Abstract
Objective
Treatments for the cognitive impairments of schizophrenia are urgently needed. We developed and tested a 12-week, group-based, manualized, Compensatory Cognitive Training (CCT) intervention targeting prospective memory, attention, learning/memory, and executive functioning. The intervention focused on compensatory strategies such as calendar use, self-talk, note-taking, and a six-step problem-solving method, and did not require computers.
Method
In a randomized controlled trial, 69 outpatients with DSM-IV primary psychotic disorders were assigned to receive standard pharmacotherapy (SP) alone or CCT + SP for 12 weeks. Assessments of neuropsychological performance and functional capacity (primary outcomes) and psychiatric symptom severity, quality of life, social skills performance, cognitive insight, and self-reported everyday functioning (secondary outcomes) were administered at baseline, post-treatment, and 3-month follow-up. Data were collected between September 2003 and August 2009.
Results
Hierarchical linear modeling analyses demonstrated significant CCT-associated effects on attention at follow-up (p=0.049), verbal memory at post-treatment and follow-up (ps≤0.039), and functional capacity (UCSD Performance-based Skills Assessment) at follow-up (p=0.004). The CCT group also differentially improved in negative symptom severity at post-treatment and follow-up (ps≤0.025) and subjective quality of life at follow-up (p=0.002).
Conclusion
Compensatory Cognitive Training, a low-tech, brief intervention, has the potential to improve not only cognitive performance, but also functional skills, negative symptoms, and self-rated quality of life in people with psychosis.
Keywords: schizophrenia, rehabilitation, cognitive remediation, memory
Introduction
Empirically supported treatments for schizophrenia and primary psychotic disorders include a variety of psychosocial interventions, such as social skills training, supported employment, and cognitive behavioral therapy.1 As awareness of the functional importance of neurocognitive impairments in schizophrenia has increased2-3, interest in pharmacological and behavioral treatments to improve cognition has grown. One such treatment, cognitive remediation or cognitive training, is defined as “a behavioral, training-based intervention that aims to improve cognitive processes (attention, memory, executive function, social cognition, or metacognition) with the goal of durability and generalization”.4
The most recent meta-analysis4 of cognitive remediation found small-to-moderate effects on cognitive tests, as well as psychosocial functioning and psychiatric symptom severity (effect sizes of .45, 42, and .18 respectively). There were no differences in effect sizes depending on intervention approach (strategy coaching vs. drill and practice), duration of treatment, or use of computers. Most commercially available interventions, however, use computerized drill and practice exercises. Furthermore, some of the effects of these interventions may be attributable to non-specific cognitive stimulation, as one well-controlled study found considerable cognitive improvements in a control group receiving training on computer software packages.5 Interventions emphasizing compensatory strategies (with or without computerized drills) have produced some of the largest effect sizes in the field6, but are less commonly used.
Our goal was to create and pilot-test a cognitive training intervention that would be brief, practical, low-tech, engaging to clients, and portable enough to be delivered in the community. Accordingly, we tested the efficacy of a 12-week, manualized Compensatory Cognitive Training (CCT) intervention designed to target four cognitive domains: 1) prospective memory, 2) attention and vigilance, 3) learning and memory, and 4) executive functioning. These domains were selected based on their degree of impairment in schizophrenia-spectrum disorders, relevance for psychosocial functioning, and potential modifiability.2,7-8 Although prospective memory (the ability to remember to do things) generally has not been targeted in cognitive training, it predicts functional capacity9, treatment attendance, and adherence.10 Our goal was to take advantage of intact abilities in schizophrenia, such as habit learning11-12 and imagery13, to bolster impaired functions. Because habit learning is also highly resistant to forgetting14, we aimed to help participants form new habits in attention, learning, and problem-solving to automate tasks and reduce the active cognitive effort usually demanded for effective performance.
