Abstract
Background
Recent studies revealed a critical role for thymic stromal lymphopoietin (TSLP) released from epithelial cells and OX40 ligand (OX40L) expressed on dendritic cells (DCs) in TH2 priming and polarization.
Objectives
We sought to determine the importance of the TSLP-OX40L axis in neonatal respiratory syncytial virus (RSV) infection.
Methods
Mice were initially infected with RSV as neonates or adults and reinfected 5 weeks later. Anti-OX40L or anti-TSLP were administered during primary or secondary infection. Outcomes included assessment of airway function and inflammation and expression of OX40L, TSLP, and IL-12.
Results
OX40L was expressed mainly on CD11c+MHC class II (MHCII)+CD11b+ DCs but not CD103+ DCs. Treatment of neonates with OX40L antibody during primary RSV infection prevented the subsequent enhancement of airway hyperresponsiveness and the development of airway eosinophilia and mucus hyperproduction on reinfection. Administration of anti-TSLP before neonatal RSV infection reduced the accumulation of lung DCs, decreased OX40L expression on lung DCs, and attenuated the enhancement of airway responses after reinfection.
Conclusions
In mice initially infected as neonates, TSLP expression induced by RSV infection is an important upstream event that controls OX40L expression, lung DC migration, and TH2 polarization, accounting for the enhanced response on reinfection.
Keywords: Respiratory syncytial virus, OX40 ligand, thymic stromal lymphopoietin
The association between respiratory syncytial virus (RSV) lower respiratory tract infection in early life and the subsequent development of persistent wheezing and asthma is well documented.1-4 There is a TH2 bias in the immune response during the neonatal period,5,6 and RSV infection during the first 3 months of life induces a TH2-biased response compared with infection in older infants.7 Previously, we demonstrated that the age at primary RSV infection dictated the subsequent consequences on reinfection.8 Initial infection of neonatal mice resulted in enhanced airway hyperresponsiveness (AHR) after reinfection, as characterized by increased AHR, airway eosinophilia, increased IL-13 levels, and mucus hyperproduction. The underlying mechanisms responsible for these significant age differences are not entirely defined. IFN-γ,9 RSV-specific IgE,10 cysteinyl leukotrienes,11 and T cells12 have all been implicated in this asthma-like TH2 response of neonates to RSV reinfection. However, the characteristics of lung dendritic cells (DCs), which are potent antigen-presenting cells (APCs), and their ability to activate and polarize T cells in neonates infected with RSV has not been studied.
OX40 ligand (OX40L; CD252) and its cognate receptor, OX40 (CD134), belong to the TNF/TNF receptor superfamily. OX40 is expressed on activated T cells, whereas OX40L is mainly expressed on APCs. OX40L/OX40 interactions are important in T-cell activation and survival and for the generation of memory T cells from activated effector T cells.13,14 In allergen-induced models of asthma, OX40- or OX40L-deficient mice had markedly attenuated TH2 responses and airway inflammation15,16; blocking OX40L significantly impaired the development of TH2 responses and abolished lung inflammation.17,18 Recently, thymic stromal lymphopoietin (TSLP), an epithelium-derived cytokine, has been identified as a key initiator of allergic airway responses.19-21 Further evidence showed that TSLP-activated DCs triggered allergic inflammatory TH2 responses through OX40L expressed on these DCs.22,23 These studies suggested that preventing OX40-OX40L interactions could be beneficial in the treatment of allergic disease.24,25 On the basis of these observations, we hypothesized that OX40L expressed on lung DCs might play a critical role during primary RSV infection, particularly in determining the asthma-like TH2 airway responses after RSV reinfection of mice initially infected as neonates. Some of the results of these studies have been previously reported in abstract form.26
METHODS
Antibodies
Rat anti-mouse OX40L (RM134L) and anti-TSLP mAb (M702) were used.19
Animals
BALB/c mice were obtained from the Jackson Laboratory (Bar Harbor, Me).
Virus preparation
Human RSVA2 Strain (catalog no. VR-1302) and HEp-2 cells (catalog no. CCL-23) were obtained from the American Type Culture Collection (Rockville, Md), and stocks of purified RSV were prepared and viral titers determined.8
RSV infection and treatments
Mice were inoculated with 106 plaque-forming units of purified RSVat the indicated age. Anti-OX40L or control antibody was administered intraperitoneally at 15 mg/kg 1 day before primary or secondary RSV infection and on days 1 and 2 after infection. Secondary RSV infection was initiated 5 weeks after primary infection. Airway function and inflammation were assessed on day 7 after either primary or secondary RSV infection. Anti-TSLP or control antibody (50 mg per pup) was administered intraperitoneally 1 day before primary RSV infection in neonates.
Assessment of airway function
Airway function was as previously described.27
Airway inflammation
Airway inflammation was assessed based on counting of cells recovered in BAL fluid.
Measurement of cytokine levels
IFN-γ, IL-4, IL-5, and IL-6 levels were measured in bronchoalveolar lavage (BAL) fluid by using commercial ELISA kits, according to the manufacturer’s instructions (eBioscience, San Diego, Calif), as was IL-13 (R&D Systems, Minneapolis, Minn).
Flow cytometry
Lung single-cell suspensions were prepared and analyzed, as previously described.28,29
Quantitative real-time PCR analysis
RNA was isolated from lungs by using the RNeasy Mini Kit (Qiagen, Valencia, Calif) and analyzed for TSLP transcript expression by using TaqMan 1-step real-time (RT) PCR Master Mix Reagents (Applied Biosystems, Foster City, Calif) with 50 ng of total RNA.
In vitro cytokine production by peribronchial lymph node cells after restimulation with RSV
Seven days after secondary RSV infection, single-cell suspensions from peribronchial lymph nodes (PBLN) were prepared, and concentrations of IL-4, IL-5, IL-6, IL-13, and IFN-γ in the supernatants were measured by using ELISA.
Statistical analysis
All results were expressed as means ± SEMs. Data were analyzed by means of ANOVA with the StatView 4.5 statistical analysis software package (Abacus Concepts, Piscataway, NJ). Student t tests and 1-way ANOVA were used to determine the level of differences, where appropriate. Nonparametric analysis with the Mann-Whitney U test was used to confirm that the statistical differences remained significant, even if the underlying distribution was uncertain. The P values for significance were set to .05 for all tests.
