Abstract
Toxicity assessment is a major challenge for cost-effective drug development, and there is great need for better tools to accurately predict adverse drug reactions. Technological advances are empowering new cell-based assays for predictive toxicology, and these assays are critically dependent on the cell source. Here we describe the properties of human induced pluripotent stem (iPS) cells that make them a promising cell source for toxicity assessment and highlight progress to date and important roadblocks remaining.
CURRENT LIMITATIONS OF PREDICTIVE TOXICOLOGY
Predicting drug toxicity remains one of the greatest limitations for efficient development of safe pharmaceuticals and is a major contributor to the high cost of drug development. Although extensive efforts at identifying the toxicity profile of compounds use a variety of in vitro and animal models prior to clinical testing, up to 40% of the drugs tested in clinical trials are abandoned because of unanticipated toxic effects.(1) Despite a steady flow of new assays, the success in bringing truly novel compounds to market has declined in recent years. Furthermore, adverse drug reactions in clinically marketed compounds remain a major cause of morbidity and mortality with some estimates suggesting adverse drug reactions as the fourth most common cause of death in the United States.(2) Therefore, new approaches are greatly needed to improve predictive toxicology
There are multiple opportunities for improvement within the current approach for drug safety assessment. The central components of a safety program required prior to dosing in human subjects are mandated by regulatory agencies and rely heavily upon characterizing toxicity in animals dosed. Animal studies are hampered by several factors, including: an imperfect correlation of toxicity patterns between animal models and human studies, cost, and a growing pressure to reduce, refine, and replace animal use if alternative approaches are available. In vitro studies have been increasingly used, especially early in clinical candidate profiling, but their inadequate predictive value is not suitable to replace animal studies and has prompted regulatory agencies to introduce initiatives to improve in vitro toxicity prediction (e.g., parts of the FDA’s Critical Path Initiative and the EU’s Seventh Research Framework Programme) as well as to develop better in vitro assays to evaluate the impact of hazardous compounds in the environment, as recommended by a 2007 National Academy of Science report.(3) However, most existing in vitro assays are substandard since they are rely on immortalized cell lines or isolated primary cells (see Table 1). Immortalized cell lines are genetically altered, typically aneuploid, and may exhibit clinically irrelevant toxic responses to compounds. Isolated cells from animal tissues lose their in vivo phenotype, can exhibit high variability from isolation to isolation, and can often only be expanded by dedifferentiation. Species differences will also potentially impact the results. Many of these limitations can be overcome by using human pluripotent stem cells. Readily expandable, karyotypically normal, human embryonic stem (ES) cells and iPS cells are promising cell sources for in vitro toxicity assays.
Table 1.
Comparison of cell preparations for in vitro toxicity testing
| Cell Source | Origin | Quantity | Genetic Stability | Genetic Diversity | Disease Model | Gene Targeting |
|---|---|---|---|---|---|---|
| Transformed cells | Immortalized or tumor cells | Unlimited | Often aneuploid with drift | Minimal | Possible and sometimes engineered | Yes, but limited |
| Primary cells | Primary tissue isolation | Limited | Diploid, stable | Moderate | Intrinsic mutations possible | No |
| ES cells | Embryo | Unlimited | Diploid, relatively stable | Moderate | For abnormal Preimpl testing or engineered | Yes |
| iPS cells | Somatic cells | Unlimited | Diploid, unknown | Great | Greatest with direct sampling of diverse patients | Yes |
FEATURES OF iPS CELLS ENABLING IN VITRO MODELS
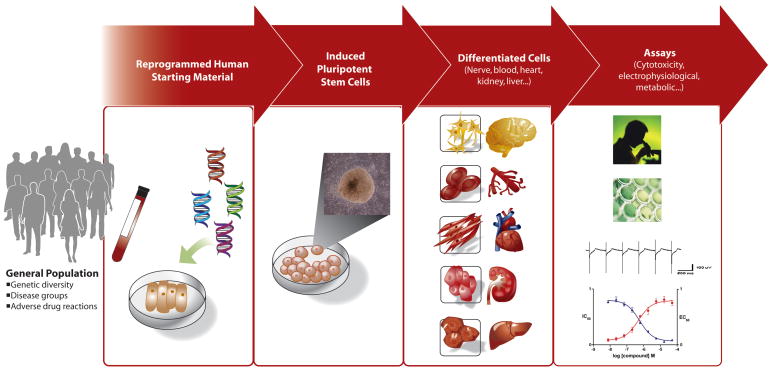
The successful reprogramming of human somatic cells to iPS cells has opened powerful new avenues for predictive in vitro models.(4, 5) iPS cells share many properties with ES cells including self-renewal and pluripotency. However, the ability to generate iPS cells from a variety of diseased and normal human phenotypes is a substantial advantage over ES cells. Access to a theoretically unlimited supply of human iPS-derived cells, such as cardiomyocytes, hepatocytes, and neurons, from a diverse population opens the door for powerful genetic and epigenetic experiments that previously were impossible to conduct. Readily obtained tissues like skin,(6–8) peripheral blood,(9) or other somatic tissues can be used to generate large libraries of genetically diverse iPS cell lines (Figure 1). Such iPS libraries can be used for “preclinical” human trials using cell-based assays that will ideally reflect the diversity of drug responses in the population. Secondly, the ability to derive iPS cells directly from patient samples enables creation of patient- and disease-specific iPS cell lines which provide powerful in vitro disease models.(6–8) Disease iPS cell lines will enable not only toxicity testing but likely will be an important tool in the drug discovery process. In addition, generation of iPS cells from patients who exhibit specific side effect profiles or idiosyncratic reaction to drugs may prove useful in screening for relatively rare but serious toxic effects. The potential of patient-specific iPS cells to be able to identify patients that would respond adversely or favorably to a drug could provide powerful new tools for personalized medicine.
