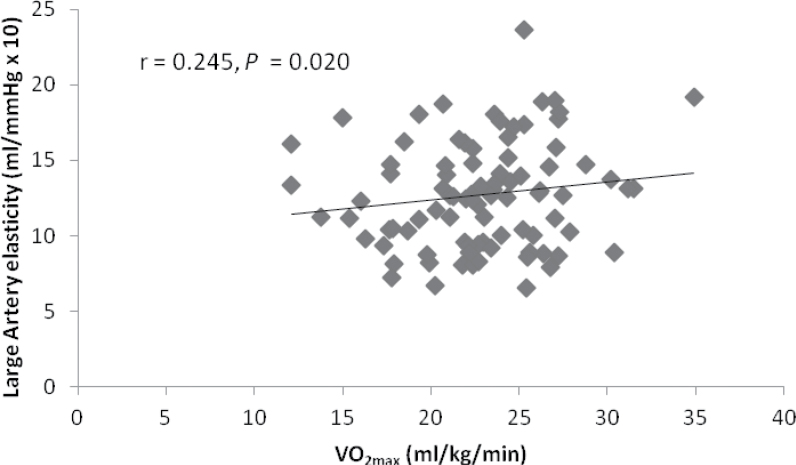
Partial correlation analyses revealed a significant positive association between VO2max and LAE (p < .05; Figure 2). No significant associations were observed between VO2max and other small artery elasticity or hemodynamic measurements.
Figure 2.
Partial correlation analysis for large artery elasticity and VO2max. (adjusted for age).
Discussion
The objective of this study was to examine associations between circulating proinflammatory cytokines (IL-6, TNF-α, and CRP) with arterial elasticity and other hemodynamic measures associated with hypertension in a cohort of healthy postmenopausal and older women aged 60 years and older. Additionally, we sought to determine if these relationships could be explained by differences in body composition. Results demonstrated that, in this sample of older postmenopausal women, TNF-α was strongly associated with a decrease in arterial elasticity and greater vascular resistance. Furthermore, the associations between elasticity were independent of differences in body composition, lipids, and age. These observations suggest that future longitudinal human studies should be conducted to determine whether TNF-α is a critical proinflammatory cytokine associated with arterial aging.
To our knowledge, this is the first study to investigate the associations of proinflammatory cytokines with hemodynamic and arterial elasticity measures in a cohort of normotensive healthy women with no known clinical CVD. Chronic inflammation is well known to play an essential role in the etiology of many CVDs, and more recent evidence suggests an involvement in vascular aging (2,22). Among the proinflammatory cytokines measured in this study, TNF-α demonstrated the strongest association with arterial elasticity, SVR, SBP, DBP, and MAP measures. Consistent with these studies, we also find that BP is related to vascular resistance. This is in agreement with several animal studies that have demonstrated a link between TNF-α and endothelial impairment during the aging process (15,23). Although we can only speculate as to potential mechanisms in which TNF-α influences arterial elasticity or hemodynamics, several animal and in vitro studies have demonstrated that TNF-α may induce oxidative stress by upregulating and/or activating NADPH oxidase, which generates reactive oxygen species and can further activate nuclear factor kappa B, resulting in upregulation of proatherogenic inflammatory mediators (14,15,24). Thus, there appears to be strong evidence linking increased TNF-α concentration during aging to arterial inflammation, endothelial dysfunction, arterial elasticity, and age-related cardiovascular pathophysiology. Interestingly, TNF-α is not independently related to arterial elasticity or resistance after adjusting for SBP, suggesting that BP changes are inherently involved in the TNF-α arterial function relationship. Further research is necessary to improve our understanding of these interrelationships.