The CCT manual incorporated ideas and materials from various sources. The prospective memory module adapted techniques from the Acquired Brain Injury program at Mesa College in San Diego regarding external aids. The attention and vigilance module adapted conversational vigilance skills from Bellack and colleagues’ social skills training manual15; the use of self-talk to improve task vigilance was informed by Meichenbaum and Cameron's work.16 The executive functioning module included categorization tasks adapted from Delahunty and Morice's manual17, which Wykes and colleagues18 have also used. Finally, a six-step problem solving method was adapted from the social skills training approach of Bellack and colleagues15, from which we also adapted the homework sheets for the manual. Multiple stakeholders (e.g., consumers, caregivers, treating clinicians, and cognitive training experts) provided feedback during the development of the CCT manual (e.g., participants requested assistance with remembering people's names, so a name-learning section was added). The CCT strategies included approaches that were both internal and external to the individual (see Table 1).
Table 1.
Domains and Strategies Included in CCT
| Prospective Memory | Calendar use; to-do lists; prioritizing tasks; linking tasks by using planned cues; automatic places; using routines to automate tasks |
| Attention and Vigilance | Eye contact, paraphrasing, asking questions during conversations; self-talk during tasks; taking breaks to refocus |
| Learning and Memory | Taking notes; association; chunking; categorization; acronyms; visual imagery; overlearning |
| Executive Functioning | Six step problem solving method; self-talk and self-monitoring while solving problems; hypothesis testing using pro and con evidence; set shifting; set maintenance |
CCT = Compensatory Cognitive Training
Because our primary goals were to improve cognition and community functioning, our primary outcome measures were cognitive tests in the four targeted domains and a performance-based test of functional capacity, the UCSD Performance-based Skills Assessment (UPSA19, a common co-primary outcome measure in cognitive treatment trials).20 We hypothesized that, compared to individuals receiving standard pharmacotherapy (SP) alone, participants who received the CCT intervention plus SP would show improvements in targeted cognitive domains and functional capacity. We also explored generalization of effects to psychiatric symptom severity, social skills performance, cognitive insight, and self-reports of cognitive problems, strategy use, everyday functioning, and quality of life.
Method
Participants
Participants initially enrolled in the study included 89 community-dwelling outpatients. Inclusion criteria were: primary psychotic disorder (including schizophrenia, schizoaffective disorder, psychotic mood disorder, or psychosis NOS), age 18 or older, and fluency in English. Exclusion criteria were: dementia, neurological conditions affecting cognition, mental retardation, substance use disorder within the past month, and participation in other intervention trials. The study was approved by the UCSD Institutional Review Board; all participants provided written informed consent to participate in the study. Sample characteristics and tests for differences between groups are presented in Table 2.
Table 2.
Baseline Group Comparison of Randomized Participants (n=69)
| CCT | SP | |||||
|---|---|---|---|---|---|---|
| n | mean (SD)/% | n | mean (SD)/% | t or χ2 | p-value | |
| Demographics | ||||||
| Age | 38 | 44.3 (10.1) | 31 | 48.8 (8.7) | 1.98 | 0.052 |
| Gender, % male | 38 | 63.2% | 31 | 67.7% | 0.16 | 0.691 |
| Education | 38 | 12.9 (1.8) | 31 | 13.0 (1.6) | 0.39 | 0.697 |
| Race/Ethnicity, % Caucasian | 38 | 63.2% | 31 | 54.8% | 0.49 | 0.484 |
| Housing, % living independently | 37 | 81.1% | 30 | 80.0% | 0.38 | 0.539 |