Figure 1.
Generation and utilization of human iPS cells in predictive toxicology. Somatic cells from skin, blood, fat or other tissues can be obtained from consenting donors for transcription factor-based reprogramming to iPS cells. The starting material can provide broad genetic diversity and represent distinct disease phenotypes as well as adverse drug reactions. The iPS cells are differentiated into specific cell lineages which can be characterized in vitro using a wide range of assays for toxicity.
DEVELOPMENTAL TOXICITY
Developing human embryos can be highly sensitive to the effects of drugs or chemicals, and determination of fetal toxicity from maternal exposure to compounds is a key component of toxicity risk assessment. Spontaneous differentiation of mouse ES cells into beating embryoid bodies is currently used by the pharmaceutical industry as an in vitro model to predict teratogenicity.(10) While other models have been assessed, the mouse embryonic stem cell test (mEST) has been deemed the most promising assay by the European Centre for the Validation of Alternative Methods (ECVAM).(11) However, recent publications have revealed a substantially lower assay performance, attributed in part to limitations to the training set used by ECVAM.(12)
While the mEST assay is successful at predicting non-developmental toxicants, an area for performance improvement is in embyrotoxic and human-specific teratogens. The recent reevaluation of the mEST by the Integrated Project ReProTect recommended new molecular end points and reporter gene approaches.(12, 13) Perhaps of most predictive gain, though, would be to integrate new molecular approaches with the use of human ES or iPS cells which would avoid cross-species extrapolation.(14) Experiments have recently been reported using human ES cells and have demonstrated predictive improvement over mEST when using molecular markers monitoring cardiac differentiation as well as metabolomics.(14, 15) Thus, the integration of advances in the human stem cells with insight into the molecular aspects of tissue differentiation is a promising approach to improve the ability to assess the risk of human teratogenicity.
HEPATOTOXICITY
Xenobiotic-induced hepatotoxicty arises from a variety of mechanisms including reactive metabolites, formation of reactive oxygen species, and inhibition of cytochrome P-450 and/or transporter activity.(16, 17) While readily available and expandable, non-hepatic cell line models only vaguely resemble hepatocytes and are suited primarily to assess generalized irreversible cytotoxic endpoints such as cell death (19). Primary human hepatocytes maintain enough metabolic and functional properties of intact liver to be useful in predictive drug-induced liver injury studies.(18, 19) However, primary human hepatocytes have sporadic and limited accessibility, high intrinsic variability, phenotypic instability, and display a rapid loss of metabolic functionality once they are placed into culture.(20) Human pluripotent stem cell-derived hepatocytes hold promise for overcoming limitations of primary hepatocyte preparations. Stem cell-derived hepatocytes exhibit CYP450 metabolism, albumin production, glycogen storage, and uptake and excretion of indocyanine green; however, current hepatic differentiation protocols result in cells that have lower enzyme activities compared to intact human liver.(21) Rapid progress in differentiation protocols and cell culture likely will provide more functionally robust hepatocytes. Further into the future, hepatocytes from iPS cells generated from specific patients afflicted with idiosyncratic hepatotoxicity may provide insight into the role of genetic diversity in drug-induced injury. Generating panels of iPS cells to reflect the diverse CYP450 profiles seen in the human population will enable studies of human drug metabolism and drug-drug interactions.