Although associations between inflammatory markers with arterial elasticity have previously been shown in young and older adults (5,25,26), these data are often confounded by other factors (impaired glucose tolerance, dyslipidemia, adiposity, smoking, or antihypertensive medication) known to influence inflammation. Our investigation included multiple inflammatory markers and vascular measures and also included a homogenous distribution of apparently healthy older women. The observation that both TNF-α and CRP were associated with a reduction in LAE and greater SVR demonstrates a potential link between inflammation and arterial aging that presents well before an increase in BP occurs. This observation is important given that hypertension-related CVDs transpire well before clinically diagnosed hypertension occurs. The following quote by the hypertension writing group in 2004 elaborately describes the new dogma linking vascular dysfunction and CVD, “Hypertension is a progressive cardiovascular syndrome arising from complex and interrelated etiologies. Early markers of the syndrome often present before blood pressure elevation is sustained; therefore hypertension cannot solely be classified by discrete blood pressure thresholds.”(27) Understanding the pathophysiological mechanisms involved in the early onset of reduced arterial elasticity and endothelial and smooth muscle cell dysfunction are essential for developing potential targets for early intervention.
Chronic inflammation is associated with adiposity, metabolic dysfunction, and CVD (28,29). Therefore, it is often difficult to determine whether inflammation is a causative factor in the progression of various cardiovascular and metabolic diseases. We did not measure specific fat depots in this study; therefore, we are unable to assess the relationship between inflammatory markers and visceral and subcutaneous fat. However, we and others have previously demonstrated that visceral fat was the primary fat depot responsible for circulating TNF-α concentration (29–31). This study revealed a significant association between TNF-α and CRP with arterial elasticity and hemodynamic measures independent of body mass index and %fat in. Although adiposity is often associated with elevated inflammation, our data suggest that the relationship between inflammatory markers and arterial function may occur independently of adiposity-induced inflammation.
The cohort of women included in this study was initially inactive and engaging in very little physical activity. It has been shown in other cross-sectional studies comparing trained and sedentary adults and intervention studies of aerobic exercise training, that maintaining or improving cardiovascular fitness may protect the arteries against age-associated decreases in arterial and endothelial dysfunction in middle-aged and older men (32,33), however, this has not been studied in healthy postmenopausal women (34). Our results revealed that cardiovascular fitness level, as measured by VO2max, was significantly positively associated with LAE in our cohort of postmenopausal women. However, given the cross-sectional nature of this study, we are unable to establish causality. These discrepancies between findings and the mechanisms underlying these sex-specific differences warrant further exploration. To date, there are few studies that have assessed the affect of exercise training on age-related vascular function in older postmenopausal women. Future studies should bridge this gap in the literature.
Strengths of this study included recruitment of a homogenous cohort of older healthy postmenopausal women with no known CVD or metabolic complications. Further strengths included robust measurements of hemodynamic variables and inflammatory markers. It is possible that the relationship between LAE and TNF-α may have been confounded by variables such as blood lipids and BP. However, there were no significant association between any blood lipid and cytokines that were significantly related to elasticity or hemodynamic measures, therefore, negating potential confounding effects. In addition, adjusting for BP did not affect the association between TNF-α and arterial elasticity. Limitations in this study include the cross-sectional design, small sample size, lack of glucose measurements, and use of dual-energy X-ray absorptiometry instead of computed tomography for body composition measurements. Future studies should focus on identifying strategies to reduce inflammation and preserve and/or restore arterial elasticity and vascular function. Additionally, longitudinal studies should be conducted in order to determine whether a cause and effect relationship exists between inflammatory markers and arterial and/or vascular function.
In conclusion, the overall implication from this study was that TNF-α and CRP may be critical proinflammatory cytokines associated with arterial aging; supporting the notion that arterial elasticity may at least in part be related to systemic inflammation. Additionally, we also showed an association between cardiovascular fitness level and arterial elasticity, which supports previous studies demonstrating an important link between fitness and vascular health. Further research is needed to determine whether reducing systemic inflammation and/or improving cardiovascular fitness can prevent or attenuate the age-associated decrease in vascular function in older postmenopausal women.
