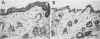Abstract
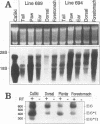
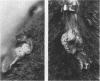
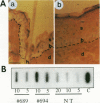
The E6 and E7 early genes of human papillomavirus type 16 have been shown in vitro to play a central role in the transforming capability of this virus. To explore their effects on differentiating epithelial cells in vivo, we used a bovine cytokeratin 10 (K10) promoter to target the expression of E6 and E7 to the suprabasal layers of the epidermis of transgenic mice. In two different lines of mice efficiently expressing the transgene, animals displayed generalized epidermal hyperplasia, hyperkeratosis and parakeratosis in the skin and the forestomach, both known to be sites of K10 expression. Northern (RNA) blot analysis revealed high levels of E6 and E7 transcripts, and in situ hybridizations localized these transcripts to the suprabasal strata of epidermis. In vivo labeling of proliferating cells showed two distinct effects of E6 and E7 expression in the epidermis: (i) an increase in the number of growing cells in the undifferentiated basal layer and (ii) abnormal proliferation of differentiated cells in the suprabasal strata. The expression of c-myc in the skin of transgenics was higher than that in control animals. The induction of c-myc transcription by topical application of tetradecanoyl phorbol acetate was prevented by simultaneous treatment with transforming growth factor beta 1 in nontransgenic skin but not in transgenic skin. In addition, transforming growth factor alpha was found to be overexpressed in the suprabasal layers of the transgenic epidermis. These findings suggest that autocrine mechanisms are involved in the development and maintenance of epidermal hyperplasia. Animals of both lines developed papillomas in skin sites exposed to mechanical irritation and wounding, suggesting that secondary events are necessary for progression to neoplasia. Collectively, these results provide new insights into the tumor promoter activities of human papillomavirus type 16 in epithelial cells in vivo.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbeit J. M., Münger K., Howley P. M., Hanahan D. Progressive squamous epithelial neoplasia in K14-human papillomavirus type 16 transgenic mice. J Virol. 1994 Jul;68(7):4358–4368. doi: 10.1128/jvi.68.7.4358-4368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailleul B., Surani M. A., White S., Barton S. C., Brown K., Blessing M., Jorcano J., Balmain A. Skin hyperkeratosis and papilloma formation in transgenic mice expressing a ras oncogene from a suprabasal keratin promoter. Cell. 1990 Aug 24;62(4):697–708. doi: 10.1016/0092-8674(90)90115-u. [DOI] [PubMed] [Google Scholar]
- Barnard J. A., Lyons R. M., Moses H. L. The cell biology of transforming growth factor beta. Biochim Biophys Acta. 1990 Jun 1;1032(1):79–87. doi: 10.1016/0304-419x(90)90013-q. [DOI] [PubMed] [Google Scholar]
- Bedell M. A., Jones K. H., Laimins L. A. The E6-E7 region of human papillomavirus type 18 is sufficient for transformation of NIH 3T3 and rat-1 cells. J Virol. 1987 Nov;61(11):3635–3640. doi: 10.1128/jvi.61.11.3635-3640.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beermann F., Ruppert S., Hummler E., Bosch F. X., Müller G., Rüther U., Schütz G. Rescue of the albino phenotype by introduction of a functional tyrosinase gene into mice. EMBO J. 1990 Sep;9(9):2819–2826. doi: 10.1002/j.1460-2075.1990.tb07470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing M., Jorcano J. L., Franke W. W. Enhancer elements directing cell-type-specific expression of cytokeratin genes and changes of the epithelial cytoskeleton by transfections of hybrid cytokeratin genes. EMBO J. 1989 Jan;8(1):117–126. doi: 10.1002/j.1460-2075.1989.tb03355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing M., Nanney L. B., King L. E., Jones C. M., Hogan B. L. Transgenic mice as a model to study the role of TGF-beta-related molecules in hair follicles. Genes Dev. 1993 Feb;7(2):204–215. doi: 10.1101/gad.7.2.204. [DOI] [PubMed] [Google Scholar]