CARDIOTOXICITY
Drug-induced cardiotoxicity takes two primary forms: electrophysiological and biochemical. Electrophysiological toxicities arise when compounds interact with ion channels or transporters to create a proarrhythmic condition whereby patients are at increased risk for developing arrhythmias including life-threatening ones such as Torsades de Pointes (TdP).(22) The most common drug-induced proarrhythmic condition manifests as an increased QT interval on the surface EKG, most frequently arising from prolongation of the action potential through block of the human ether-a-go-go (hERG) channel.(22) However, additional ion channels are known to play roles as well.(23, 24)
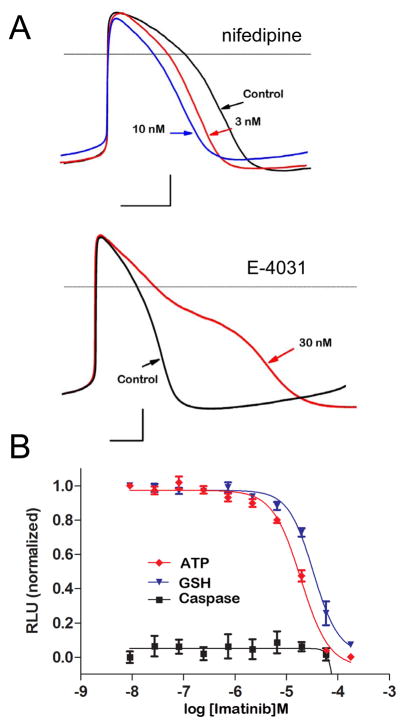
Human pluripotent stem cells can differentiate into functional cardiomyocytes that exhibit hallmark electrophysiology properties such as cardiac action potentials.(25, 26) Although the action potentials are embyronic in phenotype, these cells nonethelessexpress many of the same ion channels present in the adult human heart. Thus human stem cell-derived cardiomyocytes can be used to screen for effects of compounds on cardiac ion channels, and as predicted, drugs that block L-type Ca2+ channels shorten the plateau phase of the AP while compounds that block IKr prolong the AP duration (Figure 2A). These human cardiomyocytes hold advantages as a screening platform over traditional heterologous expression systems in which single ion channels are over-expressed, and they avoid the complications of cross-species result translation. However, a limitation of current differentiation protocols is the production of a heterogeneous mixture of cardiomyocyte cell types with predominantly ventricular-like cells but also atrial-like and nodal-like cells.(25, 26)
Figure 2.
Toxicity assays using human iPS cell-derived cardiomyocytes. (A) Electrophysiological toxicity was assayed using the perforated patch clamp technique on single human iPSC-derived cardiomyocytes. Action potential duration was shortened by the L-type Ca2+ channel blocker nifedipine (upper) and prolonged by the hERG K+ channel blocker E-4031 (lower). Scale bars; 100ms by 20mV. (B) Biochemical toxicity was assayed by measuring ATP, GSH, and Caspase activity using CellTiter-, GSH-, and Caspase-Glo® 3/7 assay kits, respectively, in a 96 well format of cultured iPS cell-derived cardiomyocytes. Imatinib (Gleevac) produced a dose-dependent decrease in cell viability (ATP decrease) and increased oxidative stress (reduction in GSH) without activating apoptotic pathways (unchanged caspase).