| Marital Status, % ever married | 38 | 42.1% | 30 | 46.7% | 0.14 | 0.707 |
| Illness Burden | ||||||
| Diagnosis | 2.09 | 0.554 | ||||
| Schizophrenia | 20 | 52.6% | 17 | 54.8% | ||
| Schizoaffective disorder | 17 | 44.7% | 13 | 41.9% | ||
| Psychosis NOS | 1 | 2.6% | 0 | 0.0% | ||
| MDD with psychotic features | 0 | 0.0% | 1 | 3.2% | ||
| Illness duration, years | 38 | 21.1 (13.5) | 31 | 25.9 (10.3) | 1.64 | 0.107 |
| Antipsychotic Medication Type | 2.73 | 0.435 | ||||
| None | 1 | 2.8% | 3 | 9.7% | ||
| Typical | 1 | 2.8% | 2 | 6.5% | ||
| Atypical | 33 | 91.7% | 24 | 77.4% | ||
| Both | 1 | 2.8% | 2 | 6.5% | ||
| Antipsychotic dose, CPZE | 29 | 479.37 (455.07) | 28 | 266.67 (246.49) | 2.20 | 0.033 |
| Clinical/Functioning Measures | ||||||
| PANSS Positive Symptoms | 38 | 16.26 (6.58) | 31 | 17.16 (6.12) | 0.58 | 0.563 |
| PANSS Negative Symptoms | 38 | 15.66 (6.24) | 31 | 14.23 (4.90) | 1.07 | 0.290 |
| HAM-D Depressive Symptoms | 38 | 12.00 (7.45) | 29 | 11.34 (6.37) | 0.38 | 0.706 |
| Functional Capacity (UPSA) | 38 | 82.44 (9.90) | 30 | 85.47 (8.42) | 1.33 | 0.187 |
| Social Skills Performance (SSPA) | 38 | 31.25 (6.24) | 31 | 31.13 (6.06) | 0.08 | 0.936 |
| Subjective Quality of Life (QOLI) | 37 | 4.16 (1.59) | 30 | 4.43 (1.38) | 0.74 | 0.465 |
| CPSA Cognitive Problems | 38 | 1.14 (0.52) | 30 | 0.92 (0.42) | 1.87 | 0.066 |
| CPSA Cognitive Strategies | 38 | 1.31 (0.43) | 30 | 1.43 (0.44) | 1.10 | 0.275 |
| Neuropsychological Raw Scores | ||||||
| Premorbid IQ estimate | 36 | 106.42 (9.66) | 31 | 107.55 (10.40) | 0.46 | 0.646 |
| Targeted Domains | ||||||
| Prospective Memory (MIST total) | 38 | 28.82 (11.74) | 31 | 29.71 (9.08) | 0.36 | 0.723 |
| Attention (maximum forward Digit Span) | 38 | 6.08 (1.36) | 31 | 6.23 (1.59) | 0.41 | 0.680 |
| Verbal Learning (HVLT-R recall total) | 38 | 24.63 (5.94) | 31 | 22.87 (5.44) | 1.27 | 0.208 |
| Verbal Memory (HVLT-R % retained) | 38 | 86.12 (14.95) | 31 | 90.94 (19.71) | 1.16 | 0.252 |
| Executive Functioning (WCST total) | 37 | 42.84 (9.72) | 31 | 40.97 (12.79) | 0.67 | 0.507 |
| Non-targeted Domains | ||||||
| Processing Speed (Digit Symbol total) | 38 | 52.03(15.37) | 31 | 52.71(13.26) | 0.20 | 0.846 |
| Working Memory (LNS total) | 38 | 8.32(2.57) | 31 | 8.39(2.56) | 0.11 | 0.909 |
| Verbal Fluency (COWAT total) | 37 | 41.3(11.45) | 31 | 41.65(11.02) | 0.13 | 0.899 |
Note. Significant differences are indicated in bold font. CCT = Compensatory Cognitive Training; CPSA = Cognitive Problems and Strategies Assessment; CPZE = chlorpromazine equivalent; COWAT = Controlled Oral Word Association Test; HAM-D = Hamilton Depression Rating Scale; HVLT-R = Hopkins Verbal Learning Test-Revised; IQ = Intelligence Quotient; LNS = Letter-Number Sequencing; MDD = Major Depressive Disorder; MIST = Memory for Intentions Screening Test; NOS = not otherwise specified; PANSS = Positive and Negative Syndrome Scale; QOLI = Quality of Life Interview; SP = standard pharmacotherapy; SSPA = Social Skills Performance Assessment; UPSA = UCSD Performance-Based Skills Assessment; WCST = Wisconsin Card Sorting Test
Sixty-nine participants completed baseline assessments and were randomized, and 51 of these participants completed the study. Our statistical models included data from the 69 participants with a baseline assessment who were randomized, thus, we present the characteristics of these 69 participants in Table 2. Study completers (n=51) included 23 CCT participants and 28 SP participants who had follow-up data. Compared to the participants who dropped out with no CCT exposure (n=28), study completers (n=51) had more education and lower daily doses of antipsychotics, but did not otherwise differ. A description of the development of the CCT intervention and effect sizes based on data from 38 of the 51 completers were published previously21, but inferential statistics have not been published.
