Abstract
This study presents a novel myoelectric pattern recognition strategy towards restoration of hand function after incomplete cervical spinal cord Injury (SCI). High density surface electromyogram (EMG) signals comprised of 57 channels were recorded from the forearm of 9 subjects with incomplete cervical SCI while they tried to perform 6 different hand grasp patterns. A series of pattern recognition algorithms with different EMG feature sets and classifiers were implemented to identify the intended tasks of each SCI subject. High average overall accuracies (>97%) were achieved in classification of 7 different classes (6 intended hand grasp patterns plus a hand rest pattern), indicating that substantial motor control information can be extracted from partially paralyzed muscles of SCI subjects. Such information can potentially enable volitional control of assistive devices, thereby facilitating restoration of hand function. Furthermore, it was possible to maintain high levels of classification accuracy with a very limited number of electrodes selected from the high density surface EMG recordings. This demonstrates clinical feasibility and robustness in the concept of using myoelectric pattern recognition techniques toward improved function restoration for individuals with spinal injury.
Index Terms: Surface EMG, myoelectic control, pattern recognition, spinal cord injury
I. Introduction
A number of robot devices have been designed to help provide rehabilitation therapy for patients with different neurological injuries such as hemiparetic stroke, cerebral palsy, multiple sclerosis, Parkinson’s disease, or spinal cord injury (SCI) [1, 2]. These robot devices can assist users to perform exercises which involve movement of the disabled limb in a passive way (as they relax) or in an active way (as they intend to contribute to the movement). While passive movements have been proved useful to improve motor abilities, voluntary user interactions are very important to promote robot-aided therapy and enhance therapeutic effect for patients with neurological disorders [3–5].
Surface electromyogram (EMG) signals contain rich motor control information from which the user’s intention can be detected with appropriate signal processing methods. Thus surface EMG can be used as voluntary command signals for controlling artificial devices. For example, EMG signals from amputee’s residual muscles have been used to control prosthesis for more than 40 years [6–9]. Myoelectric control has also been reported in robot–aided therapy for stroke subjects, primarily based on a conventional “on-off” or proportional control strategy [10, 11]. In the SCI population, EMG signals from muscles with retained voluntary contraction have been utilized as neuroprosthesis control signals using functional neuromuscular stimulation [12, 13].
Myoelectric pattern recognition has recently attracted increasing attention in the development of dexterous myoelectric control systems. The advancement in EMG feature extraction and classification techniques provides a powerful approach for identification of various movement intentions of the missing or disabled limb. This forms a foundation for development of algorithms for myoelectric control of various artificial devices (e.g., a prosthetic arm, a virtual arm or a training robot). While most of the EMG pattern recognition studies have been focused on improved myoelectric prosthesis control for individuals with different levels of upper limb amputation [6–9], very recently EMG pattern recognition has also been applied to paretic muscles of stroke subjects to extract motor control information targeting improved stroke rehabilitation [14, 15].
Individuals with incomplete SCI may retain partial volitional EMG from their muscles below the level of injury. However, a systematic evaluation of the EMG from these muscles as a control signal for artificial devices is lacking. In fact, for the SCI population EMG recording is sometimes used as an assessment approach for therapy or treatment rather than a control signal for artificial devices. As a result, it is presently unclear how much motor control information can be extracted from EMG signals of muscles partially paralyzed by SCI.
After cervical SCI, arm and hand muscles may have different levels of paralysis, and be incapable of completing upper limb movements without assistance (from therapists or robotic devices). It is very important to improve the restoration of upper limb function, due to its importance in daily activities. The specific goal of this study was to determine whether EMG signals recorded from arm and hand muscles of individuals with incomplete cervical SCI can be used to detect different hand grasp patterns. The intention of these patterns, if detectable, can be used to trigger or control a movement assistive device for upper limb voluntary exercise or rehabilitation training.
High density surface EMG recording and pattern recognition analysis methods were used in this study. We hypothesize that different hand grasp intentions can be reliably classified through high density surface EMG recording and pattern recognition analyses. Furthermore, it is possible to select only a very limited number of EMG channels from the high density recordings to maintain high levels of classification accuracy, thus demonstrating clinical feasibility and robustness in the concept of using myoelectric pattern recognition techniques to extract motor control information toward improved function restoration for incomplete SCI subjects.
