Abstract
Background
Cardiac arrest occurs in >400 000 patients in the United States per year, and mortality rates vary across the country. Whether variations in cardiac arrest outcome are the result of differences in hospital or patient characteristics remains understudied. We tested whether hospital-independent factors would account for the difference in outcome between 2 geographically distinct hospitals.
Methods and Results
Consecutive adult (age >18 years) out-of-hospital cardiac arrests were considered for analysis. The primary outcome was in-hospital mortality. Predictor variables were classified according to whether they were hospital-independent or whether they could be related to the hospital’s quality of care. Only hospital-independent variables were considered for the analysis. Sequential logistic modeling was used to assess outcome. A propensity score was derived and was used in subsequent multivariate logistic regression to predict hospital outcome. A total of 208 subjects were included. Overall mortality in the Detroit cohort was 87% in comparison with 61% in the Boston cohort (odds ratio: 4.4; 95% confidence interval: 2.2– 8.8). After sequential adjustments for baseline covariates, out-of-hospital cardiac arrest score and propensity score, city was not significantly associated with mortality (odds ratio: 1.16; 95% confidence interval: 0.45–2.97). After propensity matching there was no significant difference in the odds ratio for death between the 2 cities (odds ratio: 1.15; 95% confidence interval: 0.51–2.61).
Conclusions
In this pilot study, we found that pre- and intra-arrest conditions contribute substantially to the severity of the postarrest syndrome and on outcomes. Postarrest quality-of-care evaluations should include inherent differences in the presenting syndrome rather than a crude mortality rate.
Keywords: cardiac arrest, out-of-hospital cardiac arrest, severity of illness
Cardiac arrest occurs in >400 000 patients in the United States per year,1 a figure that will likely increase with the aging population. Overall morbidity and mortality remain high, but outcomes from cardiac arrest vary markedly across the country. Factors including geography,2,3 hospital size, intensive care unit volume,4,5 and day of the week or time of day6 potentially influence outcomes from after cardiac arrest. The wide outcome variability has been labeled an “unacceptable disparity”7 and has led to calls for reform.
In a recent position statement by the American Heart Association, authors hypothesized that postarrest outcome is driven mainly by hospital-based care quality, so much so that individual patient characteristics become irrelevant: “Large interhospital variations exist in survival to hospital discharge after admission after successful resuscitation from out-of-hospital cardiac arrest. Such differences do not appear to be explained by differences in patient characteristics, which implies that variation in hospital-based care contributes to differences in outcomes across regions.”8 If this hypothesis is true, then hospitals could be graded on the quality care provided to patients after out-of-hospital cardiac arrest (OHCA) by using unadjusted in-hospital mortality rates. Evaluating and testing this hypothesis is highly important particularly given the recent movement toward grading healthcare quality based on unadjusted mortality (or perhaps adjusted without literature-based validation of technique). In addition, recent calls for OHCA to be considered a reportable event further accentuates the need to evaluate this hypothesis, because future quality comparisons nationwide could potentially shift from process measures to outcome measures.
To test the hypothesis that outcome variation results from hospital-based care with little contribution from patient characteristics (ie, prearrest and intra-arrest conditions), we set up a pilot comparison between 2 geographically distinct cities in the United States. Through the stepwise analysis of variables that can only be determined by pre- and intra-arrest conditions, we are able to adequately test the hypothesis that patient outcome for OHCA depends mostly (or only) on postarrest care provided in the in-hospital setting.
Methods
Design, Methods, and Patients
We sought to test whether factors that unequivocally preceded hospitalization would account for the difference in outcome between 2 hospitals in different geographic locations. If the difference in outcome was completely explained by factors preceding hospitalization, then we would conclude that quality data relating to cardiac arrest must be carefully adjusted for individual patient characteristics and characteristics of the region not necessarily related to the hospital. The null hypothesis is that the variability in OHCA outcome is related to purely hospital-level performance and not significantly confounded by patient-level or regional-level characteristics.
We evaluated post– cardiac arrest patients presenting to the emergency department (ED) at 2 geographically distinct urban tertiary care hospitals: Beth Israel Deaconess Medical Center, a university teaching hospital with 50 000 annual ED visits located in Boston, Massachusetts, and Henry Ford Hospital, a university teaching hospital with 95 000 annual ED visits located in Detroit, Michigan. The study was approved by the institutional review board at each facility. A waiver of the requirement for informed consent was obtained under the regulations of each center.
Patients were identified through an electronic query of the ED registry. Consecutive adult patients (age > 18 years) presenting to the ED from September 2006 to March 2010 (Boston site) and from January 2006 to December 2009 (Detroit site) were identified. Patients who had experienced an OHCA with subsequent return of spontaneous circulation (ROSC) were considered for analysis. Patients who had a traumatic cardiac arrest or did not obtain ROSC were excluded from analysis. All patients received medical therapy in accordance with hospital guidelines for post– cardiac arrest management. Therapeutic hypothermia is available at both investigational sites and was used as part of the medical therapy when indicated, as determined by the clinical teams caring for the patients.