RESULTS
RSV infection induces OX40L expression on lung DCs
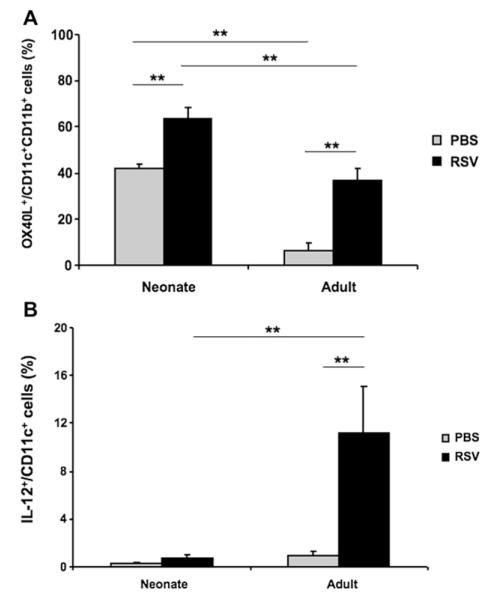
Mice were infected as neonates (<1 week of age) or at 5 weeks of age to determine the levels of OX40L expression on lung DCs after RSV infection. On each day after infection, single-cell suspensions from lung homogenates were prepared, and the frequency of lung DC subsets expressing OX40L was obtained according to the gating strategy described in Fig E1 in this article’s Online Repository at www.jacionline.org. In both age groups, RSV infection resulted in an increased percentage of OX40L+CD11c+ cells. The increased frequency of OX40L+ cells peaked 1 day after RSV infection (Fig 1, A), was maintained for up to 2 days after infection, and then returned to basal levels (data not shown). However, the pattern of OX40L expression was different in the 2 age groups either before or after RSV infection. In both age groups, OX40L was expressed mainly on CD11c+MHCII+CD11b+CD103− DCs but was significantly higher at baseline in neonatal than in adult mice, resulting in an even greater frequency of OX40L+ lung DCs in neonatal mice after RSV infection (Fig 1, A, and see Fig E2, A, in this article’s Online Repository at www.jacionline.org). In both age groups, OX40L was not expressed on CD103+ DCs before or after RSV infection (see Fig E2, B). In addition, OX40L was not expressed on CD11c+Siglec-F+ cells (data not shown). Of note, there were very few plasmacytoid DCs (pDCs) in neonates, and the frequency of OX40L+ pDCs did not change after RSV infection. In adult mice more pDCs in the lung were identified; the percentage of OX40L+ cells among this population was lower at baseline and increased on RSV infection (see Fig E3 in this article’s Online Repository at www.jacionline.org).
FIG 1.
RSV infection induces OX40L expression on lung DCs. Mice were infected as neonates or at 5 weeks of age. Age-matched control mice were inoculated with PBS. One day after RSV infection, lung single-cell suspensions were prepared, and lung DC subsets were identified following the gating strategy described in Fig E1. The percentage of OX40L+ cells among CD11c+CD11b+CD103− DCs (A) and the percentage of IL-12+ cells among CD11c+ cells (B) was analyzed by using flow cytometry, as described in the Methods section. Results are from 3 independent experiments (n = 12). **P < .01.
As shown in Fig 1, B, a small number of lung DCs expressed IL-12 at baseline in both age groups. After RSV infection, a significantly increased percentage of IL-12–expressing DCs was detected in the adult lung, whereas there were no changes in neonatal mice (Fig 1, B, and see Fig E4 in this article’s Online Repository at www.jacionline.org).
Effect of anti-OX40L on primary RSV infection in adult mice
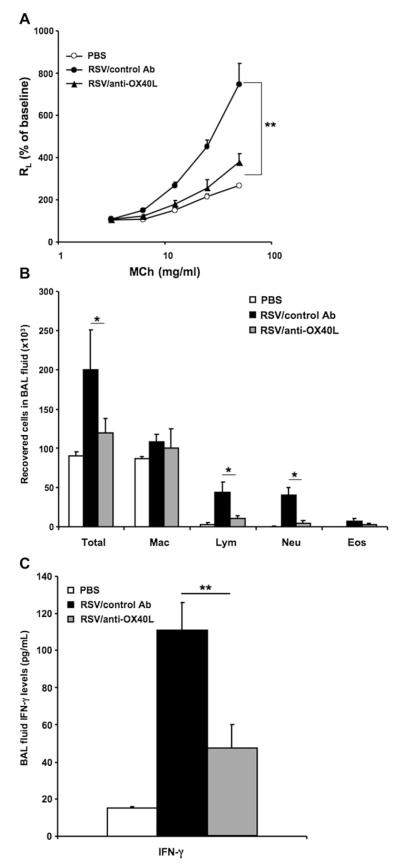
Changes in the expression pattern of OX40L in the lung after RSV infection suggested it might be involved in the development of altered airway responsiveness. To test this possibility, we determined the effects of administration of anti-OX40L on RSV-induced AHR and inflammation in vivo after primary infection. Mice were infected at 5 weeks of age; anti-OX40L or control antibody was administered intraperitoneally at 15 mg/kg 1 day before RSV infection and on days 1 and 2 after infection. As shown in Fig 2, A, in mice treated with anti-OX40L, AHR development was significantly decreased. After primary RSV infection, total cell, lymphocyte, and neutrophil numbers recovered in BAL fluid were significantly increased compared with those in noninfected mice, and injection of anti-OX40L significantly reduced lymphocyte and neutrophil numbers in BAL fluid compared with those seen in control antibody–treated mice (Fig 2, B). There were no significant differences in the numbers of macrophages or the few eosinophils detected. Although RSV infection resulted in significant increases in BAL fluid IFN-γ levels, the anti-OX40L–treated group had significantly lower levels of IFN-γ in BAL fluid (Fig 2, C); none of the other cytokines measured (IL-4, IL-5, IL-6, and IL-13) were detected after primary infection in the BAL fluid of the anti-OX40L–treated or control antibody–treated groups (data not shown).
FIG 2.
Effect of anti-OX40L on airway responsiveness to primary RSV infection in adult mice. Mice were infected on day 0 at 5 weeks of age. Anti-OX40L (RM134L) or control antibody was administered intraperitoneally at 15 mg/kg on days −1, +1, and +2. Airway responsiveness to inhaled MCh (A), BAL cellularity (B), and BAL fluid IFN-γ levels (C) were assessed on day 7 after infection. Results are from 3 independent experiments (n = 12). Data are expressed as means ± SEMs. *P < .05 and **P < .01. Ab, Antibody; Eos, eosinophil; Lym, lymphocyte; Mac, macrophage; Neu, neutrophil; RL, lung resistance.