II. Methods
A. Subjects
Nine subjects with incomplete cervical spinal injury (ASIA C or D) participated in this study. The study was approved by the Institutional Review Board of Northwestern University (Chicago, IL, USA). All our subjects were recruited from the Clinical Neuroscience Research Registry at the Rehabilitation Institute of Chicago (Chicago, IL, USA). All subjects gave their written consent before the experiment. The experimental procedures were conducted in accord with the Helsinki Declaration of 1975. Demographic and clinical measures for the subjects are detailed in Table I.
Table1.
Physical characteristics of subjects
| Subject No. |
Level of Injury |
ASIA Class |
Gender | UEMS | Age (years) |
Post-injury time (years) |
|---|---|---|---|---|---|---|
| 1 | C6 | C | M | 30 | 31 | 11 |
| 2 | C7 | C | F | 30 | 37 | 4 |
| 3 | C4 | D | M | 45 | 62 | 7 |
| 4 | C8 | D | F | 39 | 51 | 8 |
| 5 | C4 | D | M | 44 | 53 | 11 |
| 6 | C7 | C | M | 36 | 38 | 12 |
| 7 | C5 | C | M | 34 | 44 | 11 |
| 8 | C4 | D | F | 32 | 42 | 7 |
| 9 | C7 | D | M | 45 | 57 | 17 |
Neurological Injury Levels: C = cervical
ASIA = American Spinal Injury Association
UEMS: Upper Extremity Motor Score
B. Data Acquisition
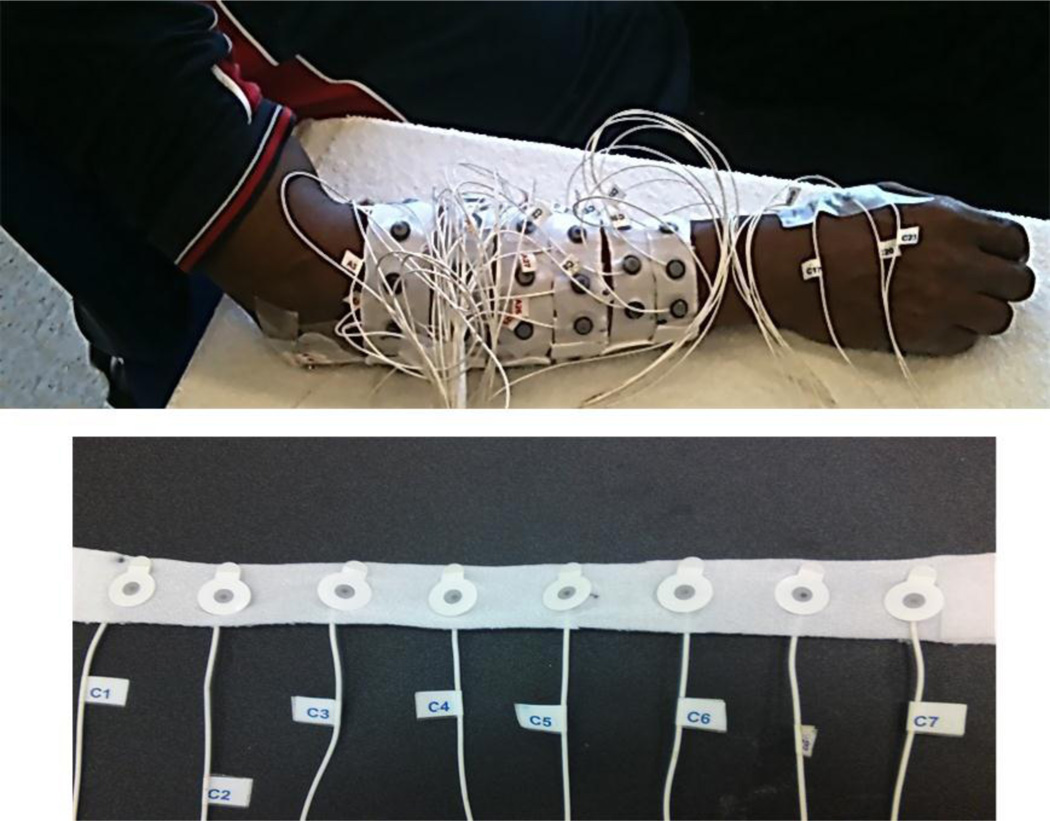
A multiple-channel surface EMG system (REFA 128 model, TMS International BV, Enschede, Netherlands) was used for surface EMG recording of the forearm and hand muscles in the weaker side of each SCI subject. As shown in Figure 1, a total of 57 surface EMG electrodes were used, among which 48 were placed in a 6×8 grid formation over the forearm, with a reference electrode located on the olecranon (each channel also had a common feedback subtraction of the average of all the recording channels). The size of each individual electrode was 10 mm in diameter while the recording surface was 5 mm in diameter. To facilitate electrode placement, 8 electrodes were equally spaced and attached to a stretchable strap custom designed for this experiment. After putting each stretchable strap around the forearm, the center to center distance between two consecutive electrodes depends on the size of the arm. The six stretchable straps were equally placed around the forearm at different locations from approximately 12.5% to 75.0% (with 12.5% increments) of the entire distance from the medial epicondyle of the humerus to the styloid process of the ulna. Before electrode placement, the skin was shaved, lightly abraded, and cleaned with alcohol; and conductive gel was applied to each electrode. In addition to 48 electrodes on the forearm, three electrodes were placed on the first dorsal interosseous (FDI), the thenar group and the hypothenar group muscles, respectively. The surface EMG signals were sampled at 2k Hz per channel, with a system band pass filter setting at 20–500 Hz.
Fig. 1.
Recording of 57-channel surface EMG from forearm and hand muscles of an SCI subject. The bottom figure shows the stretchable strap used for the recording on which 8 surface electrodes are evenly distributed.
C. Experimental Protocol
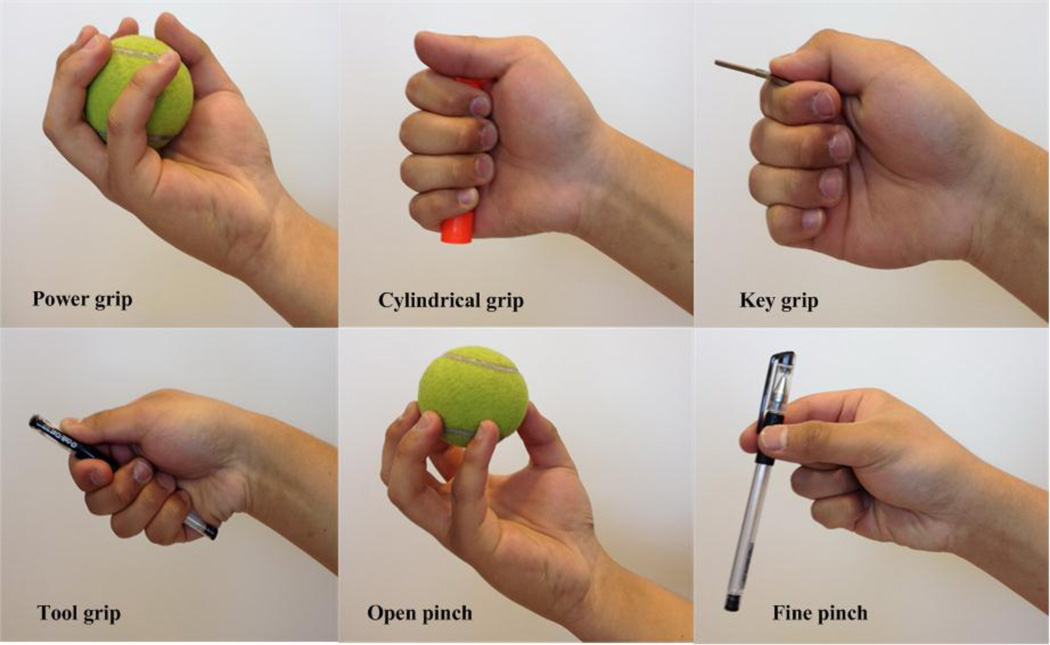
Each SCI subject was comfortably seated on a chair with the tested forearm relaxed on a height-adjustable table. The subject was instructed to perform six different grasp patterns as shown in Figure 2, including power grip, cylindrical grip, key grip, tool grip, open pinch, and fine pinch. The experiment comprised of 6 trials. Each trial contained 8 repetitions of one hand grasp pattern. For each repetition of a pattern, the subject was instructed to perform (or intend to perform) the task with a moderate or comfortable force, hold the task for 5 seconds and then relax between repetitions. Subjects did not practice the grasp patterns before or during the experiments. Each subject received auditory cues from the experimenter when to start and stop each repetition of contraction. Sufficient relaxation time between repetitions of each task was allowed to help the SCI subjects decrease muscle spasticity or involuntary EMG activity before performing the next task. The subject was allowed to sufficiently rest between trials to avoid muscular and mental fatigue. In addition to the 6 hand grasp patterns, the spontaneous EMG signal during hand relaxation was also recorded as the 7th task for each subject.
Fig. 2.
Illustrations of six different hand grasp patterns used in this study.
D. EMG Pattern Recognition Analysis
For each hand grasp pattern, the recorded EMG signal was composed of eight active segments corresponding to eight repetitions of muscle contraction. An EMG amplitude based data segmentation scheme was used to manually determine the onset and offset of the active segments for each repetition, which were the same for all the channels. For each active segment, 57-channel EMG data were further divided into a series of overlapping analysis windows (window length: 256 ms, overlapping step: 32 ms) [16, 17]. The overlapped windowing scheme was used to enhance both utilization of limited data stream and continuity of decision output by the classifier.
