Abstract
Background
The sensitized patients can develop an accelerated form of graft rejection mediated by humoral and/or T cell-mediated responses, which are resistant to currently used immunosuppression.
Methods&Results
In our model of fulminant cardiac allograft rejection in sensitized hosts, groups of wild-type (WT) and B cell-deficient (BKO) mice (B6) were challenged with skin grafts (B/c). Alloreactive CD8 T effector (Teff) activation and T memory (Tmem) differentiation during a 60-day follow-up period were reduced in the absence of B cell help. The expression of IL-2Rα, IL-7Rα, and IL-15Rα, which support/program CD8 Teff/Tmem expansion, differentiation, and survival, were selectively decreased in BKO hosts. Unlike in WT, in vivo cytotoxic activity analysis of alloreactive Tmem recall response has revealed decreased donor-type (B/c) but not third-party (C3H) cell lysis in sensitized B cell-deficient hosts. However, such impaired allo-Ag specific Tmem recall function was insufficient to markedly prolong cardiac allograft survival in sensitized BKO recipients. Indeed, despite quantitative and statistically significant differences between both animal groups, the biological impact of decreased CD8 Teff/Tmem activation and function in the sensitization phase was marginal. Indeed, cardiac allografts underwent fulminant rejection in sensitized BKO, albeit with somewhat delayed kinetics. Interestingly, unlike in naïve counterparts, the rejection cascade remained CD154 blockade-resistant, evidenced by comparable kinetics, and intra-graft cytokine gene profiles in MR1 mAb-treated sensitized WT and BKO hosts.
Conclusion
Although B cells were important for optimal alloreactive CD8 Teff/Tmem function in the sensitization phase, the fulminant rejection of cardiac allografts was B cell-independent, and CD154 blockade-resistant, as in WT hosts.
Keywords: Sensitized Recipient, Accelerated Rejection, B cells, CD8 T cells, Memory T cells
Introduction
Host sensitization to donor MHC Ags following multiple blood transfusions, previous failed grafts, or pregnancies remains one of the most critical problems in clinical transplantation (1–4). Indeed, up to 40% of sensitized patients on transplant waiting lists have decreased likelihood of a successful match, or may experience accelerated rejection (AccR), which is often irreversible or difficult to control with currently used immunosuppression (1,2).
We have long been interested in dissecting mechanism of and developing approaches to manage graft rejection in sensitized hosts (1,2,5,6). To mimic the clinical scenario in patients, who usually receive organ grafts long after the sensitizing allo-Ag and harbor memory T cells, we have developed a murine model of cardiac AccR (5–8). In this stringent model, B6 mice are challenged with B/c skin, and after resting for >40 days, i.e., until activated CD8 T effector cells (Teff: CD44highCD62Llow) move to “memory” (Tmem: CD44highCD62Lhigh) level, they receive donor-type (B/c) hearts. These cardiac allografts are rejected within 3.5 days (vs. 7 days in unprimed mice) (8). The cardinal features of AccR in this model are as follows: i/ allorective primed/memory CD8 T cells are the principal mediators (8,9); ii/ CD8 T cell sensitization requires CD154 signaling (8) and CD4 help (9–11); iii/ memory CD8 T cell-mediated AccR is CD154 blockade resistant (11,12).
B cells are critical in the mechanism of hyperacute, acute humoral, and chronic graft rejection. Alloreactive B cell activation contributes to alloimmune responses (3,13,14), consistent with amelioration of chronic (15,16) and acute (17) rejection in B cell-deficient (BKO) hosts, or the ability of donor-specific serum to restore acute rejection in B cell-deficient recipients after passive transfer (17,18). MHC class II molecules can also mediate early Ag presentation for CD4 T cell priming (19–21). Although primary CD4 cell responses develop in BKO mice (22–26), depletion of B cells reduces the activation, suggesting that B cells contribute to optimal T cell priming (19,21,27–29). Indeed, indirect allo-Ag presentation via MHC class II by recipient B cells is crucial for the progression of acute rejection (30). However, the role of B cells in T cell expansion/differentiation remains controversial. The absence of B cells had little impact on influenza virus (26), lymphocytic choriomeningitis virus (31), or male H-Y Ag (32) stimulated memory T cells. However, B cells were shown to provide essential Ag (OVA) presentation capacity in vivo, crucial for optimizing expansion and generation of Teff and Tmem cells (33,34).
This study was designed to address the role of B cell deficiency in primed/memory CD8 T cell responses and in the mechanism of cardiac allograft rejection in sensitized mouse recipients.