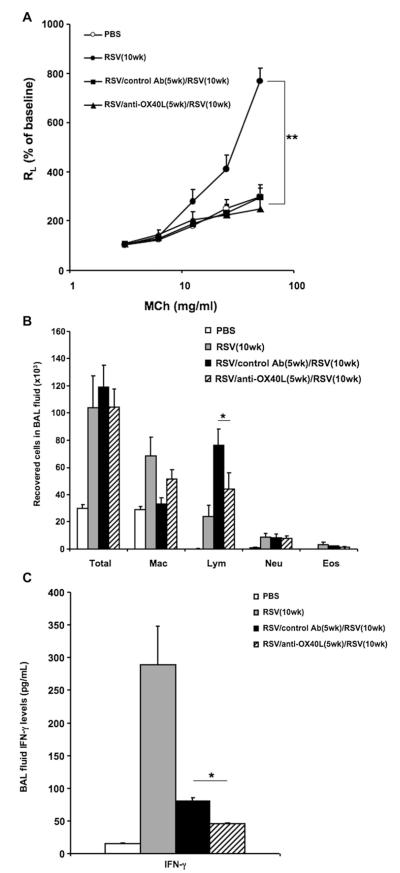
Effect of anti-OX40L during primary infection on secondary RSV infection in adult mice
In previous studies we demonstrated that neonatal RSV infection predisposes to the development of airway eosinophilia and enhanced AHR after reinfection, whereas infection at a later age protects against the development of these altered airway responses after reinfection.8 As shown in Fig 3, A, primary infected mice at 10 weeks of age had significant AHR, whereas in mice that were initially infected at 5 weeks, treated with control antibody, and reinfected 5 weeks later, AHR to inhaled methacholine (MCh) did not develop. Mice treated with anti-OX40L during primary infection similarly had no AHR on reinfection. However, compared with the mice infected (primary) at 10 weeks of age, control antibody–treated and reinfected mice had a markedly increased mononuclear cell airway inflammatory response. In parallel, the number of lymphocytes recovered in BAL fluid was also significantly increased after reinfection compared with that seen in age-matched, primary infected mice (Fig 3, B). Treatment with anti-OX40L during primary infection significantly reduced lymphocyte numbers in the BAL fluid after reinfection. IFN-γ levels in BAL fluid after reinfection were greater than those measured after primary infection, but the peak response shifted to an earlier time point after reinfection (2 days after reinfection, data not shown). As shown in Fig 3, C, 7 days after RSV reinfection, the anti-OX40L–treated mice had significantly lower levels of IFN-γ in BAL fluid compared with control antibody–treated mice. IL-4, IL-5, IL-6, and IL-13 levels in the BAL fluid were at the limit of detection and were not different in all the groups (data not shown).
FIG 3.
Effect of anti-OX40L administered during primary infection on secondary RSV infection in adult mice. Mice were initially infected with RSV at 5 weeks of age and reinfected with RSV 5 weeks later. Anti-OX40L or control antibody was administered during primary RSV infection. Airway responsiveness to inhaled MCh (A), BAL cellularity (B), and BAL fluid IFN-γ levels (C) were assessed on day 7 after reinfection. Results are from 3 independent experiments (n = 12). Data are expressed as means ± SEMs. *P < .05 and **P < .01. Ab, Antibody; Eos, eosinophil; Lym, lymphocyte; Mac, macrophage; Neu, neutrophil; RL, lung resistance.
Effect of anti-OX40L during neonatal primary infection on the response to RSV reinfection
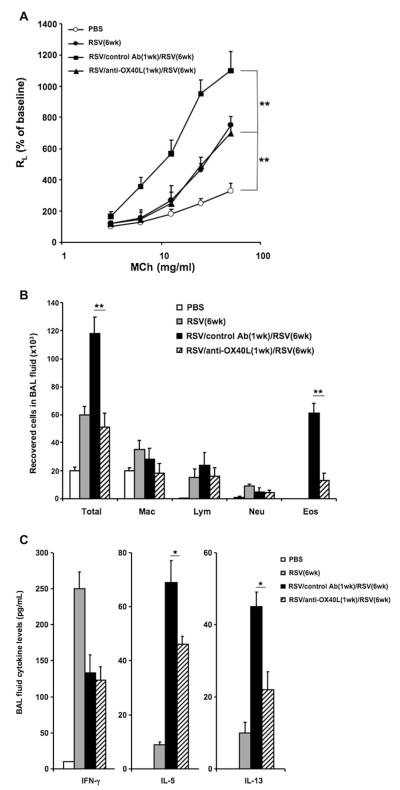
Mice were first infected with RSV shortly after birth (<1 week of age) and then reinfected 5 weeks later to determine whether anti-OX40L administered during primary infection in the neonatal period would affect the enhanced responses detected on reinfection. Anti-OX40L or control antibody was administered on days −1, +1, and +2 during primary infection. As shown in Fig 4, A, control antibody–treated mice had enhanced AHR on reinfection with RSV. Administration of anti-OX40L only during primary neonatal infection prevented the enhancement of AHR on reinfection, although these mice did have levels of AHR similar to those of the mice subjected to primary infection at 6 weeks of age.The number of total cells and eosinophils recovered in the BAL fluid were significantly increased after secondary RSV infection, and this was reduced in anti-OX40L–treated mice; there were no differences in macrophage, lymphocyte, or neutrophil numbers in the anti-OX40L–and control antibody–treated groups (Fig 4, B).
FIG 4.
Effect of anti-OX40L administered during neonatal primary infection on the response to RSV reinfection. Newborn mice were infected with RSV on day 0. Anti-OX40L or control antibody was administered on days −1, +1, and +2. Five weeks later, mice were reinfected with RSV. Airway responsiveness to inhaled MCh (A), BAL cellularity (B), and BAL fluid cytokine levels (C) were assessed on day 7 after secondary RSV infection. Results are from 3 independent experiments (n = 12). Data are expressed as means ± SEMs. *P < .05 and **P < .01. Ab, Antibody; Eos, eosinophil; Lym, lymphocyte; Mac, macrophage; Neu, neutrophil; RL, lung resistance.
After reinfection, the levels of IFN-γ and the TH2 cytokines IL-5 and IL-13 were increased in the BAL fluid after RSV reinfection compared with levels after primary infection (Fig 4, C), and as in the adult mice, the peak response of IFN-γ was shifted to an earlier time point after reinfection (2 days after reinfection, data not shown). Treatment with anti-OX40L during primary infection decreased IL-5 and IL-13 levels but did not alter IFN-γ levels (Fig 4, C). These effects of anti-OX40L treatment during primary infection were clearly different than observed when the OX40L antibody was only administered during reinfection. In essence, when administered during reinfection, there was no effect on AHR (see Fig E5, A, in this article’s Online Repository at www.jacionline.org), airway eosinophilia (see Fig E5, B), or BAL fluid cytokine levels (see Fig E5, C).