Funding
This work was supported by National Institute of Health grants R01AG027084-01, P30-DK56336, and T32DK062710-07.
Conflict of Interest
The authors have nothing to disclose.
Acknowledgments
We acknowledge David Bryan and Brandon Kane for technical assistance, Maryellen Williams and Cindy Zeng for conducting laboratory analyses, and Paul Zuckerman for project coordination.
References
- 1. Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation. 2011; 123: e18–e209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci. 2011; 120: 357–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003; 107(1):139–146 [DOI] [PubMed] [Google Scholar]
- 4. Ooi HH, Coleman PL, Duggan J, O’Meara YM. Treatment of hypertension in the elderly. Curr Opin Nephrol Hypertens. 1997; 6(5):504–509 [DOI] [PubMed] [Google Scholar]
- 5. van Bussel BC, Schouten F, Henry RM, et al. Endothelial dysfunction and low-grade inflammation are associated with greater arterial stiffness over a 6-year period. Hypertension. 2011; 58(4):588–595 [DOI] [PubMed] [Google Scholar]
- 6. Kannel WB. Historic perspectives on the relative contributions of diastolic and systolic blood pressure elevation to cardiovascular risk profile. Am Heart J. 1999; 138(3 Pt 2):205–210 [DOI] [PubMed] [Google Scholar]
- 7. Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. FASEB J. 2003; 17(9):1183–1185 [DOI] [PubMed] [Google Scholar]
- 8. Mateos-Cáceres PJ, Zamorano-León JJ, Rodríguez-Sierra P, Macaya C, López-Farré AJ. New and old mechanisms associated with hypertension in the elderly. Int J Hypertens. 2012; 2012: 150107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Belmin J, Bernard C, Corman B, Merval R, Esposito B, Tedgui A. Increased production of tumor necrosis factor and interleukin-6 by arterial wall of aged rats. Am J Physiol. 1995; 268(6 Pt 2):H2288–H2293 [DOI] [PubMed] [Google Scholar]
- 10. Csiszar A, Wang M, Lakatta EG, Ungvari Z. Inflammation and endothelial dysfunction during aging: role of NF-kappaB. J Appl Physiol. 2008; 105(4):1333–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mendall MA, Patel P, Ballam L, Strachan D, Northfield TC. C reactive protein and its relation to cardiovascular risk factors: a population based cross sectional study. BMJ. 1996; 312(7038):1061–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harris TB, Ferrucci L, Tracy RP, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999; 106(5):506–512 [DOI] [PubMed] [Google Scholar]
- 13. Bruunsgaard H, Skinhøj P, Pedersen AN, Schroll M, Pedersen BK. Ageing, tumour necrosis factor-alpha (TNF-alpha) and atherosclerosis. Clin Exp Immunol. 2000; 121(2):255–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates TNF-alpha-induced activation of coronary arterial endothelial cells: role of NF-kappaB inhibition. Am J Physiol Heart Circ Physiol. 2006; 291(4):H1694–H1699 [DOI] [PubMed] [Google Scholar]
- 15. Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-tumor necrosis factor-alpha treatment in aging. Am J Pathol. 2007; 170(1):388–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Madej A, Okopien B, Kowalski J, et al. Effects of fenofibrate on plasma cytokine concentrations in patients with atherosclerosis and hyperlipoproteinemia IIb. Int J Clin Pharmacol Ther. 1998; 36(6):345–349 [PubMed] [Google Scholar]
- 17. Paolisso G, Rizzo MR, Mazziotti G, et al. Advancing age and insulin resistance: role of plasma tumor necrosis factor-alpha. Am J Physiol. 1998; 275(2 Pt 1):E294–E299 [DOI] [PubMed] [Google Scholar]