Results
CD8 activation and differentiation
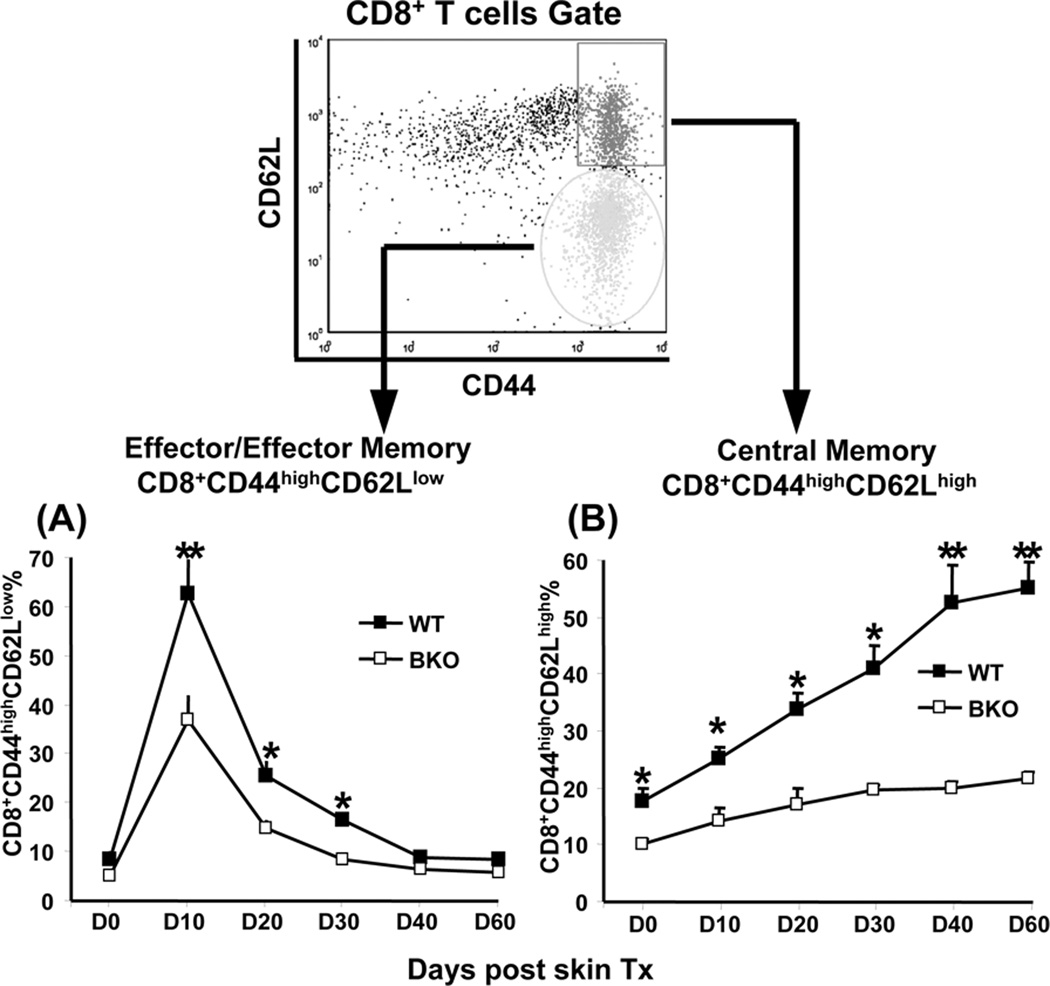
We quantified CD8 activation and kinetics by measuring PBL frequency of CD8 Teff (effector/ effector memory T cells: CD8+CD44highCD62Llow) and Tmem (central memory T cells: CD8+CD44highCD62Lhigh) in groups of B cell-proficient (WT) and BKO mice (B6), which received skin allografts (B/c). First, in agreement with others (30) we found that the survival of skin grafts was comparable in BKO and WT mice (11±1.3 and 10±1.0 days, respectively; n=10/group; not shown). Although CD8 T cells became activated in BKO recipients, their Teff frequency peak diminished after clonal expansion by day 10 after skin challenge (Fig. 1A; 36.7±5.1% vs. 62.7±6.8% of total CD8 in WT; p<0.001). Despite time-dependent decrease in both groups during the contraction phase (10–30 days after skin engraftment), the frequency of Teff remained higher in WT compared with BKO (Fig. 1A; day 20: 25.5±2.8% vs. 14.7±1.7%, and day 30: 16.5±0.6% vs. 8.5±1.1%; p<0.01). Similar Teff kinetics was noted in spleens and lymph nodes of BKO and WT mice (not shown). During the contraction period accompanied by Teff deprivation, the CD8+CD44highCD62Lhigh Tmem subset increased progressively in WT, but not BKO recipients (Fig. 1B; day 20: 33.6±2.9% vs. 17.1±2.8%, and day 30: 41.0±4.1% vs. 19.6±0.9%; p<0.001). Finally, in the memory maintenance phase (40–60 days after skin), higher numbers of Tmem were recorded in WT, compared with BKO hosts (Fig. 1 B; day 40: 52.5±6.5% vs. 19.9±1.1%, and day 60: 55.1±4.5% vs. 21.7±1.1%; p<0.001). Thus, there was a partial failure to activate CD8 Teff and generate memory repertoire in allo-Ag primed BKO mice.
Figure 1.
Disparate kinetics and frequency of primary alloreactive CD8 activation in WT vs. BKO recipients. Balb/c skin grafts were transplanted onto WT or BKO C57BL/6 mice. Alloreactive CD8 activation and its kinetics following allogeneic skin grafts were assessed by FACS analysis of the frequency of effector/effector memory T cells and central memory T cells in peripheral blood, as described in Material and Methods. (A) and (B) The averages of Teff (CD8+CD44highCD62Llow) and Tmem (CD8+CD44highCD62Lhigh) percentages were measured serially following skin grafting in WT and BKO recipients (*p<0.01, **p<0.001, n=5/group). Independent experiments were repeated four times with similar results.
IL-2Rα, IL-7Rα and IL-15Rα expression by CD8 Teff/Tmem
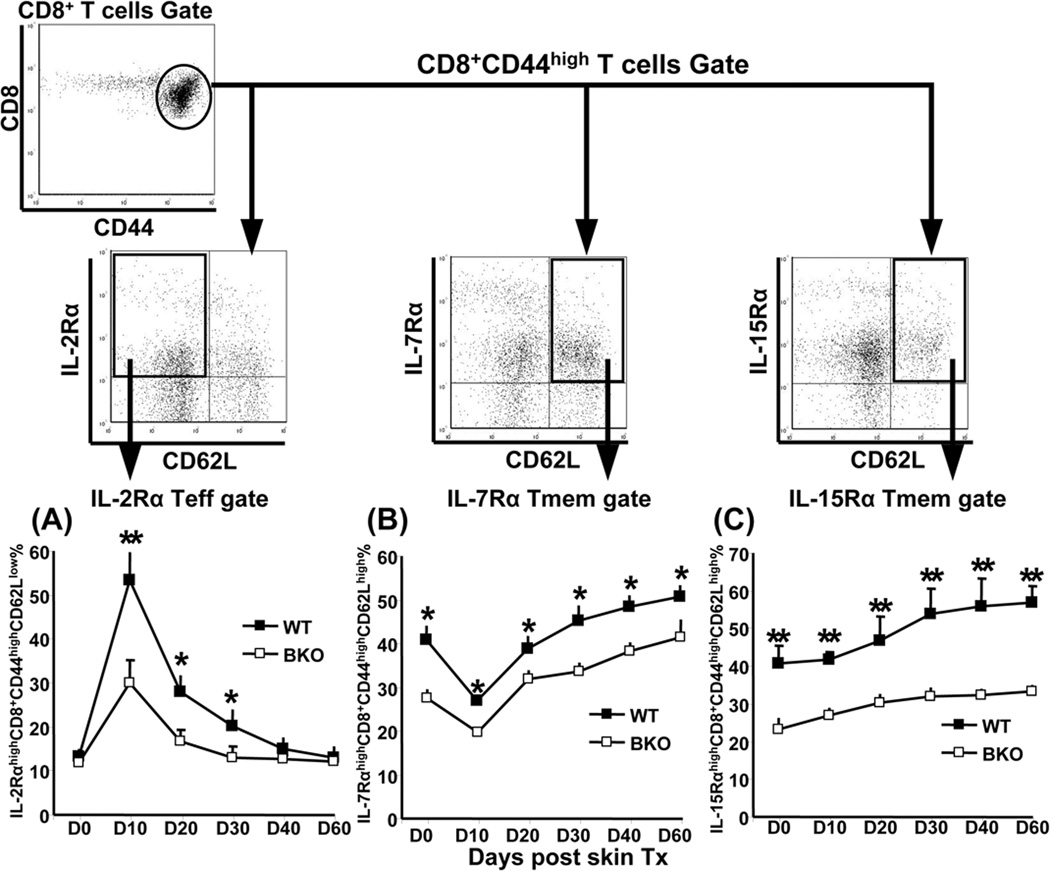
IL-2, IL-7, and IL-15, the cytokines with fundamental roles for CD8 Teff/Tmem differentiation, are critical regulators of lymphocyte homeostasis. Their disparate functions can be ascribed to distinct signaling pathways initiated by proprietary cytokine receptor chains (35–37). To elucidate the mechanism of impaired CD8 Teff/Tmem expansion, differentiation, and survival, we analyzed IL-2Rα, IL-7Rα, and IL-15Rα expression, combined with CD62L level, gated on CD8+CD44high PBLs at each checkpoint from the initial activation to the memory phase in skin-sensitized hosts. We found that