Attrition from the study (see Supplemental CONSORT diagram Figure) occurred after enrollment but before baseline assessment (n=14), after baseline assessment but before randomization (n=6), after randomization to SP (n=3), after randomization to CCT but before any exposure to the CCT intervention (n=5), or after randomization and attendance of at least one CCT session (n=10). Of those who attended at least one CCT session but later dropped out, seven attended only one session, one person each attended two, five, and ten sessions (thus, these 10 participants attended an average of 2.4 sessions). Common reasons for dropping out of the CCT intervention or the study itself were being too busy or not needing treatment for cognitive impairment, but most dropouts simply could not be contacted. Those assigned to CCT who did not drop out attended an average of 10.6 out of 12 CCT sessions (range = 6-12). There were no significant differences between participants who completed CCT and those who began CCT but later dropped out.
Procedure
Data were collected between September 2003 and August 2009. Participants were referred to the study by treating clinicians or self-referral. CCT was described to potential participants as a thinking and memory “class” to de-stigmatize the focus on cognitive impairment and to emphasize skill acquisition rather than psychotherapy. Diagnoses were confirmed via DSM-IV-based diagnostic chart reviews and/or structured diagnostic interview (Mini International Neuropsychiatric Interview).22 Following baseline assessment, participants were randomly assigned to receive CCT plus standard pharmacotherapy (CCT) or standard pharmacotherapy alone (SP). Early in the study, randomization occurred following each participant's baseline assessment, but to save time, we altered the study procedure to randomize in blocks of five, meaning that after five participants were enrolled and completed baseline assessment, they were randomized as a group to receive either CCT or SP.
The CCT intervention was delivered in groups in 12, two-hour sessions over 12 weeks in two community-based mental health clinics that followed a psychosocial rehabilitation model.1 The CCT groups consisted of five participants and two therapists; therapists were EWT and doctoral trainees trained and supervised by EWT. The structure of the CCT intervention was determined by the treatment manual, but was also intended to be interactive and personally meaningful to the participants. Sessions included a review of homework, troubleshooting of strategy use, psychoeducation and rationale for the targeted domains, demonstration and practice of each strategy, feedback on strategy use, and individualized discussion regarding implementation of the strategies in daily life. A break was provided between the first and second hours of each session. Homework was assigned to encourage real-world implementation of strategies as well as to provide an opportunity to troubleshoot any difficulties. CCT did not use computers, and strategies taught did not “train to the test” or use any of the outcome measures during training. Therapists and participants all used the treatment manual during sessions.
Participants completed outcome assessments at post-treatment and at three-month follow-up. Personnel performing the assessments were blind to group assignment and trained to a high level of reliability on symptom rating instruments (ICC ≥ 0.80). Participants were compensated for their time and travel to assessment sessions, but were not paid for attending CCT sessions. Chlorpromazine equivalent amounts in milligrams (CPZE) were used to convert antipsychotic medication dosages at baseline according to standard formulae (except clozapine and injectables).23-24
Measures
Neuropsychological measures included an estimate of premorbid intellectual functioning, the American National Adult Reading Test (ANART).25 Additional neuropsychological measures of domains targeted by the CCT strategies included:
Prospective memory: Memory for Intentions Screening Test (total score)26