- 18. Arnett DK, Evans GW, Riley WA. Arterial stiffness: a new cardiovascular risk factor? Am J Epidemiol. 1994; 140(8):669–682 [DOI] [PubMed] [Google Scholar]
- 19. Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000; 102(11):1270–1275 [DOI] [PubMed] [Google Scholar]
- 20. Cohn JN, Finkelstein S, McVeigh G, et al. Noninvasive pulse wave analysis for the early detection of vascular disease. Hypertension. 1995; 26(3):503–508 [DOI] [PubMed] [Google Scholar]
- 21. Hale S. Statistical Essays. Haemostaticks II. New York: Hafner Publishing; 1964. [Google Scholar]
- 22. Pearson T, Mensah G, Alexander R, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003; 107(3):499–511 [DOI] [PubMed] [Google Scholar]
- 23. Arenas IA, Xu Y, Davidge ST. Age-associated impairment in vasorelaxation to fluid shear stress in the female vasculature is improved by TNF-alpha antagonism. Am J Physiol Heart Circ Physiol. 2006; 290(3):H1259–H1263 [DOI] [PubMed] [Google Scholar]
- 24. Ungvari Z, Csiszar A, Edwards JG, et al. Increased superoxide production in coronary arteries in hyperhomocysteinemia: role of tumor necrosis factor-alpha, NAD(P)H oxidase, and inducible nitric oxide synthase. Arterioscler Thromb Vasc Biol. 2003; 23(3):418–424 [DOI] [PubMed] [Google Scholar]
- 25. Lieb W, Larson MG, Benjamin EJ, et al. Multimarker approach to evaluate correlates of vascular stiffness: the Framingham Heart Study. Circulation. 2009; 119(1):37–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yasmin McEniery CM, Wallace S, Mackenzie IS, Cockcroft JR, Wilkinson IB. C-reactive protein is associated with arterial stiffness in apparently healthy individuals. Arterioscler Thromb Vasc Biol. 2004; 24(5):969–974 [DOI] [PubMed] [Google Scholar]
- 27. Giles TD, Berk BC, Black HR, et al. Expanding the definition and classification of hypertension. J Clin Hypertens (Greenwich). 2005; 7(9):505–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mathieu P, Poirier P, Pibarot P, Lemieux I, Després J. Visceral obesity: the link among inflammation, hypertension, and cardiovascular disease. Hypertension. 2009; 53(4):577–584 [DOI] [PubMed] [Google Scholar]
- 29. Fisher G, Hyatt TC, Hunter GR, Oster RA, Desmond RA, Gower BA. Effect of diet with and without exercise training on markers of inflammation and fat distribution in overweight women. Obesity (Silver Spring). 2011; 19(6):1131–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Després J. Inflammation and cardiovascular disease: is abdominal obesity the missing link? Int J Obes Relat Metab Disord. 2003; 27(Suppl 3):S22–S24 [DOI] [PubMed] [Google Scholar]
- 31. Cartier A, Lemieux I, Alméras N, Tremblay A, Bergeron J, Després J. Visceral obesity and plasma glucose-insulin homeostasis: contributions of interleukin-6 and tumor necrosis factor-alpha in men. J Clin Endocrinol Metab. 2008; 93(5):1931–1938 [DOI] [PubMed] [Google Scholar]
- 32. DeSouza CA, Shapiro LF, Clevenger CM, et al. Regular aerobic exercise prevents and restores age-related declines in endothelium- dependent vasodilation in healthy men. Circulation. 2000; 102 (12):1351–1357 [DOI] [PubMed] [Google Scholar]
- 33. Newcomer SC, Leuenberger UA, Hogeman CS, Proctor DN. Heterogeneous vasodilator responses of human limbs: influence of age and habitual endurance training. Am J Physiol Heart Circ Physiol. 2005; 289(1):H308–H315 [DOI] [PubMed] [Google Scholar]
- 34. Pierce GL, Eskurza I, Walker AE, Fay TN, Seals DR. Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin Sci (Lond). 2011; 120(1):13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]