For each analysis window, a set of features was extracted to characterize the EMG data for classification of the intended hand grasp patterns. Three feature sets were investigated in this study including the time domain (TD) feature set [18], the combination of autoregressive (AR) model (6-order as suggested by Farina and Merletti [19]) coefficients and the root mean square (RMS) of the signal as a feature set (AR+RMS) [20], and the TD+AR+RMS feature set [21]. These feature sets have been shown to be effective signal representation for EMG pattern recognition with relatively low computational complexity [22–26]. The feature set was extracted on each of the 57 EMG channels and then concatenated into a feature vector.
The high density surface EMG recording resulted in very high-order n-dimensional feature vectors (n = 228 for TD feature set; n = 399 for AR+RMS feature set; n=627 for TD+AR+RMS feature set) for pattern classification. To reduce feature dimensionality, two methods, i.e. principal component analysis (PCA) [24] and uncorrelated linear discriminant analysis (ULDA) [27], were used respectively. PCA is a mathematical procedure that uses an orthogonal transformation to convert a set of correlated variables into linearly uncorrelated variables called principal components. The first principal component has the largest possible variance, and each succeeding principal component in turn has the highest variance possible under the constraint that it be orthogonal to the preceding components. ULDA employs an optimal transformation that projects high dimensional data into a lower dimensional space with minimized within-class distance and maximized between-class distance. Features in the transformed space are uncorrelated, which is attractive for feature dimension reduction.
Two classifiers were used respectively in this study. They are the linear discriminant analysis (LDA) classifier and the k-nearest neighbor (KNN) classifier (k=5) [28]. For each classifier, a post-processing method, namely the majority vote [16], was used to improve the classification performance. Majority vote takes advantage of the increased frequency of class decisions by overlapping windows. For the decision stream produced by the overlapped windowing scheme, it has been demonstrated that majority vote can be used to remove spurious misclassifications [16]. In this study, data analysis windows of 256 ms were used to extract features and produce a decision at 32 ms intervals, which allowed for 17 decisions (the current decision, the previous 8 decisions and the future 8 decisions) to be used in majority vote to choose the decision that occurs most frequently. The effect of majority vote on classification accuracy was evaluated by one-way repeated-measures ANOVA.
E. Performance Evaluation
To evaluate classification performance, an eight-fold cross-validation scheme was used. The EMG data within any random seven active segments were assigned as training dataset, and sequentially the EMG data of the remaining active segment were used as testing dataset. The performance accuracy for each intended hand pattern was the percentage of correctly classified windows over all the analysis windows in its testing dataset. An overall performance was then calculated as the percentage of correctly classified windows over all the analysis windows in the testing datasets across all hand grasp patterns. The Linear Mixed Model (LMM) in SPSS 16.0 (SPSS Inc., Chicago, IL, USA) was used to perform a Two-way Repeated Measures ANOVA (Two-way RM-ANOVA) on the classification accuracy. Bayesian Information Criteria (BIC) was used to determine the best covariance structure for repeated measurements on subjects in the RM-ANOVA model.
F. Channel Reduction
The high density surface EMG recording was used to evaluate how much control information one can extract with the maximum possible number of EMG signals from partially paralyzed muscles of SCI subjects. However, it is impractical to use the high density surface EMG as a source for real time control. Therefore, a preliminary study seeking a practical number of EMG channels was conducted using a strategy of sequential forward selection (SFS) developed in [17].
III. Experimental Results
A. The Effect of Feature Dimension on Classification Performance
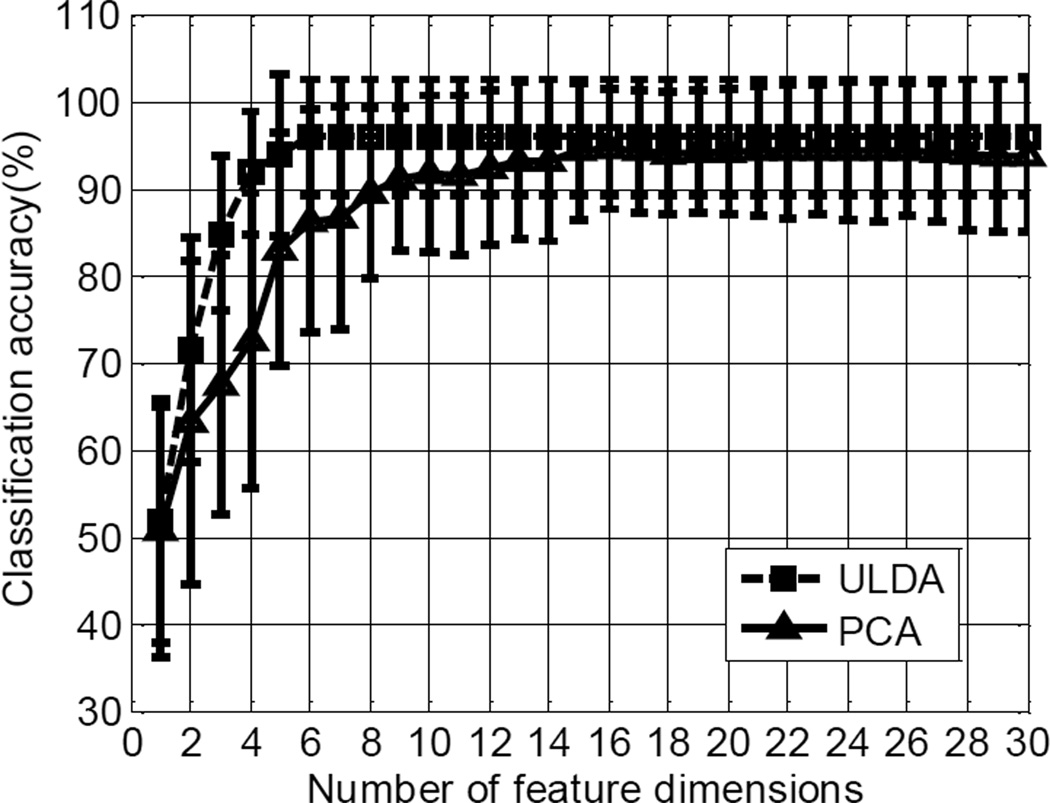
Figure 3 shows the effect of the number of feature dimensions on the classification performance averaged across all the subjects, using combination of the TD feature set and the LDA classifier as an example, where the feature dimensions were reduced via the ULDA and the PCA, respectively. The comparison between the two methods indicates that the ULDA is more efficient than the PCA in reducing feature dimensions for classification. With 7 classes used in this study, the maximum number of the linearly independent feature dimensions derived from the ULDA is 6. These 6 feature dimensions from the ULDA were used for the following classification analyses with different features sets and classifiers.
Fig. 3.
The effect of the number of feature dimensions on the classification performance averaged cross all the subjects using ULDA and PCA respectively. The TD feature set and the LDA classifier were used in this example.