in WT recipients, resting CD8 T cells constitutively expressed IL-7Rα and IL-15Rα, but not IL-2Rα (Fig. 2), which could have affected naïve T cell maturation and survival. The B/c skin grafts triggered robust CD8 T cell clonal expansion and differentiation (Fig. 1A). By day 10, we found elevated IL-2Rα (Fig. 2A) and IL-15Rα (data not shown) levels on Teff, which may control the overall clonal expansion and co-stimulatory TCR-mediated T cell proliferation. In marked contrast, by day 10, IL-7α expression on Tmem was downregulated (Fig. 2B). In the contraction phase (day 10–30), IL-2Rα expression rapidly diminished (Fig. 2A), consistent with a large scale of Teff apoptotic episodes and return to homeostasis. Tmem homeostasis results from balancing their levels (~50% of total CD8) with survival and death (Fig. 1B) that requires IL-7 and IL-15. As shown in Fig. 2B,C, the expression of IL-7Rα and IL-15Rα on Tmem was retained in the contraction and throughout memory maintenance (day 30–60), whereas IL-2Rα function was abolished (Fig. 2A). Although a similar trend for each cytokine receptor was noted in BKO hosts, their absolute levels remained lower in the absence of B cells as compared with WT (Fig. 2). Thus, decreased CD8 Teff/Tmem generation and differentiation was accompanied by suboptimal expression of IL-2Rα, IL-7Rα, and IL-15Rα in sensitized BKO recipients.
Figure 2.
Suboptimal expression of IL-2Rα on Teff (CD8+CD44highCD62Llow), and IL-7Rα/IL-15Rα on Tmem (CD8+CD44highCD62Lhigh) in allo-Ag sensitized BKO hosts, compared with WT counterparts. At time-intervals after skin grafting, RBC-free peripheral blood cells were stained with Ab against CD8a, CD44, and CD62L plus IL-2Rα, IL-7Rα, or IL-15Rα, and analyzed by four-color flow cytometry. IL-2RαhighCD62Llow (A), IL-7RαhighCD62Lhigh (B), and IL-15RαhighCD62Lhigh (C), were gated on CD8+CD44high population, and subtracted from isotype controls. The averages of percentages are shown at serial time-points post B/c skin engraftment (*p<0.01, **p<0.001, n=5/group). The experiments were repeated twice with similar results.
Donor-specific cytotoxic activity in vivo
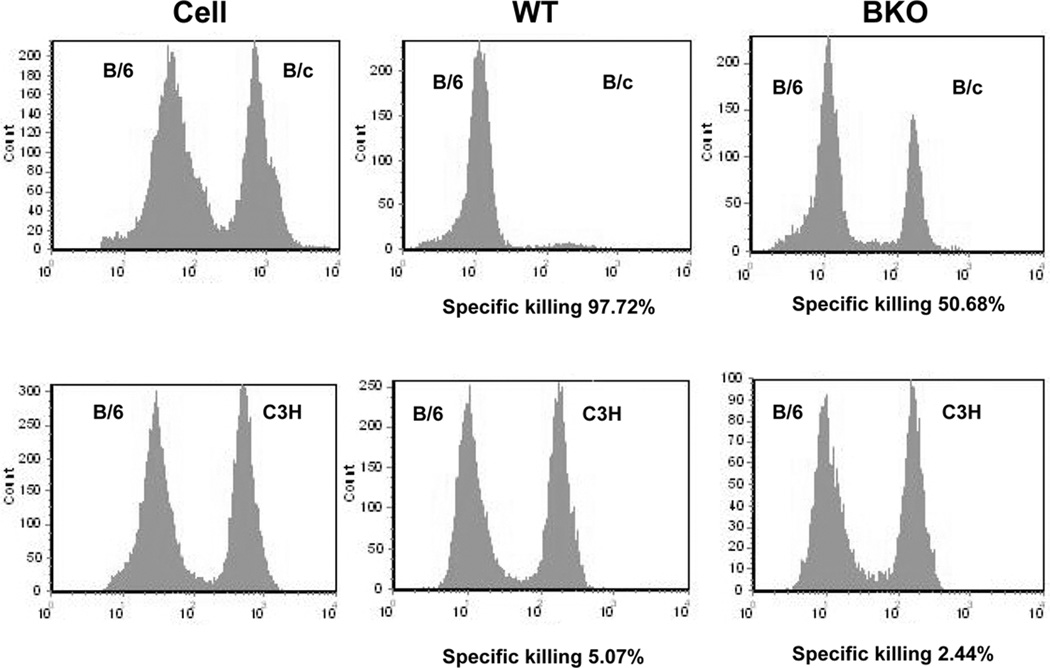
To assess donor-specific cytotoxic function in BKO sensitized mice, we performed in vivo Ag-specific cytotoxic activity assay (60 days post skin graft). WT naïve B6 splenocytes (107) labeled with low dose of CFSE were mixed with the same number of B/c splenocytes labeled with high dose of CFSE, or C3H splenocytes. Both CFSElow and CFSEhigh cell populations were mixed, and injected i.v. to cohorts of WT or BKO recipients of B/c skin grafts (day +60). The CFSElow and CFSEhigh cell frequency in host spleen was determined at 12h by analyzing Topro 3-negative viable lymphocytes. As shown in Fig. 3, reduced cytotoxic activity against B/c targets was detectable in sensitized BKO hosts, as compared with WT (average: 51.8±0.9% vs. 98.9±1.1%, p<0.001). The marginal (2–6%) killing of C3H third-party target cells in WT and BKO hosts indicates these cytotoxic activities were donor Ag-specific.