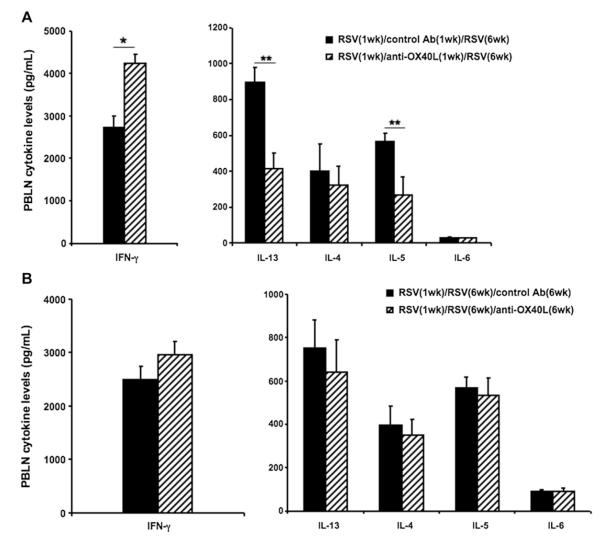
Administration of anti-OX40L affects cytokine responses in PBLNs
To further define the T-cell cytokine profiles after RSV reinfection, PBLN mononuclear cells were isolated 7 days after RSV reinfection and stimulated with UV-inactivated RSV in vitro, and levels of IL-4, IL-5, IL-6, IL-13, and IFN-γ in the culture supernatants were measured. As shown in Fig 5, A, in mice treated with anti-OX40L during primary infection, lower IL-5 and IL-13 levels but higher IFN-γ levels were detected. There were no differences in IL-4 and IL-6 levels in these 2 groups. When PBLNs were isolated from reinfected mice treated with anti-OX40L during secondary infection and restimulated with UV-RSV, there were no differences in cytokine responses compared with those seen in control antibody-treated animals (Fig 5, B). Thus unlike the effects of OX40L blockade on reinfection responses when administered during primary neonatal infection, anti-OX40L treatment was without effect on the responses to reinfection when administered only during secondary infection.
FIG 5.
Administration of anti-OX40L during neonatal infection affects cytokine responses in PBLNs. Newborn mice were infected with RSV and reinfected with RSV 5 weeks later. Anti-OX40L or control antibody was administered either during primary RSV (A) or secondary RSV (B) infection. Seven days after reinfection, PBLN cells were collected and seeded in 96-well plates and stimulated with UV-RSV. IL-4, IL-5, IL-6, IL-13, and IFN-γ levels in the supernatants were measured 3 days after culture. Results are from 3 independent experiments (n = 12). Data are expressed as means ± SEMs. *P < .05 and **P < .01. Ab, Antibody.
RSV induces TSLP expression in the lung
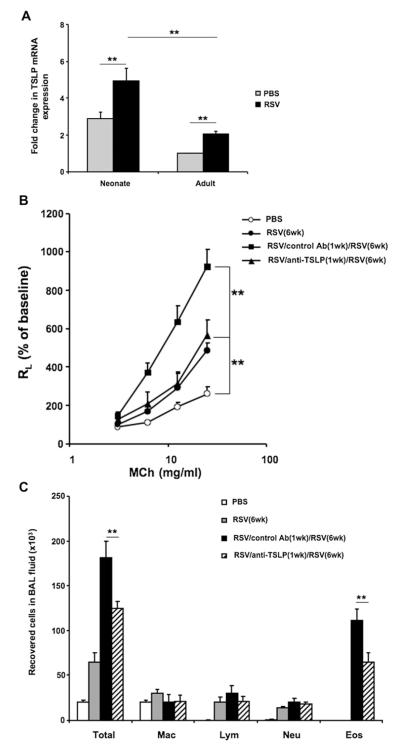
It has been shown that the TSLP-OX40L-OX40 axis plays an important role in the development of allergic inflammation both in human subjects and experimental mouse models.21,25 TSLP expression was measured before and after RSV infection in both age groups to identify potentially important events upstream of OX40L/OX40 signaling. Compared with uninfected control animals, RSV-infected lungs expressed higher levels of TSLP mRNA, as determined by using quantitative PCR in both neonates and adult mice (Fig 6, A). Of note, among the uninfected mice, TSLP expression was significantly higher in neonatal than adult mice.
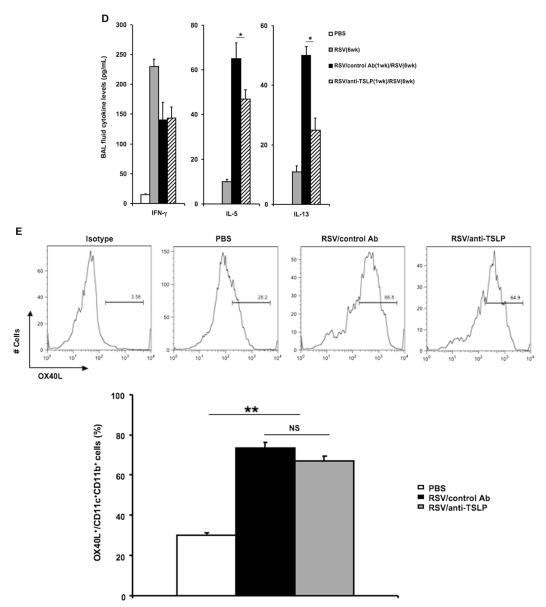
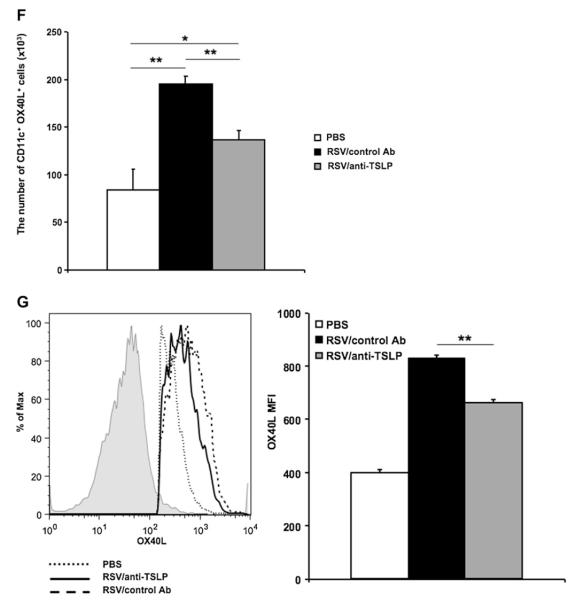
FIG 6.
RSV induces TSLP expression in the lung and neutralization of TSLP before primary infection affects the responses to RSV reinfection. A, Lung mRNA was isolated 1 day after primary infection of neonates or adult mice and TSLP mRNA levels were measured by quantitative RT-PCR. Increases in expression levels were compared with those in adult noninfected control animals. B-D, Newborn mice were infected with RSV and reinfected with RSV 5 weeks later. Anti-TSLP or control antibody was administered 1 day before neonatal infection. Seven days after reinfection, airway responsiveness to inhaled MCh (Fig 6, B), BAL cellularity (Fig 6, C), and BAL fluid cytokine levels (Fig 6, D) were assessed. E-G, Mice were infected with RSV as neonates. Anti-TSLP or control antibody was administered 1 day before infection. Lung single-cell suspensions were prepared 1 day after infection, and OX40L expression was analyzed by flow cytometry. Fig 6, E, Percentage of OX40L+ cells among lung CD11c+CD11b+ DCs. Fig 6, F, Total number of OX40L+CD11c+ cells in the lung. Fig 6, G, OX40L MFI expression on CD11c+CD11b+ DCs. Representative histogram and combined MFI depicting expression of OX40L on CD11c+CD11b+ DCs; the isotype control is shown as a shaded line. Results are from 2 to 4 independent experiments (n = 6-12). Data are expressed as means ± SEMs. **P < .01. Ab, Antibody; Eos, eosinophil; Lym, lymphocyte; Mac, macrophage; Neu, neutrophil; RL, lung resistance.