- Bravo R., Frank R., Blundell P. A., Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987 Apr 2;326(6112):515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- Celis J. E., Fey S. J., Larsen P. M., Celis A. Expression of the transformation-sensitive protein "cyclin" in normal human epidermal basal cells and simian virus 40-transformed keratinocytes. Proc Natl Acad Sci U S A. 1984 May;81(10):3128–3132. doi: 10.1073/pnas.81.10.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cid A., Auewarakul P., Garcia-Carranca A., Ovseiovich R., Gaissert H., Gissmann L. Cell-type-specific activity of the human papillomavirus type 18 upstream regulatory region in transgenic mice and its modulation by tetradecanoyl phorbol acetate and glucocorticoids. J Virol. 1993 Nov;67(11):6742–6752. doi: 10.1128/jvi.67.11.6742-6752.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran B. H., Zullo J., Verma I. M., Stiles C. D. Expression of the c-fos gene and of an fos-related gene is stimulated by platelet-derived growth factor. Science. 1984 Nov 30;226(4678):1080–1082. doi: 10.1126/science.6093261. [DOI] [PubMed] [Google Scholar]
- Crook T., Morgenstern J. P., Crawford L., Banks L. Continued expression of HPV-16 E7 protein is required for maintenance of the transformed phenotype of cells co-transformed by HPV-16 plus EJ-ras. EMBO J. 1989 Feb;8(2):513–519. doi: 10.1002/j.1460-2075.1989.tb03405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R., Hicks R., Crook T., Morris J., Vousden K. Human papillomavirus type 16 E7 associates with a histone H1 kinase and with p107 through sequences necessary for transformation. J Virol. 1993 May;67(5):2521–2528. doi: 10.1128/jvi.67.5.2521-2528.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R. Transforming growth factor alpha. Cell. 1988 Aug 26;54(5):593–595. doi: 10.1016/s0092-8674(88)80001-1. [DOI] [PubMed] [Google Scholar]
- DiGiovanni J. Multistage carcinogenesis in mouse skin. Pharmacol Ther. 1992;54(1):63–128. doi: 10.1016/0163-7258(92)90051-z. [DOI] [PubMed] [Google Scholar]
- Dyson N., Guida P., Münger K., Harlow E. Homologous sequences in adenovirus E1A and human papillomavirus E7 proteins mediate interaction with the same set of cellular proteins. J Virol. 1992 Dec;66(12):6893–6902. doi: 10.1128/jvi.66.12.6893-6902.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson N., Howley P. M., Münger K., Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989 Feb 17;243(4893):934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- Dürst M., Glitz D., Schneider A., zur Hausen H. Human papillomavirus type 16 (HPV 16) gene expression and DNA replication in cervical neoplasia: analysis by in situ hybridization. Virology. 1992 Jul;189(1):132–140. doi: 10.1016/0042-6822(92)90688-l. [DOI] [PubMed] [Google Scholar]
- Elder J. T., Fisher G. J., Lindquist P. B., Bennett G. L., Pittelkow M. R., Coffey R. J., Jr, Ellingsworth L., Derynck R., Voorhees J. J. Overexpression of transforming growth factor alpha in psoriatic epidermis. Science. 1989 Feb 10;243(4892):811–814. doi: 10.1126/science.2916128. [DOI] [PubMed] [Google Scholar]
- Glick A. B., Kulkarni A. B., Tennenbaum T., Hennings H., Flanders K. C., O'Reilly M., Sporn M. B., Karlsson S., Yuspa S. H. Loss of expression of transforming growth factor beta in skin and skin tumors is associated with hyperproliferation and a high risk for malignant conversion. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6076–6080. doi: 10.1073/pnas.90.13.6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howley P. M. Role of the human papillomaviruses in human cancer. Cancer Res. 1991 Sep 15;51(18 Suppl):5019s–5022s. [PubMed] [Google Scholar]