Figure 3.
Impaired Ag-specific cytotoxic activity in BKO sensitized recipients. Target lysis was calculated based on the incidence of CFSElow and CFSEhigh cells, as described in Material and Methods. Significantly reduced cytotoxic activity against B/c targets in sensitized BKO host, as compared with WT (51.8±0.9% vs. 98.9±1.1%, p<0.001 n=4–5/group). No effect against C3H controls in sensitized mice (WT 6.0±3.9% vs. BKO 4.0±2.0%, p>0.5, n= 4–5/group). Data from one representative experiment of five are shown.
CD154 costimulation blockade-resistant AccR
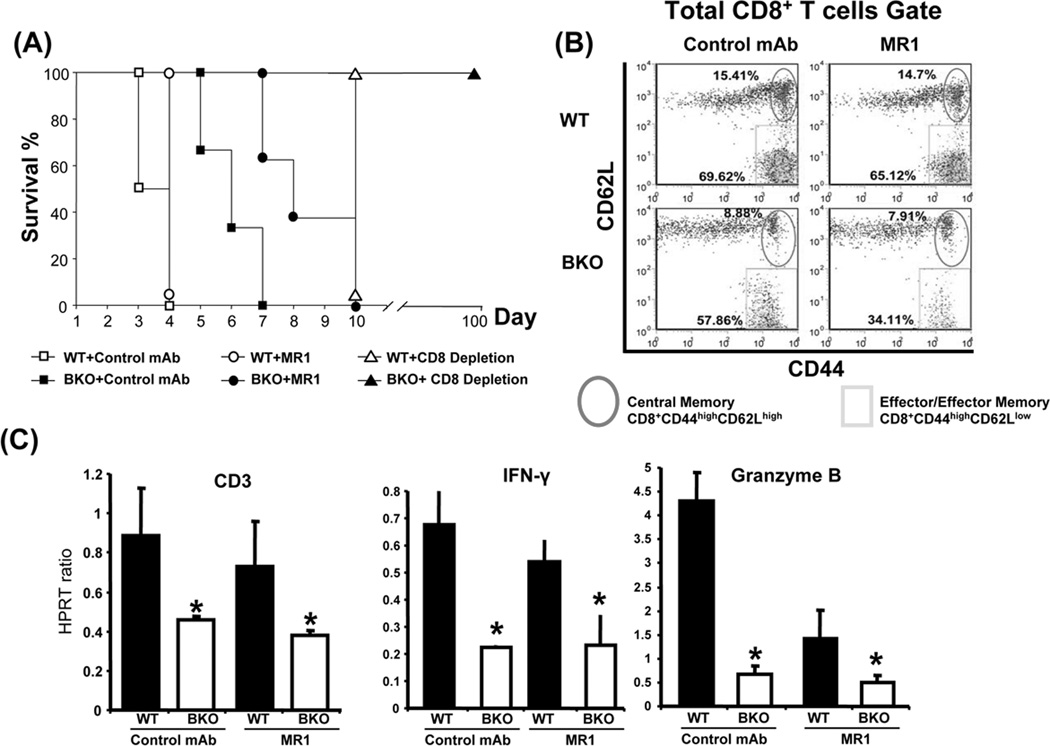
We have reported that naïve and primed/memory CD8+ T cells have differential requirement for CD154 signaling, and unlike acute rejection in naive mice, AccR in sensitized hosts remains CD154 blockade resistant (7,12). Next, groups of WT and B cell-deficient B6 mice, bearing B/c skin grafts for 60 days, were challenged with donor-type heart grafts, in conjunction with anti-CD154, anti-CD8 or control mAb treatment. As shown in Fig. 4A, the rejection of cardiac allografts was somewhat delayed in BKO, as compared with WT mice (MST=6.0 days vs. 3.5 days, p<0.001). Although unlike in WT, MR1 mAb treatment delayed cardiac allograft rejection in primed BKO mice by 2 days (MST=8.0 days vs. 6.0 days, p>0.05), it failed to produce long-term graft acceptance seen otherwise in naïve mice subjected to CD154 blockade (12). Interestingly, CD8 T cell depletion has led to long-term (>100 days) cardiac allograft survival in sensitized B cell-deficient hosts. Consistent with CD154 blockade-resistant rejection, MR1 mAb treatment reduced (but failed to abolish) the CD8 memory recall in sensitized BKO compared with WT counterparts (Fig. 4B: Teff= 35.4±2.1% vs. 60.1±5.3% p<0.01). Moreover, as shown in Fig. 4C, adjunctive MR1 mAb treatment in BKO or WT hosts did not affect intragraft expression of CD3, IFN-γ and granzyme B.
Figure 4.
Accelerated cardiac allograft rejection in sensitized WT and BKO recipients. Primed WT and BKO mice (BL6) were re-challenged with cardiac allografts (B/c) in conjunction with CD154 blockade (MR1 mAb; 0.5 mg/mouse at day 0); CD8 T cell depletion (2.43 mAb; 0.25 mg/mouse/day at day −2, −1, and 0 i.v.), or control mAb at 60 days after skin engraftment. (A) Cardiac allograft survival. Control Ab: ■ BKO (MST=6 days) vs. □ WT (3.5 days), p<0.001; CD154 blockade: ● BKO (8 days) vs. ○ WT (4 days), p<0.001; CD8 depletion: ▲ BKO (MST>100 days) vs. ∆ WT (10 days), p<0.001. N=10/group. (B) Alloreactive CD8 activation measured by flow cytometry at day 10; representative dot plots in total CD8 T cells (n=5/group). (C) Cardiac allografts were harvested at day 4 and tissue RNA samples were subjected to quantitative RT-PCR as described in Materials and Methods. Target gene expressions were calibrated by their ratios against HPRT levels of the same sample. Average expression ratios in different groups were plotted. (n=5/group; *p<0.05). Similar results were recorded in three separate experiments.