B. Classification of Intended Movements
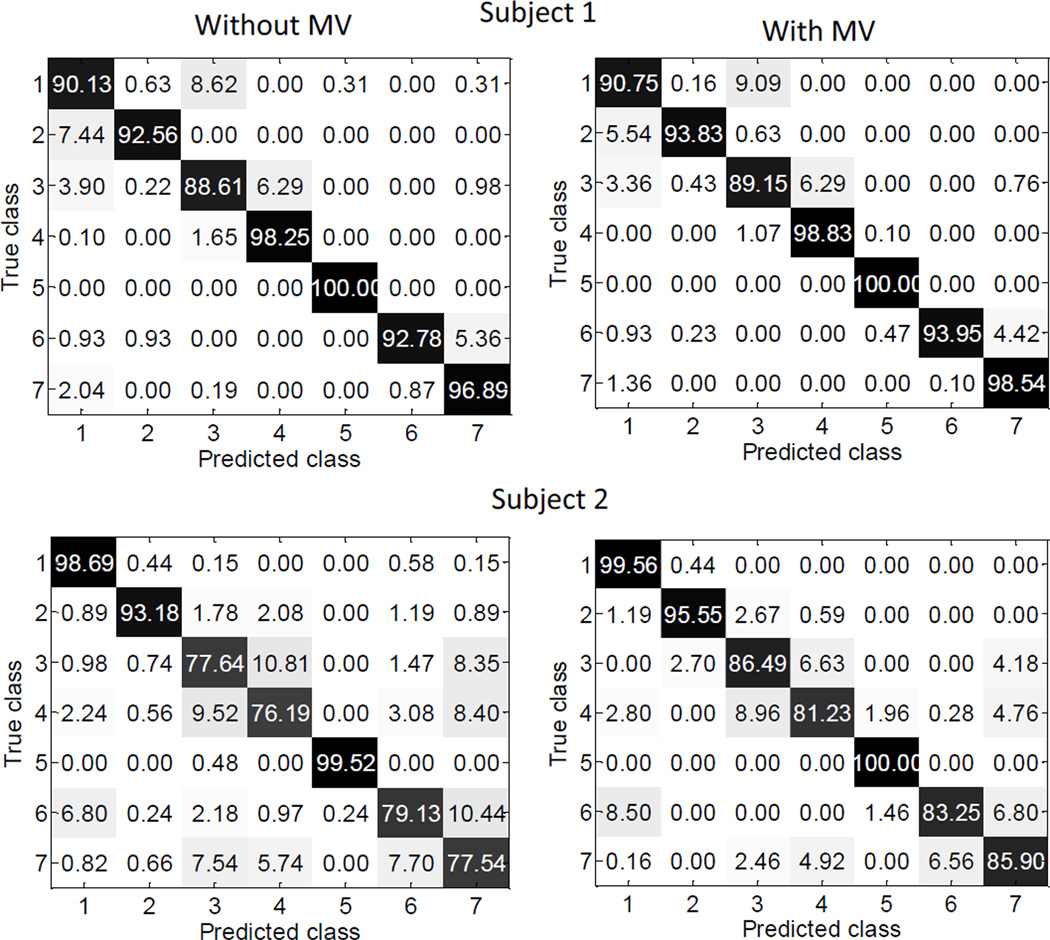
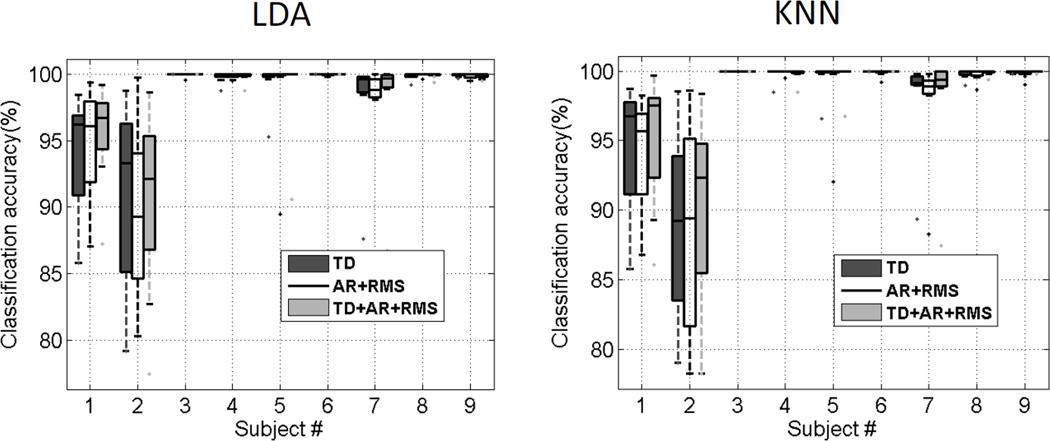
A series of pattern recognition analysis was performed using the TD, AR+RMS and TD+AR+RMS feature sets in combination with the LDA and KNN classifiers. Figure 4 shows examples of the confusion matrices to illustrate the class-to-class performance of two specific subjects (Subjects 1 and 2), with and without application of majority vote, respectively. The majority vote significantly improved the classification performance. Across all subjects the average overall classification accuracy without majority vote was 97.20 ± 4.0% while the accuracy increased to 97.93 ± 3.3% with majority vote (P<0.001). Examination of the class-to-class results revealed that the misclassifications were not consistent among subjects. Figure 5 presents the box plots of the overall classification accuracies from 9 subjects with different combinations of feature sets and classifiers, while the mean and standard deviation of each subject’s performance is demonstrated in Table II. For every combination of feature sets and classifiers, the average overall classification accuracy was higher than 97%. It was found that Compound Symmetry (CS) had the best model fit for our repeated-measures data. Pairwise comparisons using the Bonferroni correction with a family confidence coefficient of 0.95 were calculated to determine significant effects in the RM-ANOVA post-hoc tests. It was found that feature set had a significant effect on classification accuracy (F[2, 42] =5.60, P=0.01), while classifier did not significantly affect the classification performance (F[1,42] =1.26, P=0.27). The classification accuracy derived from the AR features was lower than that using the TD (P=0.38) or TD+AR+RMS (P=0.01) features; while the classification accuracy derived from the TD features was not significantly different from the TD+AR+RMS features (P=0.24). It is noted that across all subjects, the maximum difference in average overall classification accuracy was only 0.5% with different feature sets or classifiers; while for individual subjects, the maximum overall classification difference was 2.2%.
Fig. 4.
Class-to-class confusion matrices derived from Subject 1 and Subject 2, with and without application of majority vote (MV) for classification, respectively. Results are averaged as percentages. The results along the main diagonal, shaded in black, are correct classifications (accuracy) and those off the main diagonal, shaded in grey, are incorrect classifications (error rate). The TD feature set and LDA classifier were used in this example.
Fig. 5.
Box plots of the overall classification accuracies from 9 subjects with different combinations of feature sets and classifiers
Table2.