Neutralization of TSLP before primary infection affects response to RSV reinfection
Anti-TSLP or control antibody was administered 1 day before neonatal RSV infection to directly delineate the role of TSLP in the enhanced response of neonatal mice to reinfection. Compared with control antibody–treated and reinfected mice, anti-TSLP–treated mice had a significantly attenuated airway response to inhaled MCh (Fig 6, B), and total cell and eosinophil numbers in the BAL fluid were significantly reduced in the treated group (Fig 6, C). Treatment with anti-TSLP decreased IL-5 and IL-13 levels but did not alter IFN-γ levels in the BAL fluid (Fig 6, D).
Previous studies showed that TSLP promoted TH2-biased responses through induction of OX40L expression on DCs. To delineate the role of TSLP in this model, we compared OX40L expression on lung DCs with and without anti-TSLP treatment. Mice were infected with RSV as neonates, anti-TSLP or control antibody was administered 1 day before RSV infection, and single-cell suspensions from lung homogenates were prepared and analyzed by using flow cytometry 1 day after RSV infection. Compared with uninfected control animals, RSV infection induced a significantly higher percentage of CD11c+OX40L+ cells in the lung, but there were no obvious differences between the anti-TSLP–and control antibody–treated groups (Fig 6, E). However, the absolute number of CD11c+OX40L+ cells in anti-TSLP–treated mice was significantly lower than in control antibody–treated mice (Fig 6, F). Moreover, the mean fluorescence intensity (MFI) of OX40L was lower in the anti-TSLP–treated mice (Fig 6, G). Thus after infection of neonates, TSLP exerted its activity through increasing the levels (MFI) of OX40L expression on CD11c+ DCs, as well as resulting in increased numbers of CD11c+OX40L+ cells in the lung.
DISCUSSION
The interplay between RSV infection and asthma, especially the development of asthma, is not entirely clear.30 Severe RSV infection and the need for hospitalization have been linked to asthma development,2-4 but it is unclear whether reinfection plays a similar role as it does in enhancing respiratory disease severity. To approach these issues in an experimental model, we examined the responses to reinfection in mice initially infected as neonates or at a later age and demonstrated dramatic differences.8 Whereas initial infection at 5 weeks of age and reinfection 5 weeks later elicited robust airway lymphocytosis, there was no associated AHR to inhaled MCh, eosinophilia, or significant mucus hyperproduction. In contrast, when mice were initially infected as neonates and reinfected 5 weeks later, AHR was markedly enhanced and associated with airway eosinophilia, mucus hyperproduction, and increases in IL-13 levels, responses often associated with an asthma-like picture, especially in experimental models.8 To some extent, these differential responses in the age groups at the time of primary infection have been related to the capacity of the neonatal mice to have RSV-specific IgE antibodies10 and a deficiency in IFN-γ production,9 aspects also associated with RSV disease severity in human infants.31,32
To define the mechanism underlying the enhanced responsiveness in neonatally infected mice reinfected with RSV, we focused on potential differences in lung DCs given their central role as APCs in T-cell polarization. Lung DCs from neonatal mice differed from those obtained at 5 weeks of age. Neonatal lung DCs expressed higher baseline levels of OX40L and lower cytoplasmic levels of IL-12, a known inducer of IFN-γ production,33 and these differences were amplified after RSV infection, with further increases in OX40L expression in infected neonates and higher IL-12 expression in infected older mice. In the 2 age groups OX40L was mainly expressed on CD11c+MHCII+CD11b+CD103− myeloid dendritic cells (mDCs). The expression pattern of OX40L among mDCs was different in the 2 age groups; it was higher at baseline in neonates than in adult mice. After RSV infection, the expression of OX40L increased in both groups, but the increases were greater in the neonates. We did not detect OX40L expression on CD103+ DCs in either age group.
The other major difference in the 2 age groups was the number of pDCs. There were few pDCs in neonates, and they expressed higher levels of OX40L than in adult mice at baseline but did not change on RSV infection. The adult mice had more pDCs in the lung than neonatal mice, and these cells expressed lower levels of OX40L at baseline that increased on RSV infection. The pDCs are thought to be protective against asthmatic reactions to harmless inhaled antigen34 and might limit TH2 responses to RSV infection.35 Here the differences in the numbers of pDCs between neonates and adults might play a role in the age-dependent responses.
Although treatment of older mice with anti-OX40L during primary infection reduced the consequences of both primary and secondary RSVinfection, limiting the development of AHR in the former and the prominent lymphocytosis in the latter, the more dramatic effects were seen in neonatal mice. In concert with the increased expression of OX40L during neonatal infection, administration of anti-OX40L during primary but not secondary infection dramatically reduced the consequences of reinfection, preventing the enhancement of AHR, airway eosinophilia, mucus hyperproduction, and increases in levels of the TH2 cytokines IL-5 and IL-13. Moreover, the changes in cytokine levels were mirrored in the draining PBLN cells, where treatment with anti-OX40L during primary but not secondary infection resulted in a similar reduction in TH2 cytokine levels and an increase in IFN-γ production after RSV restimulation in vitro.
Together, these data positioned the OX40L-OX40 pathway as a major regulator of the skewed response of reinfected mice initially exposed to RSV in the neonatal period. However, the effects of OX40L-OX40 interaction on the polarization of TH cells is complex. Initially, the notion was that OX40 ligation led to TH2 polarization and was implicated in a number of allergic diseases. OX40L-OX40 ligation on naive CD4+ T cells was shown to preferentially lead to TH2 generation driven by early autocrine production of IL-4.36-38 OX40- or OX40L-deficient mice or anti-OX40L–treated mice had attenuated TH2 responses and airway inflammation in allergen-induced asthma models.15-18 In a similar fashion several lines of evidence in the present work suggested the involvement of OX40L in initiating the TH2-dominant asthma-like responses in the neonatal reinfection model. Although the frequency of OX40L+ lung DCs was increased in neonatal mice after RSV infection and greater than the frequency of OX40L+ lung DCs in adult mice, they were nonetheless increased in the adult mice, as well as after RSV infection, even though TH1 responses predominated. Ito et al22 have shown that in the presence of IL-12, OX40L served to increase TH1 responses, whereas in the absence of IL-12, OX40L promoted TH2 polarization. We found IL-12 expression to be lower after RSV infection in neonatal than adult mice, and this was associated with lower IFN-γ levels. Thus it might be that local conditions determine how OX40L-OX40 interactions influence the response to RSV in the lung. If the cytokine environment in neonatal mice after primary RSV infection was TH2 biased, OX40L-OX40 interactions could preferentially lead to the development of TH2-skewed responses. In adult mice the increased production of IL-12 could skew away from TH2-driven activities, resulting in enhanced TH1 responses after OX40 engagement.