Complete medical records were reviewed, and data were extracted by experienced research assistants. All data were recorded in the Utstein style. Demographics, initial cardiac arrest rhythm, intra-arrest medications, initial vital signs, and laboratory data were recorded. The presence or absence of bystander cardiopulmonary resuscitation (CPR), patient downtimes, and vasopressor dependence after ROSC were determined from the prehospital EMS record and ED documentation. Patient downtimes were defined as the sum of no-flow (complete circulatory arrest without chest compressions) and low-flow (chest compressions in progress) intervals. Hospital survival was recorded. Cerebral Performance Category scores were determined by review of the patient’s discharge summary and pertinent neurological and functional assessments at the time of discharge. The OHCA score was calculated, and the probability of poor outcome for each patient as described previously, as well.9 Data were collected by using a standard data collection form and subsequently entered into an electronic database (Microsoft Access 2003).
Outcome Measures
The primary outcome variable of interest was in-hospital mortality, defined as death for any reason before hospital discharge.
Categorization of Predictor Variables
Variables were classified according to whether they are characteristics that are independent of hospital or whether they could conceptually be related to the hospital’s quality of care. For example, the uses of therapeutic hypothermia or cardiac catheterization are hospital dependent, whereas the patient’s age or initial cardiac rhythm are independent of the hospital care. Only variables assessed to be independent of hospital care were considered for the current investigation. These included age, sex, arrest rhythm, no-flow and low-flow intervals, whether the arrest was witnessed, presence of bystander CPR, epinephrine dose in the field (before ED arrival), patient comorbid conditions (eg, history of diabetes mellitus), vital signs at the time of hospital arrival, and laboratory test results at the time of hospital arrival.
Modeling Strategy
A sequential approach was used to identify and adjust for confounding as follows.
Univariate Analysis
Unadjusted incidence of death was compared between cities by the use of the Fisher exact test, and the strength of the relationship is reported as an odds ratio.
Multivariate Analysis
The OHCA score was used to adjust for severity of the cardiac arrest event.9 The OHCA score was developed as a severity of illness scoring tool to predict poor neurological outcome and in-hospital mortality in cardiac arrest patients and includes only event characteristics that are independent of hospital care (initial cardiac rhythm, no-flow and low-flow intervals, and lactic acid and creatinine values) and has been validated externally.10 The OHCA score was calculated and treated as a continuous variable for adjustment in the logistic regression models.
A stepwise forward selection procedure was used to evaluate the association between hospital-independent predictor variables and in-hospital mortality as the outcome variable. An entry criterion of P<0.1 and a maintenance criterion of P<0.05 were used to build the model, and the selected variables were included in the multivariate model. After adjusting for covariates identified in the stepwise procedure, the patient’s city was added to the model to assess the independent relationship of city to the risk of death.
Propensity Score–Based Approaches
A propensity score was derived by using a logistic regression model with patient city as the dependent variable and only prehospital variables as independent predictors. The fitted probability from this model (ie, the propensity score), which reflected a patient’s estimated propensity to be in 1 city instead of the other, was assigned to each patient.
Following the development of the propensity score, 2 subsequent analyses were performed. First, a propensity score adjustment was made, and we assessed the residual relationship of city to mortality. In the second analysis, patients were matched based on propensity score using a “greedy” matching technique.11 In this matching approach, a patient from 1 city is randomly selected and then matched to its nearest neighbor based on the propensity score. This process is repeated until matches are attempted for each patient in both cities. Each matched pair is unique; data from unmatched patients are not included in further analyses. After propensity score matching, a conditional logistic regression was used to assess the relationship of the patient’s city to mortality risk.
Data for continuous variables are presented as means with standard deviations. Categorical variables are presented as frequencies with percentages. The differences in patient characteristics were compared by using Wilcoxon rank sum test and Fisher exact test, as appropriate. Statistical significance was set at P<0.05 for all analyses, and data analysis was performed with the use of SAS 9.2 (SAS Institute Inc, NC).
Results
Unmatched Patient Characteristics
A total of 208 patients were included in the investigation: 101 from Detroit and 107 from Boston. In Boston, 82% of the patients were white, and in Detroit 83% of the patients were black. In this population of patients with OHCA who achieved ROSC, those patients in the Boston cohort were more than twice as likely to have received bystander CPR as those in Detroit (P<0.001). Asystole was twice as likely to be the initial rhythm in the Detroit cohort as in the Boston cohort, whereas the Boston patients were twice as likely as those in Detroit to present in either ventricular tachycardia, ventricular fibrillation, or pulseless electric activity. Median downtime in Detroit was 8 minutes longer than it was in Boston (P<0.001). After ROSC, patients in Detroit and Boston had similar mean arterial pressures, although more patients in Detroit required vasopressor support (P=0.04) and patients were significantly more likely to be tachycardic (P<0.0001). Patients in Detroit also had significantly higher initial lactate levels in comparison with Boston patients (P<0.0001), and the OHCA score was significantly higher in Detroit than Boston (P<0.0001), likely reflecting the longer downtimes and more severe tissue hypoperfusion in the Detroit cohort. Additional baseline characteristics of the study cohorts are available in Table 1.