Pattern recognition results (mean ± sd) in SCI subjects, averaged across eight-fold tests for each subject. (Unit: %)
| Subject No. |
TD feature set | AR+RMS feature set | TD+AR+RMS feature set | |||
|---|---|---|---|---|---|---|
| LDA | KNN | LDA | KNN | LDA | KNN | |
| 1 | 94.0 ± 4.8 | 94.5 ± 4.7 | 94.8 ± 4.6 | 94.0 ± 4.8 | 95.5 ± 3.8 | 95.2 ± 4.9 |
| 2 | 90.9 ± 7.5 | 88.9 ± 6.8 | 89.5 ± 6.8 | 88.7 ± 7.7 | 90.6 ± 7.1 | 90.2 ± 6.9 |
| 3 | 100.0 ± 0.0 | 100.0 ± 0.0 | 99.9 ± 0.2 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 |
| 4 | 99.8 ± 0.5 | 99.8 ± 0.5 | 99.9 ± 0.2 | 99.9 ± 0.2 | 99.8 ± 0.4 | 99.8 ± 0.5 |
| 5 | 99.4 ±1.7 | 99.6 ± 1.2 | 98.7 ± 3.7 | 99.0 ± 2.8 | 99.8 ± 3.3 | 99.6 ± 1.1 |
| 6 | 100.0 ± 0.0 | 100.0 ± 0.0 | 99.9 ± 0.1 | 99.9 ± 0.3 | 100.0 ± 0.0 | 100.0 ± 0.0 |
| 7 | 97.9 ± 4.2 | 98.1 ± 3.5 | 97.5 ± 4.5 | 97.8 ± 3.7 | 98.0 ± 4.5 | 98.0 ± 4.3 |
| 8 | 99.8 ± 0.3 | 99.8 ± 0.4 | 99.9 ± 0.1 | 99.8 ± 0.4 | 99.9 ± 0.2 | 99.9 ± 0.2 |
| 9 | 99.9 ± 0.1 | 99.9 ± 0.1 | 99.9 ± 0.2 | 99.8 ± 0.4 | 99.9 ± 0.2 | 100.0 ± 0.0 |
| Average | 98.0 ± 3.3 | 97.8 ± 3.8 | 97.8 ± 3.6 | 97.7 ± 3.9 | 98.2 ± 3.2 | 98.1 ± 3.4 |
C. Preliminary Channel Reduction Analysis
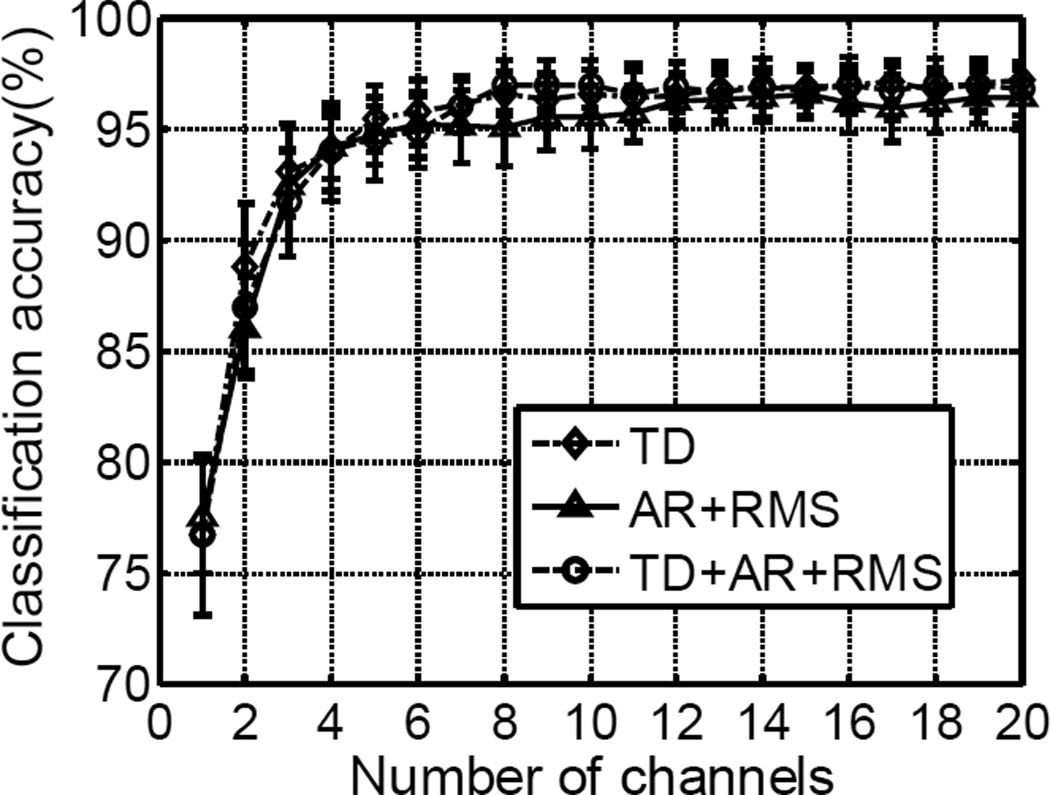
The preliminary channel selection analysis indicated that it was feasible to greatly reduce the number of EMG channels while maintaining high classification accuracy. Figure 6 shows an example of performance using the different feature sets and the LDA classifier. Across all subjects, the three EMG channels selected using the SFS method achieved higher than 90% average overall classification accuracy. With increase of the EMG channels to 8, the overall classification accuracy tended to saturate (at higher than 95%). Little improvement was achieved with further increase of EMG channels.
Fig. 6.
The classification performance with limited number of EMG channels selected using the SFS method. The LDA classifier was used with different feature sets in this example.
IV. Discussions and Conclusions
This study focuses on assessment of a novel application of myoelectric pattern recognition techniques for patients with neurological disorders. In contrast to success achieved in prosthesis control using EMG pattern recognition techniques, myoelectric pattern recognition based systems have rarely been designed for individuals with neurologic injuries. A previous study [14] assessed classification of intended hand grasp patterns of stroke subjects with untargeted placement of ten surface electrodes on paretic forearm and hand muscles, and achieved relatively low mean classification accuracies (71.3% for moderately impaired subjects and 37.9% for severely impaired subjects). Taking advantage of high density surface EMG recording, we demonstrated that high accuracies were achieved in classification of 20 different elbow, wrist, hand, and finger/thumb movements, suggesting that substantial motor control information can be extracted from paretic muscles of stroke subjects. A channel reduction analysis on the high density surface EMG further demonstrated the clinical feasibility of using such information for myoelectric control [15].
By testing a novel application of high density surface EMG recording and pattern recognition techniques in individuals with a different nature of neurologic injury from stroke (i.e. incomplete SCI), the current study further assesses the concept of applying myoelectric pattern recognition techniques for improved rehabilitation after neurologic injuries. We have demonstrated that applying pattern recognition techniques to high density surface EMG recordings achieved high accuracies in classification of 7 different intended hand patterns (6 hand grasp patterns and the hand rest pattern) of 9 incomplete SCI subjects, suggesting that substantial motor control commands can be extracted from partially paralyzed muscles following incomplete SCI. This study, together with the previous one in stroke [15], supports the concept of using myoelectric pattern recognition techniques to control EMG driven therapy or assistive devices toward improved motor recovery for individuals with neurologic disorders.