- Huszar M., Gigi-Leitner O., Moll R., Franke W. W., Geiger B. Monoclonal antibodies to various acidic (type I) cytokeratins of stratified epithelia. Selective markers for stratification and squamous cell carcinomas. Differentiation. 1986;31(2):141–153. doi: 10.1111/j.1432-0436.1986.tb00395.x. [DOI] [PubMed] [Google Scholar]
- Kanda T., Watanabe S., Yoshiike K. Immortalization of primary rat cells by human papillomavirus type 16 subgenomic DNA fragments controlled by the SV40 promoter. Virology. 1988 Jul;165(1):321–325. doi: 10.1016/0042-6822(88)90694-0. [DOI] [PubMed] [Google Scholar]
- Kondoh G., Murata Y., Aozasa K., Yutsudo M., Hakura A. Very high incidence of germ cell tumorigenesis (seminomagenesis) in human papillomavirus type 16 transgenic mice. J Virol. 1991 Jun;65(6):3335–3339. doi: 10.1128/jvi.65.6.3335-3339.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey M., Alpert S., Hanahan D. Bovine papillomavirus genome elicits skin tumours in transgenic mice. Nature. 1986 Aug 14;322(6080):609–612. doi: 10.1038/322609a0. [DOI] [PubMed] [Google Scholar]
- Lambert P. F., Pan H., Pitot H. C., Liem A., Jackson M., Griep A. E. Epidermal cancer associated with expression of human papillomavirus type 16 E6 and E7 oncogenes in the skin of transgenic mice. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5583–5587. doi: 10.1073/pnas.90.12.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. Epidermal growth factor-like transforming growth factor. II. Interaction with epidermal growth factor receptors in human placenta membranes and A431 cells. J Biol Chem. 1983 Nov 25;258(22):13614–13620. [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Moses H. L., Yang E. Y., Pietenpol J. A. TGF-beta stimulation and inhibition of cell proliferation: new mechanistic insights. Cell. 1990 Oct 19;63(2):245–247. doi: 10.1016/0092-8674(90)90155-8. [DOI] [PubMed] [Google Scholar]
- Münger K., Phelps W. C., Bubb V., Howley P. M., Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989 Oct;63(10):4417–4421. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano M., Draetta G., Jansen-Dürr P. Association of cdk2 kinase with the transcription factor E2F during S phase. Science. 1992 Feb 28;255(5048):1144–1147. doi: 10.1126/science.1312258. [DOI] [PubMed] [Google Scholar]
- Peacock J. W., Benchimol S. Mutation of the endogenous p53 gene in cells transformed by HPV-16 E7 and EJ c-ras confers a growth advantage involving an autocrine mechanism. EMBO J. 1994 Mar 1;13(5):1084–1092. doi: 10.1002/j.1460-2075.1994.tb06357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps W. C., Yee C. L., Münger K., Howley P. M. The human papillomavirus type 16 E7 gene encodes transactivation and transformation functions similar to those of adenovirus E1A. Cell. 1988 May 20;53(4):539–547. doi: 10.1016/0092-8674(88)90570-3. [DOI] [PubMed] [Google Scholar]
- Pietenpol J. A., Stein R. W., Moran E., Yaciuk P., Schlegel R., Lyons R. M., Pittelkow M. R., Münger K., Howley P. M., Moses H. L. TGF-beta 1 inhibition of c-myc transcription and growth in keratinocytes is abrogated by viral transforming proteins with pRB binding domains. Cell. 1990 Jun 1;61(5):777–785. doi: 10.1016/0092-8674(90)90188-k. [DOI] [PubMed] [Google Scholar]
- Riou G., Barrois M., Lê M. G., George M., Le Doussal V., Haie C. C-myc proto-oncogene expression and prognosis in early carcinoma of the uterine cervix. Lancet. 1987 Apr 4;1(8536):761–763. doi: 10.1016/s0140-6736(87)92795-4. [DOI] [PubMed] [Google Scholar]