Table 1.
Baseline Characteristics of the Study Cohorts
| Boston | Detroit | ||
|---|---|---|---|
| n=107 | n=101 | P | |
| Patient demographics | |||
| Age | 65.8±17.5 | 62.6±16.1 | 0.1 |
| Sex, % male | 66 | 51 | 0.02 |
| Arrest characteristics | |||
| Arrest rhythm, % | <0.0001 | ||
| VF/VT | 41 | 15 | |
| PEA | 27 | 33 | |
| Asystole | 23 | 51 | |
| Other or unknown | 8 | 2 | |
| No-flow time (out-of-hospital) | 3.5±4.5 | 5.6±4.6 | <0.0001 |
| Low-flow time (out-of-hospital) | 20.3±16.0 | 24.9±15.1 | 0.002 |
| Bystander CPR, % | 66 | 31 | <0.0001 |
| Witnessed arrest?, % | 75 | 75 | 0.9 |
| Epinephrine dose in the field | 2.8±2.4 | 4.2±2.6 | <0.0001 |
| Location, % | 0.004 | ||
| Public space | 33 | 25 | |
| Private residence | 47 | 67 | |
| Nursing home | 10 | 7 | |
| Other or unknown | 10 | 1 | |
| Patient comorbidities, % | |||
| Diabetes mellitus | 22 | 33 | 0.09 |
| Hypertension | 50 | 62 | 0.08 |
| Chronic obstructive pulmonary disease |
8 | 17 | 0.07 |
| Coronary artery disease | 30 | 21 | 0.1 |
| Congestive heart failure | 23 | 28 | 0.5 |
| ESRD | 4 | 15 | 0.005 |
| Chronic kidney disease (non-ESRD) |
10 | 18 | 0.05 |
| Cerebrovascular disease | 4 | 7 | 0.2 |
| Vital signs at hospital arrival | |||
| Heart rate | 96.6±28.8 | 115.9±34.9 | <0.0001 |
| Systolic blood pressure | 123.5±33.2 | 126.5±41.7 | 0.6 |
| Diastolic blood pressure | 70.2±22.6 | 67.2±26.7 | 0.4 |
| Mean arterial pressure | 86.0±26.2 | 87.0±29.6 | 0.9 |
| Initial laboratory values | |||
| Sodium | 139.8±4.1 | 142.4±6.2 | <0.0001 |
| Potassium | 4.4±1.1 | 4.6±1.4 | 0.3 |
| Chloride | 104.0±6.1 | 104.3±7.1 | 0.7 |
| Bicarbonate | 20.1±5.5 | 17.3±6.7 | 0.0008 |
| Blood urea nitrogen | 29.3±23.1 | 35.4±27.0 | 0.2 |
| Creatinine* | 1.3 (1.0–1.8) | 2.0 (1.4–3.8) | <0.0001 |
| Glucose | 225.3±92.1 | 250.1±186.5 | 0.5 |
| White blood cell count | 13.5±6.5 | 12.2±6.1 | 0.13 |
| Hemoglobin | 12.1±2.3 | 10.6±2.4 | <0.0001 |
| Lactate | 6.0±3.7 | 13.2±5.6 | <0.0001 |
| Outcome prediction score at hospital arrival |
|||
| OHCA score | 20.6±23.6 | 46.3±21.1 | <0.0001 |
| Predicated probability of poor outcome, % |
68.6 | 89.7 | <0.0001 |
Wilcoxon rank sum test for continuous variables; Fisher exact test for categorical data. VF indicates ventricular fibrillation; VT, ventricular tachycardia; PEA, pulseless electric activity; ESRD, end-stage renal disease; OHCA, out-of-hospital cardiac arrest; CPR, cardiopulmonary resuscitation.
Creatinine values represented as median with interquartile range.
Unmatched Association of City and Mortality
The overall mortality in the Detroit cohort was 87% in comparison with 61% in the Boston cohort (odds ratio [OR]: 4.4; 95% confidence interval [CI]: 2.2– 8.8); among those patients who survived to hospital discharge, those in the Boston cohort were nearly 4 times more likely (P<0.001) to have a favorable neurological outcome than were those in the Detroit cohort. After sequential adjustments for baseline covariates, OHCA score and propensity score, city was unassociated with mortality (OR: 1.16; 95% CI: 0.45–2.97). The decrease in OR for death to 1.16 (95% CI: 0.45–2.97) from the crude unadjusted mortality (OR: 4.4; 95% CI: 2.2– 8.8) indicates that differences in hospital-independent patient characteristics (ie, pre- and intra-arrest conditions) contribute significantly to the severity of the postarrest syndrome and affect postarrest patient outcomes.