The role of OX40L-OX40 interactions in reactivation of memory T cells in asthmatic patients also is unclear. Two studies showed that administration of anti-OX40L during the challenge phase resulted in significant decreases in activated CD4+ T-cell lung infiltration, TH2 cytokine production, and antigen-specific IgE levels.18,23 In contrast, Hoshino et al17 observed a greater suppressive effect of anti-OX40L during the sensitization phase compared with the challenge phase. In the current study we also demonstrated that anti-OX40L administered during secondary RSV infection did not alter the development of enhanced AHR or airway eosinophilia in mice initially infected as neonates. This lack of effect of OX40 blocking on “recall responses” was also demonstrated in a mouse influenza model.39 These results suggest that the skewing toward asthma-like TH2 responses and, in particular, the involvement of OX40L-OX40 interactions was likely initiated soon after primary infection in neonates, accounting for the absence of effects in the reinfection phase.
In keeping with this notion of early involvement of OX40L-OX40 interactions after primary RSV infection were the findings with TSLP. RSV infection increased the expression of TSLP in the lungs of both neonates and older mice, and the expression levels of TSLP at baseline were higher in neonates than in adult mice. Administration of anti-TSLP before neonatal RSV infection reduced the accumulation of lung DCs, decreased OX40L expression on lung DCs, and attenuated the enhancement of airway responses after reinfection, including AHR, eosinophilic airway inflammation, mucus hyperproduction, and IL-13 levels. There are several possibilities responsible for the decreased total level of DCs in the lungs of anti-TSLP–treated mice. RSVor influenza infection results in increased numbers of pulmonary DCs.40-43 Increases in DC numbers could be due to DCs migrating from the periphery to the lung. In addition, DC precursors have been described in the lung, and these precursors can give rise to CD11c+MHCII+ DCs.44 We identified a population of CD11c+MHCII−CD11bhi cells in both age groups and a population of CD11c+MHCII−CD11blow cells only seen in the neonates that might represent these precursors (data not shown). Most likely, the mechanism or mechanisms behind increased DC numbers on day 1 is a combination of factors. In human subjects TSLP receptor has been shown to be expressed on CD34+ hematopoietic progenitor cells,45 and TSLP–TSLP receptor interactions might play a role in the increased number of CD34+ progenitor cells that migrate to the site of allergic inflammation. Second, TSLP might be involved in the proliferation, differentiation, or both of local DC progenitors directly or interact through other intermediates. The end result, an increase in total DC numbers and an increase in OX40L MFI, could reflect these 2 aspects of TSLP function: chemotaxis and activation of DCs. Together, the data identified the involvement of a TSLP-OX40L-OX40 axis in the development of RSV-induced AHR and inflammation on reinfection of mice initially infected as neonates.
TSLP is an important initiator of allergic inflammation and is expressed primarily by epithelial cells at barrier surfaces, such as the lung.46 Mice that overexpressed TSLP had TH2-driven allergic inflammation.19,47-50 The receptor for TSLP is expressed on a variety of cell types, including DCs, and TSLP can polarize DCs to induce the differentiation of naive T cells into TH2 cells, at least in part through upregulation of OX40L expression.22,51 Qiao et al52 showed that RSV can stimulate primary rat epithelial cells to release TSLP. In the context of the present study, we postulated that neonatal RSV infection initiates a cascade that involves increased TSLP release from infected epithelial cells, which induces the upregulation of OX40L on lung DCs. This in turn initiates the polarization of RSV-specific T cells to a TH2 phenotype so that re-exposure to RSV triggers the expansion of RSV-specific TH2 memory cells and the enhanced development of AHR, now accompanied by eosinophilic airway inflammation, mucus hyperproduction, and IL-13 release. In adult mice this cascade was lower in intensity and potentially subverted by the increased production of IL-12, leading to increased IFN-γ production.33 Although the receptors for TSLP and OX40L (OX40) are expressed on many cell types,14,47 the protective effects of anti-TSLP, as seen with anti-OX40L, were postulated to be due to the interference of this initial interaction between epithelial cells and DCs. Notably, the development of AHR, airway eosinophilia, and mucus hyperproduction on reinfection was not inhibited completely by either anti-TSLP or anti-CD40L. In fact, the inhibitory effects were restricted to the increases in AHR seen over and above the response to primary infection. In addition, attenuation of airway eosinophilia, mucus production, and increased IL-13 levels were demonstrated. As shown previously, these enhanced components of the response to reinfection were blocked by interfering with IL-13 activity, indicating an important role for this cytokine in the enhanced allergic-like responses on reinfection.8 Together, these results imply that the response to RSV potentially involves 2 distinct pathways, with both contributing to the development of AHR. In neonates, RSV additionally triggers responses that were associated with TH2 skewing and dependent on TSLP and OX40L upregulation.
The results of the present study confirm the importance of age at initial contact with RSV and the critical role of the neonatal environment in dictating the outcome of reinfection. The host response to RSV infection can be divided into 3 phases: an epithelial phase, an innate immune response phase, and an adaptive immune response phase.53,54 The data presented here link these 3 phases of RSV-host interplay through a TSLP-OX40L-OX40 axis so that the epithelial phase is mediated by TSLP release, the innate phase is triggered through increased OX40L expression on lung DCs, and the adaptive or effector T-cell immune phase results from the initial polarization and subsequent expansion of TH2 memory cells on RSV reinfection. On the basis of these findings, novel insights into potential prophylactic approaches are apparent for the prevention of RSV-mediated long-term sequelae in infants with severe RSV bronchiolitis through interference with the initial phases of RSV infection.
Supplementary Material
Clinical implications: Neonatal RSV infection governs the response to infection, including the development of a TH2-like response and enhanced AHR.
Acknowledgments
Supported by National Institutes of Health (NIH) grants AI-77609, HL-36577, and HL-61005. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the NIH.
We thank Diana Nabighian for help in preparing the manuscript. We thank Dr Claudia V. Jakubzick for help in analyzing the lung DC subsets.