Attention: Wechsler Adult Intelligence Scale, third edition (WAIS-III) Digit Span forward maximum span (isolated from Digit Span backward to measure attention, rather than working memory, which was not targeted in CCT)27
Verbal learning and memory: Hopkins Verbal Learning Test - Revised (HVLT-R, total immediate recall and percent retained)28
Executive Functioning: Wisconsin Card Sorting Test (WCST; total correct)29
The non-targeted domains were measured as follows:
Processing speed: WAIS-III Digit Symbol total correct27
Working memory: WAIS-III Letter-Number Sequencing total correct27
Verbal Fluency: Controlled Oral Word Association Test (COWAT; Animals/Fruits/Vegetables total)30
Functional capacity was measured with the UCSD Performance-based Skills Assessment (UPSA), which uses structured role-play scenarios to measure ability in five everyday living domains (Household Chores, e.g., shopping in the context of a provided recipe; Communication, e.g., using the telephone for emergency and routine situations; Finance, e.g., making change and paying a bill by check; Transportation, e.g., planning a bus route; and Planning Recreational Activities, e.g., planning an outing).19 The UPSA total score (0-100) is moderately correlated with global neuropsychological functioning31-32, but has been shown to better predict real-world outcomes such as living independence.33
Secondary outcomes and other assessments to characterize the sample included established measures. The Social Skills Performance Assessment (SSPA), a role-play test of social skills, assessed ability in the context of neutral and confrontational social scenarios.34 The Independent Living Skills Survey (ILSS) was administered as a self-report measure of functioning.35 Psychiatric symptom severity was measured with the Positive and Negative Syndrome Scale (PANSS) and the Hamilton Depression Rating Scale (HAM-D).36-37 Cognitive insight was measured by the Beck Cognitive Insight Scale (BCIS), which includes items assessing self-reflectiveness, openness to feedback, and certainty about beliefs.38 Quality of life was measured with the Quality of Life Interview (QOLI).39 Self-reported cognitive problems and cognitive strategy use were measured by the Cognitive Problems and Strategies Assessment (CPSA; Twamley, unpublished; included in Appendix).
Data analyses
All variables were inspected for normality; no data transformations were needed. Study hypotheses were tested using hierarchical linear modeling (HLM), an intent-to-treat method using all available data points. The age of the two groups was close to significantly different (see Table 2), therefore age was added to the HLM analyses to test whether the group by time effects varied by age. Although CPZE dosage was significantly different between the groups (see Table 2), it was not added to the models because it was not available for all participants, due to the conversion formula restrictions. Time was modeled as a discrete parameter, as there were only three time points, and the baseline assessment was used as the reference time point. A random intercept for individuals was included in all models. The level 1 parameters were group (CCT and SP, with SP as the reference category), age (grand mean centered), and time; the level 2 parameter was individuals. For primary outcomes and for secondary outcome variables with significant HLM results, Cohen's d effect sizes were then calculated using group differences in change scores (ns with complete data ranged from 42-48). All statistical models were computed with and without outliers. The models were also run without the subjects with primary psychotic disorders other than schizophrenia or schizoaffective disorder; the results did not change, so results from the entire sample are reported below.
Results
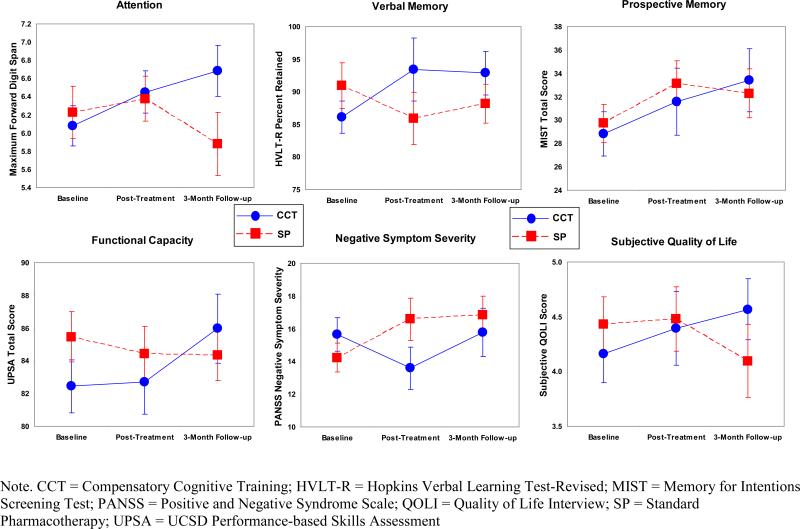
Table 3 presents all significant and borderline significant (p=0.05) model parameter estimates and test statistics for the primary and secondary outcome measures, and Figure 1 provides graphs of these group by time interactions. Other models are presented in the Supplementary Table.