Discussion
We analyzed as to whether and how the modulation of CD8 T cell differentiation and function may affect fulminant cardiac allograft rejection in a stringent model of skin-sensitized mouse recipients in the presence or absence B cells. In addition to quantitative reduction of activated Teff and Tmem, several unrecognized defects were revealed in allo-Ag primed BKO hosts. First, after the primary allo-Ag challenge in the absence of B cell help, CD8 Teff/Tmem exhibited similar kinetics of transient Teff and sustained Tmem, but decreased effector and memory phenotype, compared with WT. Second, BKO hosts had suboptimal expression of IL-2Rα, IL-7Rα and IL-15Rα, known to control and support CD8 Teff/Tmem expansion, differentiation, and survival. Third, the impaired allo-Ag specific Tmem recall function reduced donor type cell lysis in sensitized BKO recipients. Fourth, despite quantitative statistical disparities between WT and BKO groups, the biological significance of these immune defects was marginal. Indeed, sensitized B cell-deficient mice were still able to reject donor-type cardiac allografts in an accelerated fashion (MST=6 days) compared with B cell-deficient unprimed recipients (MST=14 days). Fifth, unlike in naïve mice, the rejection cascade in sensitized recipients remained CD154 blockade resistant, as evidenced by comparable AccR kinetics, and intra-graft cytokine gene expression profiles in MR1 mAb-treated WT and BKO hosts. Thus, this study provides evidence that B cells are not obligatory to facilitate fulminant accelerated-type cardiac allograft rejection in skin-sensitized recipients.
Comparable allogeneic skin graft rejection kinetics, but markedly prolonged cardiac allograft survival in BKO vs. WT hosts (17,30), has been taken as evidence that B cells make important contribution in the vascularized allograft rejection cascade. Nonetheless, the BKO immune system, which lacks mature B cells during ontogeny, variably affects the alloreactive CD8 differentiation at different post-transplant stages. There is no doubt that the absence of humoral immunity modifies the immune environment. Indeed, we found lower expression of IL-7Rα and IL-15Rα on CD8 Teff/Tmem in BKO mice vs. WT, which indicates that T cell development and maturation may have been affected in B cell-deficient milieu. It is well established that B cells can act as APCs in the alloreactive CD4 T cell activation (19–21,30). The absence of B cell-mediated MHC class II indirect Ag presentation disrupts normal progression of acute rejection (30), and may affect clonal expansion and memory T cell formation (33,34). Consistent with these findings, our data document significantly reduced CD8 Teff peak in primed BKO mice. Moreover, Tmem generation, differentiation, and survival were diminished in BKO hosts, as compared with WT. Interestingly, consistent with our findings, others have reported that co-transfer of activated T cells and B cells into naïve hosts improved transferred T cell survival and differentiation into T memory phenotype (38).
After leaving the thymus, mature naïve CD8 T cells circulate in the blood and home to lymphoid organs, while IL-7Rα and IL-15Rα signals support their maturation and survival (39,40). Upon Ag encounter, the cells undergo robust clonal expansion, develop effector function in the presence of IL-2Rα and IL-15Rα (41,42), establish and maintain long-lived memory following Ag clearance with IL-7Rα and IL-15Rα expression (39,43). The cytokine signaling supports T cell programming (i.e., clonal expansion/memory maintenance), and promotes T cell function. To elucidate the mechanism of effector/memory phenotype changes, we examined IL-2Rα, IL-7Rα, and IL-15Rα expression following CD8 T cell activation in BKO recipients. Interestingly, suboptimal frequency of IL-2Rα, IL-7Rα and IL-15Rα may have led to impaired Teff/Tmem activation and differentiation in skin-sensitized BKO, compared with WT mice.
Antigen specificity and accelerated immune response to repeated Ag stimulation are the most important features of Tmem recall (8–12,44). Indeed, graft infiltration with Tmem during recall response occurs within 24h (45), and may require only low costimulation signaling (8–12,44). To address whether host sensitization affects alloreactive recall function in BKO mice, we used in vivo cytotoxic activity assay. The otherwise vigorous Ag-specific recall cytotoxicity in WT mice was significantly impaired in BKO mice sensitized with allogeneic skin. Although memory-like NK cells following IL-15 stimulation may play a conditional role in the recall response in sensitized WT recipients (46), their contribution might be dispensable in BKO hosts because of the diminished IL-15 communication and deficiency in the B cell cooperation (47).
CD8 Tmem are the principal mediators in our clinically relevant model of cardiac allograft rejection in skin-sensitized mice (8,9). In addition: 1/ primed/memory CD8+ T cells require CD4 help, and 2/ AccR by primed/memory CD8+ T cells is CD154 blockade-resistant (9–12). Here, we evaluated as to whether and how Tmem recall affected allograft survival in the absence of B cells. Indeed, despite significantly impaired Tmem generation/function, B cell deficiency only marginally delayed cardiac allograft rejection in sensitized BKO vs. WT recipients (6 days and 3.5 days). However, transient depletion of CD8 T cells prior to cardiac engraftment resulted in long term (>100 day) allograft survival in skin-sensitized B cell-deficient hosts. In contrast, CD4 T cell depletion failed to prevent graft rejection in sensitized BKO hosts (MST=8 days). This data confirms the paramount role of CD8 T cell primed/memory responses in our model system.
In contrast to unprimed hosts (12), AccR in WT and BKO sensitized mice remained CD154 blockade resistant, with cardiac allograft survival extended just by two days (MST=8 days). Unlike in naïve mice, this therapy decreased but failed to abolish circulating CD8 Teff/Tmem in B cell-deficient sensitized animals. In parallel, intragraft CD3 T cell infiltration, CD8-derived IFN-γ and cytotoxic granzyme B expression remained comparable with or without CD154 blockade. Thus, although B cell deficiency impaired quantitatively alloreactive CD8 T cell primed/memory responses, this defect was insufficient to appreciably prolong cardiac allograft survival in our stringent AccR model. Recent identification of IL-15 induced B cell subset that can suppress immune response independent of Th2 function (48) may provide a potential explanation for the incompatibility between significantly impaired Tmem generation/function and marginally delayed graft rejection in sensitized BKO in our study. Important from both mechanistic and practical stand-points, the rejection of cardiac allografts in B cell-deficient and WT sensitized hosts, unlike in naïve mice, was CD154 blockade resistant. In agreement with our data, comparable cardiac allograft prolongation of just few days was reported in sensitized mice deficient of CD27-CD70 signaling (49), which is required for B cell activation (50).