In particular, the high accuracies achieved in classification of the intended hand grasp patterns imply a great potential for intuitive control of assistive devices towards improved restoration of hand function, which is very important due to its importance in daily activities. Indeed, a survey showed that regaining arm and hand function would be most important to quadriplegics in terms of improving quality of life [29]. While robotic training of the lower extremity has been extensively studied in the recovery of gait function after SCI [30–33], several very recent studies have performed upper extremity robotic training in incomplete cervical SCI and demonstrated the effectiveness of such training for upper limb function improvement [34–36]. In this regard, the novel myoelectric pattern recognition strategy proposed in this study has great potential for improvement of hand function after incomplete SCI by promoting EMG-driven artificial devices for active and dexterous robotic training.
The six hand patterns used in this study were selected from the Cutkosky’s grasp taxonomy [37] based on the usage frequency of different grasp types. The typical activities of daily living can be accomplished using a finite set of predefined grasps. A recent study has shown that a small number of grasp types comprises the majority of typical daily activities. The six selected hand patterns (i.e. power grip, cylindrical grip, key grip, tool grip, open pinch, and fine pinch) comprise nearly 80% of the daily usage time [38] and thus can be viewed as shortcuts for the main daily operations of every human. Because of this, the latest prosthetic arm, the DEKA arm, also chose to have these six hand patterns [39]. Compared with other movements (such as wrist rotation or flexion/extension), the hand patterns are more complex and difficult to decode using conventional myoelectric control techniques (i.e. based on EMG amplitude). Thus these patterns were selected to test the proposed myoelectric pattern recognition strategy. We acknowledge that development of a practical assistive device for cervical SCI subjects may involve elbow and wrist movements in addition to hand patterns. The control of such a device may require a pattern recognition strategy or a combination of pattern recognition and conventional strategies. Further studies are necessary to determine the most appropriate control strategy.
Similar to our previous studies [15, 17], a preliminary channel selection analysis indicates that it was possible to maintain high levels of classification accuracy with only a very limited number of EMG channels. This demonstrates clinical feasibility and robustness of the proposed myoelectric pattern recognition strategy. Although with high density surface EMG much redundant information exists among different channels, such recording can be used to optimize electrode number and location to facilitate implementation of a practical myoelectric control system.
Compared with myoelectric prosthesis control, there are also several factors that are unique to myoelectric control for individuals with SCI. For example, it is likely that the voluntary EMG activities are contaminated by involuntary muscle contractions as a result of muscle spasticity. As in our previous study with hemiparetic stroke subjects [15], additional rest periods were allowed for SCI subjects to decrease the muscle spasticity or involuntary EMG activities. It is currently unclear how relatively high levels of muscle spasticity may affect the classification performance. A previous study indicated that the classification performance may not be compromised as long as the interference (e.g., electrocardiography artifacts) was consistently present in specific channels for the tested classes [40]. Further experimental studies are necessary to investigate the effect of strong muscle spasticity on classification performance by comparing the classification accuracies in presence or absence of such spasticity. In addition to classification accuracy, involuntary EMG activity may also compromise muscle activity onset detection using conventional amplitude threshold based methods, thus making it a challenging task to automatically detect onset or offset of a muscle activity. Appropriate methods other than conventional amplitude thresholding should be developed for automatic onset detection. This is particularly important for implementation of a myoelectric system for subjects with neurological disorders (e.g., stroke, SCI) using a conventional control strategy, or using a combination of conventional and pattern recognition control strategies. We have shown that voluntary EMG bursts can be distinguished from spurious background spikes in the nonlinear dynamic or complexity domain. Based on this, a muscle activity onset detection method was developed against those spurious background spikes (such as spontaneous tonic spikes) [41, 42].
This study is currently limited by only recording from incomplete SCI subjects (ASIA C and D). A recent study [43] indicates that EMG signals from lower extremities of patients with motor complete cervical SCI can be detected in response to voluntary movement attempts, and thus it is feasible to use these signals as a command source for motor neuroprosthetic control. The study reports that significant EMG activity was evident in 89% of the 192 examined muscles of 12 clinically complete cervical SCI patients during their attempted movements of the foot and lower limb [43]. For clinical complete cervical SCI, it would be interesting to examine whether high density surface EMG can be used to sense isometric muscular activity in the upper limb muscles even no movement can be produced. The muscular activity, if captured by high density surface EMG, can be potential command signals for a myoelectric system with conventional or pattern recognition control strategy. This would promote the restoration of upper limb function for clinically complete SCI subjects (ASIA A and B).
Finally, it is worth noting the SCI subjects in this study had varying levels of impairment (Injury level: C4-C8; ASIA class: C and D; UEMS: 30–45), and the class-to-class errors are not consistent among different subjects. In particular, the first two subjects (ASIA C) with the worst classification performance had the lowest UEMS (30 for both) among all the subjects. This suggests that the EMG activity and classification performance may be affected by different levels or degrees of functional impairment. The variance in daily activity and therapeutic interventions may also contribute to the different EMG patterns and their classification. It follows that the myoelectric pattern recognition control system should be individually customized for SCI subjects. The design should consider motor control features of each specific subject. The selection of target tasks should also reflect the degree of functional impairment and injury level, and the need of each specific subject.
In conclusion, this study presents a novel framework toward hand function restoration using high density surface EMG recording and pattern recognition analysis for individuals with incomplete cervical SCI. High accuracies can be obtained in classification of seven intended hand movements, and the classification performance can be maintained with a very limited number of EMG channels selected from the high density surface EMG recording. This suggests that with myoelectric pattern recognition techniques substantial motor control information can be extracted from partially paralyzed muscles of SCI subjects. Such information will potentially enable volitional control of assistive devices, thereby facilitating function restoration for individuals with spinal injury.
Acknowledgments
This work was supported in part by the National Institutes of Health under Grant R21NS075463 and Grant R24HD050821.
Biographies

Jie Liu received the B.S. and M.S. degrees in mechanical manufacturing and automation from Harbin Institute of Technology, Harbin, Heilongjiang, China in 1998 and 2000, respectively, and the Ph.D. degree in mechanical engineering from Beijing University of Aeronautics and Astronautics, Beijing, China, in 2003.
He was a Marie Curie Transfer of Knowledge fellow at Unilever R&D, Port Sunlight, UK, and a postdoctoral fellow in the BrainGate Project at Brown University, Providence, RI, USA. He is currently a Research Associate at the Rehabilitation Institute of Chicago, Chicago, IL, USA. His research interests include electromyography signal processing, control of robots and assistive devices.

Ping Zhou (S’01–M’05–SM’07) received the B.S. degree in electrical engineering and the M.S. degree in biomedical engineering from the University of Science and Technology of China, Hefei, China, in 1995 and 1999, respectively, and the Ph.D. degree in biomedical engineering from Northwestern University, Evanston, IL, in 2004. His Ph.D. dissertation project was performed as part of the Sensory Motor Performance Program (SMPP), Rehabilitation Institute of Chicago, Chicago, IL, USA.
From 2004 to 2006, he was a Research Associate in the Neural Engineering Center for Artificial Limbs (NECAL), Rehabilitation Institute of Chicago. After that he has been a Research Scientist in NECAL and later in SMPP at the Rehabilitation Institute of Chicago. He has been an Adjunct Assistant Professor since 2006 in the Department of Physical Medicine and Rehabilitation, Northwestern University, Chicago, USA, and a Professor since 2012 in the Institute of Biomedical Engineering, University of Science and Technology of China, Hefei, China. His current research interests include biomedical signal (in particular, EMG) processing, motor unit pathophysiology in neurologic disorders, noninvasive electrodiagnosis of neuromuscular diseases, computational modeling of neuromuscular systems, myoelectric prosthesis control, and assistive devices.