Unlike electrophysiological toxicity, biochemical cardiotoxicity is less well understood.(27) Anthracyclines such as doxorubicin are anti-cancer therapeutics that exhibit classical cardiotoxicity through mechanisms that appear to be independent of its DNA binding ability, potentially through increased reactive oxygen species (ROS) activity.(28) Small molecule kinase inhibitors (KIs) are another class of anti-neoplastic compounds with reported cardiotoxic side effects.(27) KIs target specific and/or multiple kinase pathways present in the cancerous cells, yet show cardiotoxic side effects as many of these same pathways are also expressed in the heart.(27) Human iPS cell-derived cardiomyocytes recapitulate many of the in vivo cardiomyocyte functions and may be an ideal system for assessing multiple facets of toxicity as shown in Figure 2B where application of Imatinib (Gleevac) caused a decrease in viability and glutathione levels, without inducing caspase-mediated apoptosis. As the mechanisms underlying cardiotoxicity are poorly understood and often unpredictable, testing in systems that closely mimic the in vivo condition with appropriate measurable endpoints is imperative. In vivo-like systems would be expected to detect changes to tissue-specific functionality such a contractility. Native-like cardiotoxicity models employing cardio-specific biomarkers such as the release of cardiac troponin may prove to be more sensitive than the traditional irreversible endpoints such as ATP, LDH, or XTT.(29) Likewise, toxicity of the intended therapeutic target should be examined in a relevant cell system as one needs to know the ability of redundant pathways to compensate for the reduced activity of the targeted pathway and/or the extent to which the unwanted toxicity can be engineered out or overcome.(27)
FUTURE CHALLENGES AND OPPORTUNITIES
Substantial challenges remain before the full promise of these cells in predictive toxicology can be realized. The technology to generate iPS cells will continue its rapid evolution to facilitate the efficient production of libraries of optimally reprogrammed iPS cells. Although progress has been made in the differentiation protocols, the preparations can vary and can contain heterogeneous mixturesof cell types. Thus refining protocols to obtain the desired cell type will continue to be a focus of effort such as through the use of tissue and lineage specific promoters coupled with antibiotic resistance genes.(30). Likewise, the advent of directed differentiation protocols towards other adult cell types such as spleen, kidney, and others will broaden the landscape of potential toxicity assays. Furthermore, the relative maturity of the iPS cell-derived cell lineages remains a potential limitation, since most cell types maintain a more embryonic phenotype in culture. Future efforts to combine various cell types and construct complex tissue and organ models for testing may be particularly powerful.
CONCLUSIONS
Utilizing human iPS cell-derived cell lineages early in the drug development pipeline holds promise to enable early, rapid, and more comprehensive toxicity screening across general populations as well as targeted and at risk populations than current technologies. There remain substantial challenges before the full promise of iPS cells for predictive toxicology can be realized including more effective ways to differentiate cells to the desired lineage or tissue constructs in addition to optimizing assays with these preparations. Progress using iPS cell-derived cardiomyocytes and hepatocytes is beginning to validate the approach for the two most common areas of toxicity encountered in drug development. Ultimately, using iPS cell derivatives throughout the drug discovery pipeline can be expected to reduce late phase compound attrition and thus cost as well as increase consumer safety.
Acknowledgments
The authors gratefully acknowledge Joleen Rau for assistance with illustrations.
Footnotes
CONFLICT OF INTEREST / DISCLOSURES
TJK is a co-founder and consultant for Cellular Dynamics International which is a company that performs toxicity testing with cellular products derived from human iPS cells.
References
- 1.Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004;3:711–5. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- 2.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279:1200–5. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 3.Applications of Toxicogenomic Technologies to Predictive Toxicology and Risk Assessment. The National Academies Press; Washington, D.C: 2007. [PubMed] [Google Scholar]
- 4.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Yu J, Thomson JA. Pluripotent stem cell lines. Genes Dev. 2008;22:1987–97. doi: 10.1101/gad.1689808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–86. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moretti A, Bellin M, Welling A, Jung CB, Lam JT, Bott-Flugel L, et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med. 2010;363:1397–409. doi: 10.1056/NEJMoa0908679. [DOI] [PubMed] [Google Scholar]