Abbreviations used
- AHR
Airway hyperresponsiveness
- APC
Antigen-presenting cell
- BAL
Bronchoalveolar lavage
- DC
Dendritic cell
- MCh
Methacholine
- mDC
Myeloid dendritic cell
- MFI
Mean fluorescence intensity
- MHCII
MHC class II
- OX40L
OX40 ligand
- PBLN
Peribronchial lymph node
- pDC
Plasmacytoid dendritic cell
- RSV
Respiratory syncytial virus
- RT
Real-time
- TSLP
Thymic stromal lymphopoietin
Footnotes
Disclosure of potential conflict of interest: S. F. Ziegler has received research support from the National Institutes of Health (NIH), has received lecture fees from the American Academy of Allergy, Asthma & Immunology, and has received travel expenses from the Federation of Clinical Immunology Societies. E. W. Gelfand has received research support from the NIH. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Collins PL, Graham BS. Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol. 2008;82:2040–55. doi: 10.1128/JVI.01625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sigurs N, Gustafsson PM, Bjarnason R, Lundberg F, Schmidt S, Sigurbergsson F, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171:137–41. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- 3.Rooney JC, Williams HE. The relationship between proved viral bronchiolitis and subsequent wheezing. J Pediatr. 1971;79:744–7. doi: 10.1016/s0022-3476(71)80385-2. [DOI] [PubMed] [Google Scholar]
- 4.Sly PD, Hibbert ME. Childhood asthma following hospitalization with acute viral bronchiolitis in infancy. Pediatr Pulmonol. 1989;7:153–8. doi: 10.1002/ppul.1950070307. [DOI] [PubMed] [Google Scholar]
- 5.Adkins B. T-cell function innewborn mice and humans. Immunol Today. 1999;20:330–5. doi: 10.1016/s0167-5699(99)01473-5. [DOI] [PubMed] [Google Scholar]
- 6.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–64. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 7.Kristjansson S, Bjarnarson SP, Wennergren G, Palsdottir AH, Arnadottir T, Haraldsson A, et al. Respiratory syncytial virus and other respiratory viruses during the first 3 months of life promote a local TH2-like response. J Allergy Clin Immunol. 2005;116:805–11. doi: 10.1016/j.jaci.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Dakhama A, Park JW, Taube C, Joetham A, Balhorn A, Miyahara N, et al. The enhancement or prevention of airway hyperresponsiveness during reinfection with respiratory syncytial virus is critically dependent on the age at first infection and IL-13 production. J Immunol. 2005;175:1876–83. doi: 10.4049/jimmunol.175.3.1876. [DOI] [PubMed] [Google Scholar]
- 9.Lee YM, Miyahara N, Takeda K, Prpich J, Oh A, Balhorn A, et al. IFN-γ production during initial infection determines the outcome of reinfection with respiratory syncytial virus. Am J Respir Crit Care Med. 2008;177:208–18. doi: 10.1164/rccm.200612-1890OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dakhama A, Lee YM, Ohnishi H, Jing X, Balhorn A, Takeda K, et al. Virus-specific IgE enhances airway responsiveness on reinfection with respiratory syncytial virus in newborn mice. J Allergy Clin Immunol. 2009;123:138–45. doi: 10.1016/j.jaci.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Han J, Jia Y, Takeda K, Shiraishi Y, Okamoto M, Dakhama A, et al. Montelukast during primary infection prevents airway hyperresponsiveness and inflammation after reinfection with respiratory syncytial virus. Am J Respir Crit Care Med. 2010;182:455–63. doi: 10.1164/rccm.200912-1811OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tregoning JS, Yamaguchi Y, Harker J, Wang B, Openshaw PJ. The role of T cells in the enhancement of respiratory syncytial virus infection severity during adult reinfection of neonatally sensitized mice. J Virol. 2008;82:4115–24. doi: 10.1128/JVI.02313-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Croft M, So T, Duan W, Soroosh P. The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol Rev. 2009;229:173–91. doi: 10.1111/j.1600-065X.2009.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Croft M. Control of immunity by the TNFR-related molecule OX40 (CD134) Annu Rev Immunol. 2010;28:57–78. doi: 10.1146/annurev-immunol-030409-101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jember AG, Zuberi R, Liu FT, Croft M. Development of allergic inflammation in a murine model of asthma is dependent on the costimulatory receptor OX40. J Exp Med. 2001;193:387–92. doi: 10.1084/jem.193.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arestides RS, He H, Westlake RM, Chen AI, Sharpe AH, Perkins DL, et al. Costimulatory molecule OX40L is critical for both Th1 and Th2 responses in allergic inflammation. Eur J Immunol. 2002;32:2874–80. doi: 10.1002/1521-4141(2002010)32:10<2874::AID-IMMU2874>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Hoshino A, Tanaka Y, Akiba H, Asakura Y, Mita Y, Sakurai T, et al. Critical role for OX40 ligand in the development of pathogenic Th2 cells in a murine model of asthma. Eur J Immunol. 2003;33:861–9. doi: 10.1002/eji.200323455. [DOI] [PubMed] [Google Scholar]
- 18.Salek-Ardakani S, Song J, Halteman BS, Jember AG, Akiba H, Yagita H, et al. OX40 (CD134) controls memory T helper 2 cells that drive lung inflammation. J Exp Med. 2003;198:315–24. doi: 10.1084/jem.20021937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–53. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 20.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005;202:829–39. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huston DP, Liu YJ. Thymic stromal lymphopoietin: a potential therapeutic target for allergy and asthma. Curr Allergy Asthma Rep. 2006;6:372–6. doi: 10.1007/s11882-996-0006-7. [DOI] [PubMed] [Google Scholar]
- 22.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–23. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seshasayee D, Lee WP, Zhou M, Shu J, Suto E, Zhang J, et al. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J Clin Invest. 2007;117:3868–78. doi: 10.1172/JCI33559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang YH, Liu YJ. OX40-OX40L interactions: a promising therapeutic target for allergic diseases? J Clin Invest. 2007;117:3655–7. doi: 10.1172/JCI34182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang YH, Liu YJ. Thymic stromal lymphopoietin, OX40-ligand, and interleukin-25 in allergic responses. Clin Exp Allergy. 2009;39:798–806. doi: 10.1111/j.1365-2222.2009.03241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han J, Dakhama A, Shiraishi Y, Okamoto M, Takeda K, Gelfand EW. In vivo blockade of OX40 ligand prevents respiratory syncytial virus (RSV)-induced airway hyperresponsiveness (AHR) and inflammation. J Allergy Clin Immunol. 2010;125:AB62. [Google Scholar]