Propensity-Matched Analysis
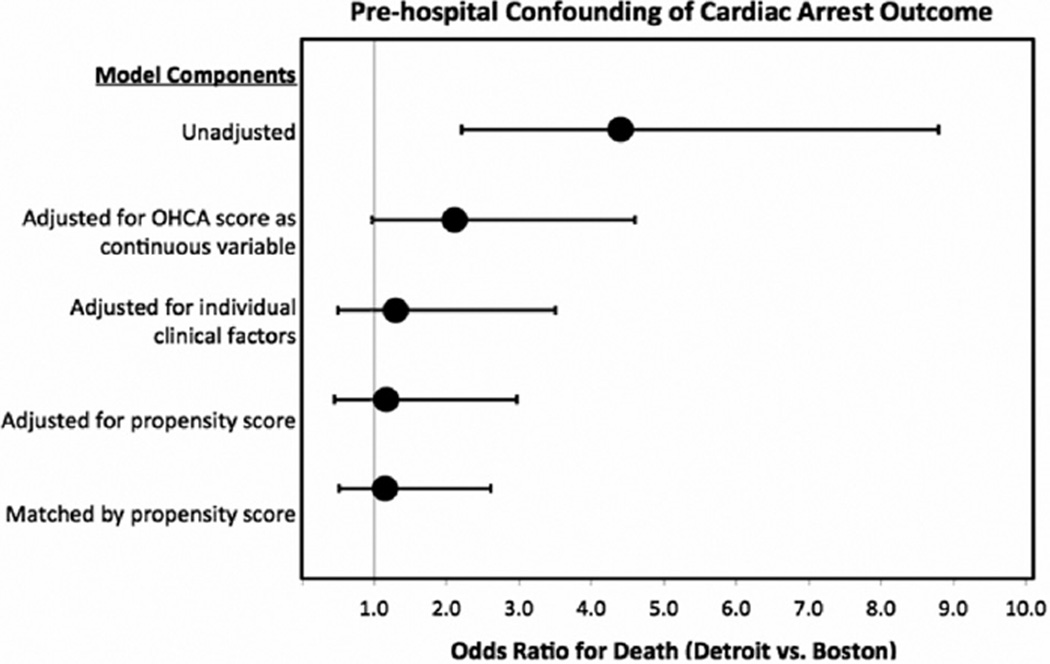
There were 78 cardiac arrest patients matched successfully by propensity score. After matching, patients in Detroit and Boston were significantly more alike in terms of baseline characteristics (Table 2), and by comparing the difference in mean covariate values before and after matching, we estimate an overall 84% bias reduction in measured covariates.12 There was no significant difference between the 2 cities based on OHCA score or predicted probability for poor outcome and there was no significant difference in the OR for death between the 2 cities (OR: 1.15; 95% CI: 0.51–2.61; Figure). The complete results of the sequential modeling strategy are shown in Table 3.
Table 2.
Propensity-Matched Characteristics of the Study Cohort
| Boston | Detroit | ||
|---|---|---|---|
| n=39 | n=39 | P | |
| Patient demographics | |||
| Age | 64.2±20.5 | 66.6±16.9 | 0.9 |
| Sex, % male | 67 | 46 | 0.1 |
| Arrest characteristics | |||
| Arrest rhythm, % | 0.3 | ||
| VF/VT | 15 | 15 | |
| PEA | 36 | 49 | |
| Asystole | 41 | 36 | |
| Other or unknown | 8 | 0 | |
| No-flow time (out-of-hospital) | 4.7±5.4 | 5.2±4.8 | 0.4 |
| Low-flow time (out-of-hospital) | 23.9±17.0 | 24.7±14.9 | 0.4 |
| Bystander CPR, % | 67 | 67 | 1.0 |
| Witnessed arrest?, % | 77 | 85 | 0.4 |
| Epinephrine dose in the field | 3.4±2.9 | 3.5±2.1 | 0.5 |
| Location, % | 0.4 | ||
| Public space | 26 | 39 | |
| Private residence | 51 | 49 | |
| Nursing home | 13 | 10 | |
| Other or unknown | 10 | 3 | |
| Patient comorbidities, % | |||
| Diabetes mellitus | 23 | 38 | 0.1 |
| Hypertension | 44 | 77 | 0.003 |
| Chronic obstructive pulmonary disease |
5 | 13 | 0.2 |
| Coronary artery disease | 33 | 31 | 0.8 |
| Congestive heart failure | 18 | 36 | 0.07 |
| ESRD | 5 | 26 | 0.01 |
| Chronic kidney disease (non-ESRD) |
13 | 26 | 0.2 |
| Cerebrovascular disease | 3 | 5 | 0.6 |
| Vital signs at hospital arrival | |||
| Heart rate | 104.6±26.3 | 110.7±36.3 | 0.5 |
| Systolic blood pressure | 126.0±39.4 | 129.8±36.8 | 0.4 |
| Diastolic blood pressure | 74.1±26.5 | 70.0±26.5 | 0.6 |
| Mean arterial pressure | 88.0±30.8 | 98.7±33.3 | 0.2 |
| Initial laboratory values | |||
| Sodium | 140.6±5.1 | 142.7±6.9 | 0.2 |
| Potassium | 4.7±1.1 | 4.1±1.0 | 0.2 |
| Chloride | 104.8±7.5 | 105.8±7.6 | 0.7 |
| Bicarbonate | 17.5±3.8 | 18.6±7.3 | 0.4 |
| Blood urea nitrogen | 27.2±17.0 | 33.1±23.1 | 0.5 |
| Creatinine* | 1.4 (1.0–2.0) | 2.0 (1.3–3.5) | 0.008 |
| Glucose | 227.4±106.4 | 224±116 | 0.7 |
| White blood cell count | 14.8±7.6 | 10.8±4.9 | 0.01 |
| Hemoglobin | 11.9±2.1 | 11.1±2.1 | 0.02 |
| Lactate | 8.5±3.6 | 9.4±5.6 | 0.8 |
| Outcome prediction score at hospital arrival |
|||
| OHCA score | 27.2±23.2 | 36.4±22.3 | 0.08 |
| Predicated probability of poor outcome, % |
75 | 83 | 0.08 |
Wilcoxon rank sum test for continuous variables; Fisher exact test for categorical data. VF indicates ventricular fibrillation; VT, ventricular tachycardia; PEA, pulseless electric activity; ESRD, end-stage renal disease; OHCA, out-of-hospital cardiac arrest; CPR, cardiopulmonary resuscitation.