Footnotes
Personal use of this material is permitted. However, permission to use this material for any other purposes must be obtained from the IEEE by sending an email to pubs-permissions@ieee.org.
Contributor Information
Jie Liu, Sensory Motor Performance Program (SMPP), Rehabilitation Institute of Chicago (RIC), Chicago, IL 60611 USA.
Ping Zhou, SMPP, RIC and the Department of Physical Medicine and Rehabilitation, Northwestern University, Chicago, IL 60611 USA, and the Institute of Biomedical Engineering, University of Science and Technology of China, Hefei, 230027 China (phone: 312-238-1365; p-zhou@northwestern.edu).
References
- 1.Krebs H, Dipietro L, Levy-Tzedek S, Fasoli S, Rykman-Berland A, Zipse J, Fawcett J, Stein J, Poizner H, Lo A, Volpe B, Hogan N. A paradigm shift for rehabilitation robotics. IEEE Eng. Med. Biol.Mag. 2008 Jul-Aug;vol. 27(no. 4):61–70. [Google Scholar]
- 2.Marchal-Crespo L, Reinkensmeyer DJ. Review of control strategies for robotic movement training after neurologic injury. J Neuroeng Rehabil. 2009 Jun;6:20. doi: 10.1186/1743-0003-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu XL, Tong KY, Song R, Zheng XJ, Leung WW. A comparison between electromyography-driven robot and passive motion device on wrist rehabilitation for chronic stroke. Neurorehabil. Neural Repair. 2009 Oct;vol. 23(no. 8):837–846. doi: 10.1177/1545968309338191. [DOI] [PubMed] [Google Scholar]
- 4.Van Peppen RP, Kwakkel G, Wood-Dauphinee S, Hendriks HJ, Van der Wees PJ, Dekker J. The impact of physical therapy on functional outcomes after stroke: What’s the evidence? Clin. Rehabil. 2004 Dec;vol. 18(no. 8):833–862. doi: 10.1191/0269215504cr843oa. [DOI] [PubMed] [Google Scholar]
- 5.Cauraugh J, Light K, Kim S, Thigpen M, Behrman A. Chronic motor dysfunction after stroke: recovering wrist and finger extension by electromyography-triggered neuromuscular stimulation. Stroke. 2000 Jun;vol. 31, vol. 6:1360–1364. doi: 10.1161/01.str.31.6.1360. [DOI] [PubMed] [Google Scholar]
- 6.Scott RN. Myoelectric control of prostheses. Arch Phys Med Rehabil. 1966 Mar;47(3):174–181. [PubMed] [Google Scholar]
- 7.Parker PA, Scott RN. Myoelectric control of prostheses. Crit Rev Biomed Eng. 1986;13(4):283–310. Review. [PubMed] [Google Scholar]
- 8.Zecca M, Micera S, Carrozza MC, Dario P. Control of multifunctional prosthetic hands by processing the electromyographic signal. Crit Rev Biomed Eng. 2002;30(4–6):459–485. doi: 10.1615/critrevbiomedeng.v30.i456.80. Review. [DOI] [PubMed] [Google Scholar]
- 9.Parker P, Englehart K, Hudgins B. Myoelectric signal processing for control of powered limb prostheses. J Electromyogr Kinesiol. 2006 Dec;16(6):541–548. doi: 10.1016/j.jelekin.2006.08.006. Review. [DOI] [PubMed] [Google Scholar]
- 10.Dipietro L, Ferraro M, Palazzolo JJ, Krebs HI, Volpe BT, Hogan N. Customized interactive robotic treatment for stroke: EMG-triggered therapy. IEEE Trans. Neural Syst. Rehabil. Eng. 2005 Sep;vol. 13(no. 3):325–334. doi: 10.1109/TNSRE.2005.850423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu XL, Tong KY, Song R, Zheng XJ, Lui KH, Leung WW, Ng S, Au-Yeung SS. Quantitative evaluation of motor functional recovery process in chronic stroke patients during robot-assisted wrist training. J Electromyogr. Kinesiol. 2009 Aug;vol. 19(no. 4):639–650. doi: 10.1016/j.jelekin.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Au AT, Kirsch RF. EMG-based prediction of shoulder and elbow kinematics in able-bodied and spinal cord injured individuals. IEEE Trans. Rehabil. Eng. 2000 Dec;vol. 8(no. 4):471–480. doi: 10.1109/86.895950. [DOI] [PubMed] [Google Scholar]
- 13.Hincapie JG, Kirsch RF. Feasibility of EMG-based neural network controller for an upper extremity neuroprosthesis. IEEE Trans.Neural Sys. Rehabil. Eng. 2009 Feb;vol. 17(no. 1):80–90. doi: 10.1109/TNSRE.2008.2010480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SW, Wilson KM, Lock BA, Kamper DG. Subject-specific myoelectric pattern classification of functional hand movements for stroke survivors. IEEE Trans. Neural Syst. Rehabil. Eng. 2011 Oct;vol. 19(no. 5):558–566. doi: 10.1109/TNSRE.2010.2079334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Zhou P. High density myoelectric pattern recognition towards improved stroke rehabilitation. IEEE Trans. Biomed. Eng. 2012 Jun;vol. 59(no. 6):1649–1657. doi: 10.1109/TBME.2012.2191551. [DOI] [PubMed] [Google Scholar]
- 16.Englehart K, Hudgins B. A robust, real-time control scheme for multifunction myoelectric control. IEEE Trans. Biomed. Eng. 2003 Jul;vol. 50(no. 7):848–854. doi: 10.1109/TBME.2003.813539. [DOI] [PubMed] [Google Scholar]
- 17.Zhou P, Lowery MM, Englehart KB, Huang H, Li G, Hargrove L, Dewald JP, Kuiken TA. Decoding a new neural machine interface for control of artificial limbs. J Neurophysiol. 2007 Nov;vol. 98(no. 5):2974–2982. doi: 10.1152/jn.00178.2007. [DOI] [PubMed] [Google Scholar]
- 18.Hudgins B, Parker PA, Scott R. A newstrategy for multifunction myoelectric control. IEEE Trans. Biomed. Eng. 1993 Jan;vol. 40(no. 1):82–94. doi: 10.1109/10.204774. [DOI] [PubMed] [Google Scholar]
- 19.Farina D, Merletti R. Comparison of algorithms for estimation of EMG variables during voluntary isometric contractions. J. Electromyogr. Kinesiol. 2000 Oct;vol. 10(no. 5):337–349. doi: 10.1016/s1050-6411(00)00025-0. [DOI] [PubMed] [Google Scholar]
- 20.Graupe D, Salahi J, Kohn KH. Multifunctional prosthesis and orthosis control via microcomputer identification of temporal pattern differences in single-site myoelectric signals. J Biomed Eng. 1982 Jan;4(1):17–22. doi: 10.1016/0141-5425(82)90021-8. [DOI] [PubMed] [Google Scholar]