In summary, the absence of B cells impaired quantitatively but failed to abolish alloreactive CD8 Teff/Tmem function in sensitized mice. Although B cells were important for alloreactive CD8 Teff/Tmem activation, differentiation, and survival, they were dispensable in the fulminant AccR cascade and in the mechanism of CD154 blockade resistant rejection in sensitized hosts.
Materials and Methods
Animals and grafting techniques
We used wild-type (WT) BALB/c (B/c; H-2d), C57BL/6 (B6; H-2b), C3H/HeJ (C3H; H-2k) and B cell-deficient (C57BL/6-Igh-6tm1Cgn; BKO: IgM μ chain deficiency; H-2b) male mice (8–12 wk, 20–25 g; The Jackson Laboratory, Bar Harbor, ME). We confirmed the absence of mature B cells and alloantibody responses in BKO mice (data nor shown). Animals were housed in UCLA facilities under pathogen-free conditions, and received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by NIH (publication 86-23 revised 1985).
Orthotopic full-thickness skin grafts (~0.5cm in diameter) from B/c donors were sutured bilaterally onto the flanks of WT or BKO B6 recipients. These were challenged 60 days later with heterotopic B/c heart transplants (8–12). Graft survival was assessed by palpation of ventricular activity. Cardiac allografts, spleens and lymph nodes were harvested; tail vein blood samples were taken for the measurement of CD8 T cell activation/differentiation.
Ab therapy
Anti-mouse CD154 mAb (MR1; BioXCell) or control hamster Ig was administered at the time of cardiac engraftment (day 0; 0.5mg/mouse i.v.). Anti-CD8 depleting mAb (2.43; BioXCell, West Lebanon, NH) was given prior to heart transplant (0.25 mg/mouse/day at day −2, −1, and 0 i.v.).
Flow cytometry
RBC-free PBLs were prepared, as described (8). After Fc blocking with rat IgG, one million cells were stained with rat anti-mouse CD8a-FITC (53-6.7), CD44-PE (IM7), and CD62L-APC (MEL-14) (eBioscience, San Diego, CA). The cells were stained with IL-2Rα-PE-Cy5 (PC61.5), IL-7Rα-PE-Cy5 (A7R34) (eBioscience), or IL-15Rα-Biotin (R&D Systems, Minneapolis, MN), followed by the secondary Ab streptavidin-PE-Cy5. Four-color flow cytometry was performed on a FACS-Calibur cytometer (BD Biosciences, Mountain View, CA). Cells in lymphocyte gate stained positive for CD8a were analyzed. Teff were identified as CD8+CD44high CD62Llow; Tmem as CD8+CD44high CD62Lhigh. The IL-2Rα, IL-7Rα, or IL-15Rα, concomitant with CD62L, were gated on CD8+CD44high T cells. IL-2Rα expressed on Teff was identified as IL-2RαhighCD8+CD44highCD62Llow, IL-7Rα/IL-15Rα levels on Tmem as IL-7RαhighCD8+CD44highCD62Lhigh/IL-15RαhighCD8+CD44high CD62Lhigh.
In vivo cytotoxic activity assay
In vivo cytotoxic activity against donor-specific targets was assessed in skin-sensitized mice. B6 splenocytes (107/mL) labeled with low concentration (0.5μmol/L) of carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) were used as syngeneic control (CFSElow). B/c splenocytes labeled with high concentration (5μmol/L) of CFSE were used as donor targets (CFSEhigh). C3H splenocytes served as third-party control (CFSEhigh). Ten million cells of CFSElow (B6) and CFSEhigh (B/c or C3H) populations were mixed, and injected i.v. into WT or BKO mice (day 60 post skin transplant). Twelve hours later, spleen CFSElow and CFSEhigh cells were stained with Topro 3 (1nM), and analyzed by flow cytometry. Topro 3-negative, identified as viable lymphocytes, were analyzed for CFSE intensity. Percent specific lysis of target cells was calculated as: [1-(CFSEhigh events / CFSElow events)] × 100%.
Quantitative RT-PCR
RNA (2.5ug) was reverse-transcribed into complementary DNA with the SuperScript III firststrand synthesis system (Invitrogen, Carlsbad, CA). Quantitative PCR was performed with the DNA engine with the Chromo 4 detector (MJ Research, Waltham, MA), as described (11). Target gene expressions were calculated by their ratios to the housekeeping gene hypoxanthine-guanine phosphoribosyl transferase (HPRT). Primers to amplify a specific mouse gene fragments were published (11).
Statistical analysis
All values are expressed as mean ± standard deviation (SD). Data were analyzed with an unpaired, two-tailed Student’s t-test, with P<0.05 as statistically significant.
Acknowledgments
This work is supported by: NIH Grant RO1 AI23847 (JWKW), and The Dumont Research Foundation. HJ is the recipient of American Society of Transplantation Fellowship Grant.
Abbreviations
- AccR
Accelerated Rejection
- Ab
Antibody
- Ag
Antigen
- BKO
B cell-knockout
- Teff
effector/effector memory T cells
- Tmem
Central memory T cells
- Ig
immunoglobulin
- mAb
monoclonal antibody
- WT
wide-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author’s specific contribution:
HJ participated in research design; performed skin transplantation, cytological/molecular experiments, analyzed the data and wrote the manuscript. XS and FG performed heart transplantations. RWB serves as senior discussant and provided partial funding. YZ contributed to experimental design. JWKW participated in research design, finalized the manuscript, and sponsored the project.