Creatinine values represented as median with interquartile range.
Figure.
This figure illustrates that substantial mortality differences are present before adjusting for prehospital confounding features. Adjustment with the OHCA score does not adequately account for these prehospital differences; however, adjusting for individual clinical factors and by propensity score suggests that prehospital confounders contribute substantially to mortality differences between cities. OHCA indicates out-of-hospital cardiac arrests.
Table 3.
Predicted Odds Ratio for Death (Detroit Versus Boston) With Sequentially Sophisticated Modeling
| Model | Odds Ratio for Death |
|---|---|
| Unadjusted | 4.4 (95% CI: 2.2– 8.8) |
| Adjusted for OHCA score as continuous variable | 2.1 (95% CI: 0.96–4.6) |
| Adjusted for individual clinical factors | 1.3 (95% CI: 0.49–3.5) |
| Adjusted for propensity score | 1.16 (95% CI: 0.45–2.97) |
| Matched by propensity score | 1.15 (95% CI: 0.51–2.61) |
The unadjusted model includes only city. The adjusted model adjusted for OHCA score only. The clinical factors model includes city, rhythm, lactate, and no-flow time. The propensity score models include only propensity score and city. OHCA indicates out-of-hospital cardiac arrest; CI, confidence interval.
Discussion
These findings indicate the substantial impact of patient characteristics before hospital admission on the ultimate post– cardiac arrest outcome. In sum, unadjusted in-hospital mortality rates were markedly different between Boston and Detroit. However, by using variables that would reflect only pre- and intra-arrest conditions (independent of hospital care), we found that the city is no longer associated with mortality. These findings refute the concept that patient characteristics play little role in the outcome of survivors of cardiac arrest.
Regarding prearrest conditions, patients in the Detroit cohort had significantly higher creatinine levels at the time of arrival in the ED (2.0 [1.0 –1.8] mg/dL versus 1.3 [1.4 –3.8] mg/dL in Boston). Given the acute nature of cardiac arrest, the finding of initial creatinine elevation in the Detroit in comparison with the Boston cohort likely represents underlying renal disease rather than acute injury (and is likely an indicator of more severe comorbid disease in the Detroit group). Regardless, the elevation in creatinine on presentation indicates an organ failure disadvantage in the Detroit cohort that occurred either pre- or intra-arrest and not during the postarrest course. In addition to creatinine levels, patients in Detroit had statistically significantly lower hemoglobin levels. This finding also likely represents chronic disease in the population in Detroit in comparison with the population in Boston. Again, regardless of the underlying reason, the low value of hemoglobin represents a process that occurred before the postarrest phase and would again seem to support a disadvantage or at least a marker for disadvantage in the Detroit population.