- 21.Huang Y, Englehart KB, Hudgins B, Chan AD. A Gaussian mixture model based classification scheme for myoelectric control of powered upper limb prostheses. IEEE Trans. Biomed. Eng. 2005 Nov;vol. 52(no. 11):1801–1811. doi: 10.1109/TBME.2005.856295. [DOI] [PubMed] [Google Scholar]
- 22.Hargrove LJ, Englehart K, Hudgins B. A comparison of surface and intramuscular myoelectric signal classification. IEEE Trans Biomed Eng. 2007 May;vol. 54(no. 5):847–853. doi: 10.1109/TBME.2006.889192. [DOI] [PubMed] [Google Scholar]
- 23.Hargrove LJ, Scheme EJ, Englehart KB, Hudgins BS. Multiple binary classifications via linear discriminant analysis for improved controllability of a powered prosthesis. IEEE Trans. Neural Syst. Rehabil. Eng. 2010 Feb;vol. 18(no. 1):49–57. doi: 10.1109/TNSRE.2009.2039590. [DOI] [PubMed] [Google Scholar]
- 24.Hargrove L, Li G, Englehart K, Hudgins B. Principal components analysis preprocessing for improved classification accuracies in pattern-recognition-based myoelectric control. IEEE Trans. Biomed. Eng. 2009 May;vol. 56(no. 5):1407–1414. doi: 10.1109/TBME.2008.2008171. [DOI] [PubMed] [Google Scholar]
- 25.Huang H, Zhou P, Li G, Kuiken TA. Spatial filtering improves EMG classification accuracy following targeted muscle reinnervation. Ann. Biomed. Eng. 2009 Sep;vol. 37(no. 9):1849–1857. doi: 10.1007/s10439-009-9737-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou P, Suresh NL, Rymer WZ. Surface electromyogram analysis of the direction of isometric torque generation by the first dorsal interosseous muscle. J. Neural Eng. 2011 Jun;vol. 8(no. 3):036028. doi: 10.1088/1741-2560/8/3/036028. [DOI] [PubMed] [Google Scholar]
- 27.Ye J, Li T, Xiong T, Janardan R. Using uncorrelated discriminant analysis for tissue classification with gene expression data. IEEE Trans. Computat. Biol. Bioinformat. 2004 Oct-Dec;vol. 1:181–190. doi: 10.1109/TCBB.2004.45. [DOI] [PubMed] [Google Scholar]
- 28.Duda RO, Hart PE, Stork DG. Pattern Classification. 2nd ed. New York: Wiley-Interscience; 2001. [Google Scholar]
- 29.Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004 Oct;21(10):1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 30.Dietz V, Wirz M, Jensen L. Locomotion in patients with spinal cord injuries. Phys Ther. 1997 May;77(5):508–516. doi: 10.1093/ptj/77.5.508. Review. [DOI] [PubMed] [Google Scholar]
- 31.Wirz M, Zemon DH, Rupp R, Scheel A, Colombo G, Dietz V, Hornby TG. Effectiveness of automated locomotor training in patients with chronic incomplete spinal cord injury: a multicenter trial. Arch Phys Med Rehabil. 2005 Apr;86(4):672–680. doi: 10.1016/j.apmr.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 32.van Hedel HJ, Dietz V. Rehabilitation of locomotion after spinal cord injury. Restor Neurol Neurosci. 2010;28(1):123–134. doi: 10.3233/RNN-2010-0508. Review. [DOI] [PubMed] [Google Scholar]
- 33.Mehrholz J, Kugler J, Pohl M. Locomotor training for walking after spinal cord injury. Spine (Phila Pa 1976) 2008 Oct 1;33(21):E768–E777. doi: 10.1097/BRS.0b013e3181849747. Review. [DOI] [PubMed] [Google Scholar]
- 34.Yozbatiran N, Berliner J, O'Malley MK, Pehlivan AU, Kadivar Z, Boake C, Francisco GE. Robotic training and clinical assessment of upper extremity movements after spinal cord injury; a single case report. J Rehabil Med. 2012 Feb;vol. 44:186–188. doi: 10.2340/16501977-0924. [DOI] [PubMed] [Google Scholar]
- 35.Zariffa J, Kapadia N, Kramer J, Taylor P, Alizadeh-Meghrazi M, Zivanovic V, Albisser U, Willms R, Townson A, Curt A, Popovic M, Steeves J. Relationship between clinical assessments of function and measurements from an upper-limb robotic rehabilitation device in cervical spinal cord injury. IEEE Trans Neural Syst Rehabil Eng. 2011 Dec 23; doi: 10.1109/TNSRE.2011.2181537. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Zariffa J, Kapadia N, Kramer JL, Taylor P, Alizadeh-Meghrazi M, Zivanovic V, Willms R, Townson A, Curt A, Popovic MR, Steeves JD. Feasibility and efficacy of upper limb robotic rehabilitation in a subacute cervical spinal cord injury population. Spinal Cord. 2012 Mar;50(3):220–226. doi: 10.1038/sc.2011.104. [DOI] [PubMed] [Google Scholar]
- 37.Cutkosky MR. On grasp choice, grasp models, and the design of hands for manufacturing tasks. IEEE Trans. Robot. Autom. 1989 Jun;vol. 5(no. 3):269–279. [Google Scholar]
- 38.Zheng JZ, Rosa SDL, Dollar AM. An investigation of grasp type and frequency in daily household and machine shop tasks; Proc. IEEE Int. Conf. Robotics and Automation; 2011. May, pp. 4169–4175. [Google Scholar]
- 39.Resnik L. Research update: VA study to optimize DEKA arm. J.Rehabil. Res. Develop. 2010;vol. 47:ix–x. [PubMed] [Google Scholar]
- 40.Hargrove L, Zhou P, Englehart K, Kuiken TA. The effect of ECG interference on pattern-recognition-based myoelectric control for targeted muscle reinnervated patients. IEEE Trans. Biomed. Eng. 2009 Sep;vol. 56(no. 9):2197–2201. doi: 10.1109/TBME.2008.2010392. [DOI] [PubMed] [Google Scholar]
- 41.Zhou P, Barkhaus PE, Zhang X, Rymer WZ. Characterizing the complexity of spontaneous motor unit patterns of amyotrophic lateral sclerosis using approximate entropy. J. Neural Eng. 2011 Dec;vol. 8(no. 6):066010. doi: 10.1088/1741-2560/8/6/066010. [DOI] [PubMed] [Google Scholar]
- 42.Zhang X, Zhou P. Sample entropy analysis of surface EMG for improved muscle activity onset detection against spurious background spikes. Journal of Electromyography and Kinesiology. 2012 Dec;vol. 22(no. 6.):901–907. doi: 10.1016/j.jelekin.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moss CW, Kilgore KL, Peckham PH. A novel command signal for motor neuroprosthetic control. Neurorehabil. Neural Repair. 2011 Jun;vol. 25(no. 9):847–854. doi: 10.1177/1545968311410067. [DOI] [PubMed] [Google Scholar]