- 8.Ebert AD, Yu J, Rose FF, Jr, Mattis VB, Lorson CL, Thomson JA, et al. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–80. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown ME, Rondon E, Rajesh D, Mack A, Lewis R, Feng X, et al. Derivation of induced pluripotent stem cells from human peripheral blood T lymphocytes. PLoS One. 5:e11373. doi: 10.1371/journal.pone.0011373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heuer J, Bremer S, Pohl I, Spielmann H. Development of an in vitro embryotoxicity test using murine embryonic stem cell cultures. Toxicol In Vitro. 1993;7:551–6. doi: 10.1016/0887-2333(93)90064-c. [DOI] [PubMed] [Google Scholar]
- 11.Genschow E, Spielmann H, Scholz G, Pohl I, Seiler A, Clemann N, et al. Validation of the embryonic stem cell test in the international ECVAM validation study on three in vitro embryotoxicity tests. Altern Lab Anim. 2004;32:209–44. doi: 10.1177/026119290403200305. [DOI] [PubMed] [Google Scholar]
- 12.Paquette JA, Kumpf SW, Streck RD, Thomson JJ, Chapin RE, Stedman DB. Assessment of the Embryonic Stem Cell Test and application and use in the pharmaceutical industry. Birth Defects Res B Dev Reprod Toxicol. 2008;83:104–11. doi: 10.1002/bdrb.20148. [DOI] [PubMed] [Google Scholar]
- 13.Schenk B, Weimer M, Bremer S, van der Burg B, Cortvrindt R, Freyberger A, et al. The ReProTect Feasibility Study, a novel comprehensive in vitro approach to detect reproductive toxicants. Reprod Toxicol. 2010;30:200–18. doi: 10.1016/j.reprotox.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Adler S, Pellizzer C, Hareng L, Hartung T, Bremer S. First steps in establishing a developmental toxicity test method based on human embryonic stem cells. Toxicol In Vitro. 2008;22:200–11. doi: 10.1016/j.tiv.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 15.West PR, Weir AM, Smith AM, Donley EL, Cezar GG. Predicting human developmental toxicity of pharmaceuticals using human embryonic stem cells and metabolomics. Toxicol Appl Pharmacol. 2010;247:18–27. doi: 10.1016/j.taap.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Lechon MJ, Castell JV, Donato MT. The use of hepatocytes to investigate drug toxicity. Methods Mol Biol. 2010;640:389–415. doi: 10.1007/978-1-60761-688-7_21. [DOI] [PubMed] [Google Scholar]
- 17.Gunawan BK, Kaplowitz N. Mechanisms of drug-induced liver disease. Clin Liver Dis. 2007;11:459–75. v. doi: 10.1016/j.cld.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Dambach DM, Andrews BA, Moulin F. New technologies and screening strategies for hepatotoxicity: use of in vitro models. Toxicol Pathol. 2005;33:17–26. doi: 10.1080/01926230590522284. [DOI] [PubMed] [Google Scholar]
- 19.Xu JJ, Diaz D, O’Brien PJ. Applications of cytotoxicity assays and pre-lethal mechanistic assays for assessment of human hepatotoxicity potential. Chem Biol Interact. 2004;150:115–28. doi: 10.1016/j.cbi.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Lechon MJ, Donato MT, Castell JV, Jover R. Human hepatocytes as a tool for studying toxicity and drug metabolism. Curr Drug Metab. 2003;4:292–312. doi: 10.2174/1389200033489424. [DOI] [PubMed] [Google Scholar]
- 21.Guguen-Guillouzo C, Corlu A, Guillouzo A. Stem cell-derived hepatocytes and their use in toxicology. Toxicology. 2010;270:3–9. doi: 10.1016/j.tox.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 22.Kannankeril PJ, Roden DM. Drug-induced long QT and torsade de pointes: recent advances. Curr Opin Cardiol. 2007;22:39–43. doi: 10.1097/HCO.0b013e32801129eb. [DOI] [PubMed] [Google Scholar]
- 23.Lacerda AE, Kuryshev YA, Chen Y, Renganathan M, Eng H, Danthi SJ, et al. Alfuzosin delays cardiac repolarization by a novel mechanism. J Pharmacol Exp Ther. 2008;324:427–33. doi: 10.1124/jpet.107.128405. [DOI] [PubMed] [Google Scholar]
- 24.Towart R, Linders JT, Hermans AN, Rohrbacher J, van der Linde HJ, Ercken M, et al. Blockade of the I(Ks) potassium channel: an overlooked cardiovascular liability in drug safety screening? J Pharmacol Toxicol Methods. 2009;60:1–10. doi: 10.1016/j.vascn.2009.04.197. [DOI] [PubMed] [Google Scholar]
- 25.He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. CircRes. 2003;93:32–9. doi: 10.1161/01.RES.0000080317.92718.99. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104:e30–41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng H, Force T. Molecular mechanisms of cardiovascular toxicity of targeted cancer therapeutics. Circ Res. 2010;106:21–34. doi: 10.1161/CIRCRESAHA.109.206920. [DOI] [PubMed] [Google Scholar]
- 28.Ferreira AL, Matsubara LS, Matsubara BB. Anthracycline-induced cardiotoxicity. Cardiovasc Hematol Agents Med Chem. 2008;6:278–81. doi: 10.2174/187152508785909474. [DOI] [PubMed] [Google Scholar]
- 29.Urbanova D, Urban L, Carter A, Maasova D, Mladosievicova B. Cardiac troponins--biochemical markers of cardiac toxicity after cytostatic therapy. Neoplasma. 2006;53:183–90. [PubMed] [Google Scholar]
- 30.Klug MG, Soonpaa MH, Koh GY, Field LJ. Genetically selected cardiomyocytes from differentiating embronic stem cells form stable intracardiac grafts. JClinInvest. 1996;98:216–24. doi: 10.1172/JCI118769. [DOI] [PMC free article] [PubMed] [Google Scholar]