To further assess prearrest conditions, patient charts in both cohorts were examined for documented past history of diabetes mellitus, renal disease, chronic obstructive pulmonary disease, and cardiovascular disease. There was a higher percentage of baseline diabetes mellitus and renal disease in the Detroit cohort, whereas in Boston there was a higher incidence of coronary artery disease, although these trends were not statistically significant. A documented past history of disease is more subject to errors of omission than is a measured laboratory value. For example, a patient without a primary care doctor may have uncontrolled hypertension and coronary artery disease; however, this condition would now remain undocumented. The Detroit trend toward more chartidentified diabetes mellitus and renal disease is consistent with the statistically significant hard laboratory values of higher creatinine and lower hemoglobin.
Regarding intra-arrest conditions, large differences were manifest between the Detroit and Boston cohorts on initial arrival at their respective university teaching hospital EDs. Before any in-hospital interventions, the Detroit patients were half as likely to have received bystander CPR. Once CPR was initiated, patients in Detroit required CPR for significantly longer periods of time (24.9 minutes versus 20.3 minutes). The initial rhythm in Detroit in comparison with Boston was twice as likely to be asystole, one of the strongest predictors of poor outcome from cardiac arrest.13 In contrast, the initial rhythm in the Boston cohort was more likely to be ventricular tachycardia/ventricular fibrillation.
In terms of the postarrest syndrome, severity can perhaps best be judged by parameters of tissue perfusion and measures of shock. Recently, we evaluated lactic acid levels, the need for vasopressors, and the percentage of patients with the most severe lactate-hypotension index between centers.14 Patients in Detroit were statistically significantly more likely to have the combination of high lactate plus requirement for vasopressors, which is a marker of very high mortality in comparison with either parameter alone. High lactic acid levels have been associated with worse outcomes in sepsis, trauma, burns, and post– cardiac arrest.15–18 Overall, patients in Detroit appear to have a more severe postarrest syndrome than those in Boston, even before the initiation of any postarrest interventions. Finally, patients in Detroit had a higher percentage of patients requiring vasopressors (73% versus 60% in Boston, P=0.04).
The abovementioned differences are all differences in characteristics that are independent of hospital care. With sequential adjustment for covariates, OHCA score, and the propensity score in this sample, there remained no significant difference in risk for death between the Detroit and Boston cohorts. The decrease in OR for death with sequential adjustments indicates that the severity of the postarrest syndrome depends on pre- and intra-arrest conditions. Furthermore, when patients in the 2 cities were matched by propensity score, there was no difference in risk for death. These results suggest that evaluations of the quality of postarrest care across different hospital systems on the basis of crude measures of mortality or other outcome variables are inadequate, because these parameters depend on conditions that precede hospital care. However, this does not mean that variations in quality of hospital care are irrelevant. Other investigations suggest better outcomes are associated with hospitals that provide advanced cardiac care services,19 with higher volumes of postarrest patients,4,5 or with transfer to critical care– capable centers.20 These investigations differ from our current investigation because they compare centers with expertise (ie, tertiary care or cardiac catheterization laboratory availability) versus centers with less expertise. In our investigation, we compared 2 equivalent hospitals in that they are both large, urban tertiary care centers. Thus, this literature provides support for the American Heart Association’s call for regional centers for postarrest care, and the findings reported here are not inconsistent with the promotion of designated regionalized Cardiac Arrest Centers.21,22
However, what the findings reported here do suggest is that the best possible postarrest care may have different results if provided to different patients on a different spectrum of critical illness. If the severity of the postarrest syndrome is driven by prearrest and intra-arrest conditions and hospitals are evaluated on strict metrics of outcomes data, then medical centers that provide excellent care may end up unfairly judged. This is not a merely academic point in an era when quality assurance measures tend to directly or indirectly steer money away from hospitals that receive poor grades in certain areas. In fact, the American Heart Association policy statement on cardiac arrest centers suggests that this very “payment for performance” approach be applied to cardiac arrest resuscitation to drive improvements in outcome.8 If quality of postarrest care is to be graded between centers, perhaps evaluating process or availability of advanced services (eg, cardiac catheterization) would be more appropriate, although challenges in this area remain as well, given the lack of consensus or strong evidence surrounding many postarrest interventions.
Previous reports have indicated that socioeconomic differences could affect the outcomes from cardiac arrest.23 There are significant differences in certain socioeconomic characteristics between the 2 cities included in this investigation. Specifically, Boston had a greater rate of high school completion (85.8% versus 77.4% in Detroit), attainment of a bachelor’s degree or higher (44.3% in Boston versus 12.0% in Detroit), and average annual household income ($75 308 in Boston versus $36 206 in Detroit).24 Although we did not specifically evaluate reasons for differences in prearrest conditions, these socioeconomic factors could theoretically be contributory.