- Rose-John S., Fürstenberger G., Krieg P., Besemfelder E., Rincke G., Marks F. Differential effects of phorbol esters on c-fos and c-myc and ornithine decarboxylase gene expression in mouse skin in vivo. Carcinogenesis. 1988 May;9(5):831–835. doi: 10.1093/carcin/9.5.831. [DOI] [PubMed] [Google Scholar]
- Rowson K. E., Mahy B. W. Human papova (wart) virus. Bacteriol Rev. 1967 Jun;31(2):110–131. doi: 10.1128/br.31.2.110-131.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer J., Rentrop M., Nischt R., Kinjo M., Winter H. The intermediate filament system of the keratinizing mouse forestomach epithelium: coexpression of keratins of internal squamous epithelia and of epidermal keratins in differentiating cells. Cell Tissue Res. 1988 Jul;253(1):221–229. doi: 10.1007/BF00221757. [DOI] [PubMed] [Google Scholar]
- Smotkin D., Prokoph H., Wettstein F. O. Oncogenic and nononcogenic human genital papillomaviruses generate the E7 mRNA by different mechanisms. J Virol. 1989 Mar;63(3):1441–1447. doi: 10.1128/jvi.63.3.1441-1447.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotkin D., Wettstein F. O. Transcription of human papillomavirus type 16 early genes in a cervical cancer and a cancer-derived cell line and identification of the E7 protein. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4680–4684. doi: 10.1073/pnas.83.13.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoler M. H., Rhodes C. R., Whitbeck A., Wolinsky S. M., Chow L. T., Broker T. R. Human papillomavirus type 16 and 18 gene expression in cervical neoplasias. Hum Pathol. 1992 Feb;23(2):117–128. doi: 10.1016/0046-8177(92)90232-r. [DOI] [PubMed] [Google Scholar]
- Tommasino M., Adamczewski J. P., Carlotti F., Barth C. F., Manetti R., Contorni M., Cavalieri F., Hunt T., Crawford L. HPV16 E7 protein associates with the protein kinase p33CDK2 and cyclin A. Oncogene. 1993 Jan;8(1):195–202. [PubMed] [Google Scholar]
- Vassar R., Fuchs E. Transgenic mice provide new insights into the role of TGF-alpha during epidermal development and differentiation. Genes Dev. 1991 May;5(5):714–727. doi: 10.1101/gad.5.5.714. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Kanda T., Yoshiike K. Human papillomavirus type 16 transformation of primary human embryonic fibroblasts requires expression of open reading frames E6 and E7. J Virol. 1989 Feb;63(2):965–969. doi: 10.1128/jvi.63.2.965-969.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S., Weinberg W., Liao X., Peters K. G., Blessing M., Yuspa S. H., Weiner R. L., Williams L. T. Targeted expression of a dominant-negative FGF receptor mutant in the epidermis of transgenic mice reveals a role of FGF in keratinocyte organization and differentiation. EMBO J. 1993 Jul;12(7):2635–2643. doi: 10.1002/j.1460-2075.1993.tb05924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werness B. A., Levine A. J., Howley P. M. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990 Apr 6;248(4951):76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- Xynos F. P., Klos D. J., Hamilton P. D., Schuette V., Fernandez-Pol J. A. Expression of transforming growth factor alpha mRNA in benign and malignant tissues derived from gynecologic patients with various proliferative conditions. Anticancer Res. 1992 Jul-Aug;12(4):1115–1120. [PubMed] [Google Scholar]
- von Knebel Doeberitz M., Oltersdorf T., Schwarz E., Gissmann L. Correlation of modified human papilloma virus early gene expression with altered growth properties in C4-1 cervical carcinoma cells. Cancer Res. 1988 Jul 1;48(13):3780–3786. [PubMed] [Google Scholar]
- zur Hausen H. Viruses in human cancers. Science. 1991 Nov 22;254(5035):1167–1173. doi: 10.1126/science.1659743. [DOI] [PubMed] [Google Scholar]