The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
References
- 1.Kupiec-Weglinski JW. Graft rejection in sensitized recipients. Ann Transplant. 1996;1(1):34–40. [PubMed] [Google Scholar]
- 2.Kupiec-Weglinski JW, Hancock WW. Transplantation Biology: Cellular and Molecular Aspects. New York: Raven Press; 1996. Hyperacute and accelerated graft rejection; pp. 541–556. [Google Scholar]
- 3.Baid S, Saidman SL, Tolkoff-Rubin N, Williams WW, Delmonico FL, Cosimi AB, et al. Managing the highly sensitized transplant recipient and B cell tolerance. Curr Opin Immunol. 2001;13(5):577–581. doi: 10.1016/s0952-7915(00)00262-4. [DOI] [PubMed] [Google Scholar]
- 4.Ogura K, Terasaki PI, Johnson C, Mendez R, Rosenthal JT, Ettenger R, et al. The significance of a positive flow cytometry crossmatch test in primary kidney transplantation. Transplantation. 1993;56(2):294–298. doi: 10.1097/00007890-199308000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Hancock WW, DiStefano R, Braun P, Schweizer RT, Tilney NL, Kupiec-Weglinski JW. Cyclosporine and anti-interleukin 2 receptor monoclonal antibody therapy suppress accelerated rejection of rat cardiac allografts through different effector mechanisms. Transplantation. 1990;49(2):416–421. doi: 10.1097/00007890-199002000-00037. [DOI] [PubMed] [Google Scholar]
- 6.Sablinski T, Hancock WW, Tilney NL, Kupiec-Weglinski JW. Biology of vascularized organ allograft rejection in sensitized recipients. Transplant Rev. 1990;4:108–120. [Google Scholar]
- 7.Hancock WW, Gao W, Shemmeri N, Shen XD, Gao F, Busuttil RW, et al. Immunopathogenesis of accelerated allograft rejection in sensitized recipients: humoral and nonhumoral mechanisms. Transplantation. 2002;73(9):1392–1397. doi: 10.1097/00007890-200205150-00006. [DOI] [PubMed] [Google Scholar]
- 8.Zhai Y, Shen XD, Gao F, Coito AJ, Wasowska BA, Salama A, et al. The CD154-CD40 T cell costimulation pathway is required for host sensitization of CD8(+) T cells by skin grafts via direct antigen presentation. J Immunol. 2002;169(3):1270–1276. doi: 10.4049/jimmunol.169.3.1270. [DOI] [PubMed] [Google Scholar]
- 9.Zhai Y, Meng L, Busuttil RW, Sayegh MH, Kupiec-Weglinski JW. Activation of alloreactive CD8+ T cells operates via CD4-dependent and CD4-independent mechanisms and is CD154 blockade sensitive. J Immunol. 2003;170(6):3024–3028. doi: 10.4049/jimmunol.170.6.3024. [DOI] [PubMed] [Google Scholar]
- 10.Zhai Y, Wang Y, Wu Z, Kupiec-Weglinski JW. Defective alloreactive CD8 T cell function and memory response in allograft recipients in the absence of CD4 help. J Immunol. 2007;179(7):4529–4534. doi: 10.4049/jimmunol.179.7.4529. [DOI] [PubMed] [Google Scholar]
- 11.Wu Z, Wang Y, Gao F, Shen X, Zhai Y, Kupiec-Weglinski JW. Critical role of CD4 help in CD154 blockade-resistant memory CD8 T cell activation and allograft rejection in sensitized recipients. J Immunol. 2008;181(2):1096–1102. doi: 10.4049/jimmunol.181.2.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhai Y, Meng L, Gao F, Busuttil RW, Kupiec-Weglinski JW. Allograft rejection by primed/memory CD8+ T cells is CD154 blockade resistant: therapeutic implications for sensitized transplant recipients. J Immunol. 2002;169(8):4667–4673. doi: 10.4049/jimmunol.169.8.4667. [DOI] [PubMed] [Google Scholar]
- 13.Nordin AA, Cerottini JC, Brunner KT. The antibody response of mice to allografts as determined by a plaque assay with allogeneic target cells. Eur J Immunol. 1971;1(1):55–56. doi: 10.1002/eji.1830010111. [DOI] [PubMed] [Google Scholar]
- 14.Waller M, Pierce JC, Lee HM, Levinson HJ. Humoral antibody responses following transplantation in man. Transplantation. 1975;19(3):210–218. doi: 10.1097/00007890-197503000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Russell PS, Chase CM, Colvin RB. Accelerated atheromatous lesions in mouse hearts transplanted to apolipoprotein-E-deficient recipients. Am J Pathol. 1996;149(1):91–99. [PMC free article] [PubMed] [Google Scholar]
- 16.Shi C, Lee WS, He Q, Zhang D, Fletcher DL, Jr, Newell JB, et al. Immunologic basis of transplant-associated arteriosclerosis. Proc Natl Acad Sci U S A. 1996;93(9):4051–4056. doi: 10.1073/pnas.93.9.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brändle D, Joergensen J, Zenke G, Bürki K, Hof RP. Contribution of donor-specific antibodies to acute allograft rejection: evidence from B cell-deficient mice. Transplantation. 1998;65(11):1489–1493. doi: 10.1097/00007890-199806150-00014. [DOI] [PubMed] [Google Scholar]
- 18.Wasowska BA, Qian Z, Cangello DL, Behrens E, Van Tran K, Layton J, et al. Passive transfer of alloantibodies restores acute cardiac rejection in IgKO mice. Transplantation. 2001;71(6):727–736. doi: 10.1097/00007890-200103270-00007. [DOI] [PubMed] [Google Scholar]
- 19.Ron Y, Sprent J. T cell priming in vivo: a major role for B cells in presenting antigen to T cells in lymph nodes. J Immunol. 1987;138(9):2848–2856. [PubMed] [Google Scholar]
- 20.Wilson JL, Cunningham AC, Kirby JA. Alloantigen presentation by B cells: analysis of the requirement for B-cell activation. Immunology. 1995;86(3):325–330. [PMC free article] [PubMed] [Google Scholar]
- 21.Janeway CA, Jr, Ron J, Katz ME. The B cell is the initiating antigen-presenting cell in peripheral lymph nodes. J Immunol. 1987;138(4):1051–1055. [PubMed] [Google Scholar]