There are limitations that must be considered when interpreting the results of this investigation. First, the study was retrospective in design. Cases of cardiac arrest subjects were identified through an electronic query of the ED records, which may introduce selection bias. Data collection was standardized across the 2 sites to minimize potential data acquisition biases. Second, there may be residual confounding following propensity score matching and multivariate analysis. We attempted to reduce confounding in the data through application of propensity score matching and multivariate adjustments for variables that were assessed to be significant confounders. Although propensity scores are powerful for reducing bias in observational studies, it is difficult to remove all bias, and it is not possible for a matching scheme to balance unmeasured confounders. Third, a number of subjects (64%) were unmatched with our matching scheme. Compared with unmatched subjects, the matched subjects had longer low-flow intervals, more frequent pulseless electric activity/asystole, and a higher incidence of end-stage renal disease. However, all other measured characteristics were the same between matched and unmatched cohorts, suggesting that these results are generalizable beyond our matching scheme. Finally, the data in this study were collected at 2 large academic medical centers, and the results may not be fully generalizable to smaller community hospitals.
Conclusions
The initial severity of the postarrest syndrome reflects prearrest and intra-arrest conditions and factors that are unassociated with hospital care and that contribute significantly to outcomes in this population. Evaluation of postarrest quality of care should include inherent differences in the presenting syndrome rather than a crude mortality rate.
CLINICAL PERSPECTIVE.
The American Heart Association has suggested that improvements in cardiac arrest outcomes might be driven by grading hospitals on in-hospital mortality rates. By comparing out-of-hospital cardiac arrest patients in Boston and in Detroit, this pilot study explores whether outcomes are heavily influenced by prearrest and intra-arrest conditions independent of hospital care. This study suggests that the severity of a postarrest syndrome is largely reflective of prearrest and intra-arrest conditions. This has implications for any plan to grade hospitals on outcomes from cardiac arrest if such grades would be based on crude mortality rates.
Acknowledgments
The authors thank Francesca Montillo for her administrative support in preparation of this manuscript.
Sources of Funding
The project described was supported, in part, by Grant Number UL1 RR025758-Harvard Clinical and Translational Science Center, from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. Dr Donnino is supported by NHLBI (1K02HL107447-01A1). Additionally, Dr Donnino was funded by the American Heart Association during the time of this investigation (0735533T). Dr Cocchi was funded by the American Heart Association during the time of this investigation (10CRP2640126).
Footnotes
Disclosures
None.
References
- 1.Garza AG, Gratton MC, Salomone JA, Lindholm D, McElroy J, Archer R. Improved patient survival using a modified resuscitation protocol for out-of-hospital cardiac arrest. Circulation. 2009;119:2597–2605. doi: 10.1161/CIRCULATIONAHA.108.815621. [DOI] [PubMed] [Google Scholar]
- 2.Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, Rea T, Lowe R, Brown T, Dreyer J, Davis D, Idris A, Stiell I. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eisenberg MS, Horwood BT, Cummins RO, Reynolds-Haertle R, Hearne TR. Cardiac arrest and resuscitation: a tale of 29 cities. Ann Emerg Med. 1990;19:179–186. doi: 10.1016/s0196-0644(05)81805-0. [DOI] [PubMed] [Google Scholar]
- 4.Carr BG, Goyal M, Band RA, Gaieski DF, Abella BS, Merchant RM, Branas CC, Becker LB, Neumar RW. A national analysis of the relationship between hospital factors and post-cardiac arrest mortality. Intensive Care Med. 2009;35:505–511. doi: 10.1007/s00134-008-1335-x. [DOI] [PubMed] [Google Scholar]
- 5.Carr BG, Kahn JM, Merchant RM, Kramer AA, Neumar RW. Inter-hospital variability in post-cardiac arrest mortality. Resuscitation. 2009;80:30–34. doi: 10.1016/j.resuscitation.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Peberdy MA, Ornato JP, Larkin GL, Braithwaite RS, Kashner TM, Carey SM, Meaney PA, Cen L, Nadkarni VM, Praestgaard AH. Survival from in-hospital cardiac arrest during nights and weekends. JAMA. 2008;299:785. doi: 10.1001/jama.299.7.785. [DOI] [PubMed] [Google Scholar]