- 27.Takeda K, Hamelmann E, Joetham A, Shultz LD, Larsen GL, Irvin CG, et al. Development of eosinophilic airway inflammation and airway hyperresponsiveness in mast cell-deficient mice. J Exp Med. 1997;186:449–54. doi: 10.1084/jem.186.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jakubzick C, Randolph GJ. Methods to study pulmonary dendritic cell migration. Methods Mol Biol. 2010;595:371–82. doi: 10.1007/978-1-60761-421-0_24. [DOI] [PubMed] [Google Scholar]
- 29.Dalod M, Salazar-Mather TP, Malmgaard L, Lewis C, Asselin-Paturel C, Brière F, et al. Interferon alpha/beta and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J Exp Med. 2002;195:517–28. doi: 10.1084/jem.20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han J, Takeda K, Gelfand EW. The role of RSV infection in asthma initiation and progression: findings in a mouse model. Pulm Med. 2011;2011:748038. doi: 10.1155/2011/748038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welliver RC, Wong DT, Sun M, Middleton E, Jr, Vaughan RS, Ogra PL. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med. 1981;305:841–6. doi: 10.1056/NEJM198110083051501. [DOI] [PubMed] [Google Scholar]
- 32.Bont L, Heijnen CJ, Kavelaars A, van Aalderen WM, Brus F, Draaisma JM, et al. Local interferon-gamma levels during respiratory syncytial virus lower respiratory tract infection are associated with disease severity. J Infect Dis. 2001;184:355–8. doi: 10.1086/322035. [DOI] [PubMed] [Google Scholar]
- 33.Trinchieri G, Scott P. The role of interleukin 12 in the immune response, disease and therapy. Immunol Today. 1994;15:460–3. doi: 10.1016/0167-5699(94)90189-9. [DOI] [PubMed] [Google Scholar]
- 34.de Heer HJ, Hammad H, Soullié T, Hijdra D, Vos N, Willart MA, et al. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200:89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smit JJ, Lindell DM, Boon L, Kool M, Lambrecht BN, Lukacs NW. The balance between plasmacytoid DC versus conventional DC determines pulmonary immunity to virus infections. PLoS One. 2008;3:e1720. doi: 10.1371/journal.pone.0001720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohshima Y, Yang LP, Uchiyama T, Tanaka Y, Baum P, Sergerie M, et al. OX40 costimulation enhances interleukin-4 (IL-4) expression at priming and promotes the differentiation of naive human CD4(1) T cells into high IL-4-producing effectors. Blood. 1998;92:3338–45. [PubMed] [Google Scholar]
- 37.Flynn S, Toellner KM, Raykundalia C, Goodall M, Lane P. CD4 T cell cytokine differentiation: the B cell activation molecule, OX40 ligand, instructs CD4 T cells to express interleukin 4 and upregulates expression of the chemokine receptor, Blr-1. J Exp Med. 1998;188:297–304. doi: 10.1084/jem.188.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.So T, Song J, Sugie K, Altman A, Croft M. Signals from OX40 regulate nuclear factor of activated T cells c1 and T cell helper 2 lineage commitment. Proc Natl Acad Sci U S A. 2006;103:3740–5. doi: 10.1073/pnas.0600205103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Humphreys IR, Walzl G, Edwards L, Rae A, Hill S, Hussell T. A critical role for OX40 in T cell-mediated immunopathology during lung viral infection. J Exp Med. 2003;198:1237–42. doi: 10.1084/jem.20030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lukens MV, Kruijsen D, Coenjaerts FE, Kimpen JL, van Bleek GM. Respiratory syncytial virus-induced activation and migration of respiratory dendritic cells and subsequent antigen presentation in the lung-draining lymph node. J Virol. 2009;83:7235–43. doi: 10.1128/JVI.00452-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smit JJ, Rudd BD, Lukacs NW. Plasmacytoid dendritic cells inhibit pulmonary immunopathology and promote clearance of respiratory syncytial virus. J Exp Med. 2006;203:1153–9. doi: 10.1084/jem.20052359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beyer M, Bartz H, Hörner K, Doths S, Koerner-Rettberg C, Schwarze J. Sustained increases in numbers of pulmonary dendritic cells after respiratory syncytial virus infection. J Allergy Clin Immunol. 2004;113:127–33. doi: 10.1016/j.jaci.2003.10.057. [DOI] [PubMed] [Google Scholar]
- 43.McGill J, Van Rooijen N, Legge KL. Protective influenza-specific CD8 T cell responses require interactions with dendritic cells in the lungs. J Exp Med. 2008;205:1635–46. doi: 10.1084/jem.20080314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang H, Peters N, Laza-Stanca V, Nawroly N, Johnston SL, Schwarze J. Local CD11c+ MHC class II- precursors generate lung dendritic cells during respiratory viral infection, but are depleted in the process. J Immunol. 2006;177:2536–42. doi: 10.4049/jimmunol.177.4.2536. [DOI] [PubMed] [Google Scholar]
- 45.Allakhverdi Z, Comeau MR, Smith DE, Toy D, Endam LM, Desrosiers M, et al. CD34+ hemopoietic progenitor cells are potent effectors of allergic inflammation. J Allergy Clin Immunol. 2009;123:472–8. doi: 10.1016/j.jaci.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 46.Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol. 2010;11:289–93. doi: 10.1038/ni.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Headley MB, Zhou B, Shih WX, Aye T, Comeau MR, Ziegler SF. TSLP conditions the lung immune environment for the generation of pathogenic innate and antigen-specific adaptive immune responses. J Immunol. 2009;182:1641–7. doi: 10.4049/jimmunol.182.3.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jessup HK, Brewer AW, Omori M, Rickel EA, Budelsky AL, Yoon BR, et al. Intradermal administration of thymic stromal lymphopoietin induces a T cell- and eosinophil-dependent systemic Th2 inflammatory response. J Immunol. 2008;181:4311–9. doi: 10.4049/jimmunol.181.6.4311. [DOI] [PubMed] [Google Scholar]
- 49.Yoo J, Omori M, Gyarmati D, Zhou B, Aye T, Brewer A, et al. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202:541–9. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou B, Headley MB, Aye T, Tocker J, Comeau MR, Ziegler SF. Reversal of thymic stromal lymphopoietin-induced airway inflammation through inhibition of Th2 responses. J Immunol. 2008;181:6557–62. doi: 10.4049/jimmunol.181.9.6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ebner S, Nguyen VA, Forstner M, Wang YH, Wolfram D, Liu YJ, et al. Thymic stromal lymphopoietin converts human epidermal Langerhans cells into antigen-presenting cells that induce proallergic T cells. J Allergy Clin Immunol. 2007;119:982–90. doi: 10.1016/j.jaci.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 52.Qiao J, Li A, Jin X. TSLP from RSV-stimulated rat airway epithelial cells activates myeloid dendritic cells. Immunol Cell Biol. 2011;89:231–8. doi: 10.1038/icb.2010.85. [DOI] [PubMed] [Google Scholar]
- 53.Schwarze J. Lung dendritic cells in respiratory syncytial virus bronchiolitis. Pediatr Infect Dis J. 2008;27(suppl):S89–91. doi: 10.1097/INF.0b013e318168b6f0. [DOI] [PubMed] [Google Scholar]
- 54.Smyth RL, Openshaw PJ. Bronchiolitis. Lancet. 2006;368:312–22. doi: 10.1016/S0140-6736(06)69077-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.