- 22.Epstein MM, Di Rosa F, Jankovic D, Sher A, Matzinger P. Successful T cell priming in B cell-deficient mice. J Exp Med. 1995;182(4):915–922. doi: 10.1084/jem.182.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips JA, Romball CG, Hobbs MV, Ernst DN, Shultz L, Weigle WO. CD4+ T cell activation and tolerance induction in B cell knockout mice. J Exp Med. 1996;183(4):1339–1344. doi: 10.1084/jem.183.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ronchese F, Hausmann B. B lymphocytes in vivo fail to prime naive T cells but can stimulate antigen-experienced T lymphocytes. J Exp Med. 1993;177(3):679–690. doi: 10.1084/jem.177.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sunshine GH, Jimmo BL, Ianelli C, Jarvis L. Strong priming of T cells adoptively transferred into scid mice. J Exp Med. 1991;174(6):1653–1656. doi: 10.1084/jem.174.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Topham DJ, Tripp RA, Hamilton-Easton AM, Sarawar SR, Doherty PC. Quantitative analysis of the influenza virus-specific CD4+ T cell memory in the absence of B cells and Ig. J Immunol. 1996;157(7):2947–2952. [PubMed] [Google Scholar]
- 27.Hayglass KT, Naides SJ, Scott CF, Jr, Benacerraf B, Sy MS. T cell development in B cell-deficient mice. IV. The role of B cells as antigen-presenting cells in vivo. J Immunol. 1986;136(3):823–829. [PubMed] [Google Scholar]
- 28.Kurt-Jones EA, Liano D, HayGlass KA, Benacerraf B, Sy MS, Abbas AK. The role of antigen-presenting B cells in T cell priming in vivo. Studies of B cell-deficient mice. J Immunol. 1988;140(11):3773–3778. [PubMed] [Google Scholar]
- 29.Moulin V, Andris F, Thielemans K, Maliszewski C, Urbain J, Moser M. B lymphocytes regulate dendritic cell (DC) function in vivo: increased interleukin 12 production by DCs from B cell-deficient mice results in T helper cell type 1 deviation. J Exp Med. 2000;192(4):475–482. doi: 10.1084/jem.192.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noorchashm H, Reed AJ, Rostami SY, Mozaffari R, Zekavat G, Koeberlein B, et al. B cell-mediated antigen presentation is required for the pathogenesis of acute cardiac allograft rejection. J Immunol. 2006;177(11):7715–7722. doi: 10.4049/jimmunol.177.11.7715. [DOI] [PubMed] [Google Scholar]
- 31.Asano MS, Ahmed R. CD8 T cell memory in B cell-deficient mice. J Exp Med. 1996;183(5):2165–2174. doi: 10.1084/jem.183.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Rosa F, Matzinger P. Long-lasting CD8 T cell memory in the absence of CD4 T cells or B cells. J Exp Med. 1996;183(5):2153–2163. doi: 10.1084/jem.183.5.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linton PJ, Harbertson J, Bradley LM. A critical role for B cells in the development of memory CD4 cells. J Immunol. 2000;165(10):5558–5565. doi: 10.4049/jimmunol.165.10.5558. [DOI] [PubMed] [Google Scholar]
- 34.Crawford A, Macleod M, Schumacher T, Corlett L, Gray D. Primary T cell expansion and differentiation in vivo requires antigen presentation by B cells. J Immunol. 2006;176(6):3498–3506. doi: 10.4049/jimmunol.176.6.3498. [DOI] [PubMed] [Google Scholar]
- 35.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 36.Schluns KS, Lefrançois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3(4):269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 37.Dai Z, Konieczny BT, Lakkis FG. The dual role of IL-2 in the generation and maintenance of CD8+ memory T cells. J Immunol. 2000;165(6):3031–3036. doi: 10.4049/jimmunol.165.6.3031. [DOI] [PubMed] [Google Scholar]
- 38.Ng YH, Oberbarnscheidt MH, Chandramoorthy HC, Hoffman R, Chalasani G. B cells help alloreactive T cells differentiate into memory T cells. Am J Transplant. 2010;10(9):1970–1980. doi: 10.1111/j.1600-6143.2010.03223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1(5):426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 40.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9(5):669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 41.Li XC, Demirci G, Ferrari-Lacraz S, Groves C, Coyle A, Malek TR, et al. IL-15 and IL-2: a matter of life and death for T cells in vivo. Nat Med. 2001;7(1):114–118. doi: 10.1038/83253. [DOI] [PubMed] [Google Scholar]
- 42.Vella AT, Dow S, Potter TA, Kappler J, Marrack P. Cytokine-induced survival of activated T cells in vitro and in vivo. Proc Natl Acad Sci U S A. 1998;95(7):3810–3815. doi: 10.1073/pnas.95.7.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oh S, Perera LP, Burke DS, Waldmann TA, Berzofsky JA. IL-15/IL-15Ralpha-mediated avidity maturation of memory CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101(42):15154–15159. doi: 10.1073/pnas.0406649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valujskikh A, Baldwin WM, 3rd, Fairchild RL. Recent progress and new perspectives in studying T cell responses to allografts. Am J Transplant. 2010;10(5):1117–1125. doi: 10.1111/j.1600-6143.2010.03087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schenk AD, Nozaki T, Rabant M, Valujskikh A, Fairchild RL. Donor-reactive CD8 memory T cells infiltrate cardiac allografts within 24-h posttransplant in naive recipients. Am J Transplant. 2008;8(8):1652–1661. doi: 10.1111/j.1600-6143.2008.02302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A. 2009;106(6):1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haddad EA, Senger LK, Takei F. An accessory role for B cells in the IL-12-induced activation of resting mouse NK cells. J Immunol. 2009;183(6):3608–3615. doi: 10.4049/jimmunol.0901391. [DOI] [PubMed] [Google Scholar]
- 48.Rafei M, Hsieh J, Zehntner S, Li M, Forner K, Birman E, et al. A granulocyte-macrophage colony-stimulating factor and interleukin-15 fusokine induces a regulatory B cell population with immune suppressive properties. Nat Med. 2009;15(9):1038–1045. doi: 10.1038/nm.2003. [DOI] [PubMed] [Google Scholar]
- 49.Yamaura K, Boenisch O, Watanabe T, Ueno T, Vanguri V, Yang J, et al. Differential requirement of CD27 costimulatory signaling for naïve versus alloantigen-primed effector/memory CD8+ T cells. Am J Transplant. 2010;10(5):1210–1220. doi: 10.1111/j.1600-6143.2010.03089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arens R, Nolte MA, Tesselaar K, Heemskerk B, Reedquist KA, van Lier RA, et al. Signaling through CD70 regulates B cell activation and IgG production. J Immunol. 2004;173(6):3901–3908. doi: 10.4049/jimmunol.173.6.3901. [DOI] [PubMed] [Google Scholar]