- 7.Eisenberg M, White RD. The unacceptable disparity in cardiac arrest survival among American communities. Ann Emerg Med. 2009;54:258–260. doi: 10.1016/j.annemergmed.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Nichol G, Aufderheide TP, Eigel B, Neumar RW, Lurie KG, Bufalino VJ, Callaway CW, Menon V, Bass RR, Abella BS, Sayre M, Dougherty CM, Racht EM, Kleinman ME, O’Connor RE, Reilly JP, Ossmann EW, Peterson E. Regional systems of care for out-of-hospital cardiac arrest: a policy statement from the American Heart Association. Circulation. 2010;121:709–729. doi: 10.1161/CIR.0b013e3181cdb7db. [DOI] [PubMed] [Google Scholar]
- 9.Adrie C, Cariou A, Mourvillier B, Laurent I, Dabbane H, Hantala F, Rhaoui A, Thuong M, Monchi M. Predicting survival with good neurological recovery at hospital admission after successful resuscitation of out-of-hospital cardiac arrest: the OHCA score. Eur Heart J. 2006;27:2840–2845. doi: 10.1093/eurheartj/ehl335. [DOI] [PubMed] [Google Scholar]
- 10.Hunziker S, Bivens MJ, Cocchi MN, Miller J, Salciccioli J, Howell MD, Donnino MW. International validation of the out-of-hospital cardiac arrest score in the united states. Crit Care Med. 2011;39:1670–1674. doi: 10.1097/CCM.0b013e318218a05b. [DOI] [PubMed] [Google Scholar]
- 11.Parsons LS. Reducing bias in a propensity score matched-pair sample using greedy matching techniques. SUGI 26 Proceedings. 2001;26:214–226. [Google Scholar]
- 12.D’Agostino RB., Jr Propensity scores in cardiovascular research. Circulation. 2007;115:2340–2343. doi: 10.1161/CIRCULATIONAHA.105.594952. [DOI] [PubMed] [Google Scholar]
- 13.Sasson C, Rogers MA, Dahl J, Kellermann AL. Predictors of survival from out-of-hospital cardiac arrest: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2010;3:63–81. doi: 10.1161/CIRCOUTCOMES.109.889576. [DOI] [PubMed] [Google Scholar]
- 14.Cocchi MN, Miller J, Hunziker S, Carney E, Salciccioli J, Farris S, Joyce N, Zimetbaum P, Howell MD, Donnino MW. The association of lactate and vasopressor need for mortality prediction in survivors of cardiac arrest. Minerva Anestesiol. 2011;77:1063–1071. [PubMed] [Google Scholar]
- 15.Andel D, Kamolz LP, Roka J, Schramm W, Zimpfer M, Frey M, Andel H. Base deficit and lactate: early predictors of morbidity and mortality in patients with burns. Burns. 2007;33:973–978. doi: 10.1016/j.burns.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Donnino MW, Miller J, Goyal N, Loomba M, Sankey SS, Dolcourt B, Sherwin R, Otero R, Wira C. Effective lactate clearance is associated with improved outcome in post-cardiac arrest patients. Resuscitation. 2007;75:229–234. doi: 10.1016/j.resuscitation.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen HB, Rivers EP, Knoblich BP, Jacobsen G, Muzzin A, Ressler JA, Tomlanovich MC. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med. 2004;32:1637–1642. doi: 10.1097/01.ccm.0000132904.35713.a7. [DOI] [PubMed] [Google Scholar]
- 18.Howell MD, Donnino M, Clardy P, Talmor D, Shapiro NI. Occult hypoperfusion and mortality in patients with suspected infection. Intensive Care Med. 2007;33:1892–1899. doi: 10.1007/s00134-007-0680-5. [DOI] [PubMed] [Google Scholar]
- 19.Stub D, Smith K, Bray JE, Bernard S, Duffy SJ, Kaye DM. Hospital characteristics are associated with patient outcomes following out-ofhospital cardiac arrest. Heart. 2011;97:1489–1494. doi: 10.1136/hrt.2011.226431. [DOI] [PubMed] [Google Scholar]
- 20.Kajino K, Iwami T, Daya M, Nishiuchi T, Hayashi Y, Kitamura T, Irisawa T, Sakai T, Kuwagata Y, Hiraide A, Kishi M, Yamayoshi S. Impact of transport to critical care medical centers on outcomes after out-of-hospital cardiac arrest. Resuscitation. 2010;81:549–554. doi: 10.1016/j.resuscitation.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Sunde K, Pytte M, Jacobsen D, Mangschau A, Jensen LP, Smedsrud C, Draegni T, Steen PA. Implementation of a standardised treatment protocol for post resuscitation care after out-of-hospital cardiac arrest. Resuscitation. 2007;73:29–39. doi: 10.1016/j.resuscitation.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Donnino MW, Rittenberger JC, Gaieski D, Cocchi MN, Giberson B, Peberdy MA, Abella BS, Bobrow BJ, Callaway C. The development and implementation of cardiac arrest centers. Resuscitation. 2011;82:974–978. doi: 10.1016/j.resuscitation.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 23.Merchant RM, Becker LB, Yang F, Groeneveld PW. Hospital racial composition: A neglected factor in cardiac arrest survival disparities. Am Heart J. 2011;161:705–711. doi: 10.1016/j.ahj.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.United States Census Bureau. [Accessed December 31, 2010];The American Community Survey. http://2010.census.gov/