Table 3.
Hierarchical Linear Models
| Attention (maximum forward Digit Span) | Verbal Memory (HVLT-R Percent Retained) | Prospective Memory (MIST Total Score) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EE | SE | t | df | p-value | EE | SE | t | df | p-value | EE | SE | t | df | p-value | |
| Intercept | 6.22 | 0.26 | 23.96 | 101.53 | <0.001 | 91.66 | 3.21 | 28.52 | 12.73 | <0.001 | 3.38 | 1.95 | 15.60 | 82.60 | <0.001 |
| Time | |||||||||||||||
| Post-treatment | 0.16 | 0.27 | 0.59 | 92.67 | 0.554 | -6.32 | 3.92 | -1.61 | 95.35 | 0.110 | 1.79 | 1.50 | 1.19 | 86.63 | 0.237 |
| 3 Month Follow-up | -0.14 | 0.26 | -0.53 | 92.14 | 0.596 | -4.38 | 3.80 | -1.15 | 94.01 | 0.253 | 1.74 | 1.45 | 1.20 | 86.30 | 0.232 |
| Group (CCT) | 0.00 | 0.02 | 0.17 | 101.53 | 0.867 | -.29 | 0.23 | -1.27 | 12.73 | 0.207 | -0.27 | 0.14 | -1.96 | 82.60 | 0.054 |
| Age | -0.13 | 0.35 | -.38 | 101.53 | 0.708 | -6.13 | 4.39 | -1.40 | 12.73 | 0.164 | -2.12 | 2.66 | -0.80 | 82.60 | 0.428 |
| Age*Time | |||||||||||||||
| Post-treatment | 0.00 | 0.02 | 0.21 | 97.63 | 0.833 | .51 | 0.30 | 1.69 | 102.19 | 0.094 | 0.21 | 0.12 | 1.77 | 89.40 | 0.080 |
| 3 Month Follow-up | -0.02 | 0.02 | -1.01 | 96.85 | 0.317 | .61 | 0.29 | 2.14 | 10.29 | 0.035 | 0.26 | 0.11 | 2.30 | 89.24 | 0.024 |
| Group*Time | |||||||||||||||
| Post-treatment | 0.38 | 0.39 | 0.98 | 96.67 | 0.330 | 13.76 | 5.69 | 2.42 | 10.71 | 0.017 | 1.75 | 2.22 | 0.79 | 88.91 | 0.431 |
| 3 Month Follow-up | 0.75 | 0.38 | 1.99 | 96.20 | 0.049 | 11.53 | 5.51 | 2.09 | 99.59 | 0.039 | 4.25 | 2.13 | 1.99 | 88.75 | 0.050 |
| Functional Capacity (UPSA Total Score) | Negative Symptom Severity (PANSS) | Subjective Quality of Life (QOLI) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EE | SE | t | df | p-value | EE | SE | t | df | p-value | EE | SE | t | df | p-value | |
| Intercept | 85.40 | 1.64 | 52.16 | 95.51 | <0.001 | 13.89 | 1.04 | 13.33 | 98.16 | <0.001 | 4.50 | 0.27 | 16.43 | 92.56 | <0.001 |
| Time | |||||||||||||||
| Post-treatment | -0.90 | 1.58 | -0.57 | 88.49 | 0.569 | 2.16 | 0.98 | 2.19 | 94.39 | 0.031 | 0.01 | 0.26 | 0.03 | 87.65 | 0.977 |
| 3 Month Follow-up | -1.48 | 1.52 | -0.97 | 88.75 | 0.335 | 2.75 | 0.96 | 2.86 | 94.02 | 0.005 | -0.42 | 0.25 | -1.68 | 87.92 | 0.096 |
| Group (CCT) | -0.07 | 0.11 | -0.58 | 93.90 | 0.563 | 0.13 | 0.07 | 1.82 | 98.16 | 0.073 | -0.04 | 0.02 | -2.00 | 91.00 | 0.048 |
| Age | -3.09 | 2.22 | -1.39 | 94.50 | 0.168 | 2.04 | 1.42 | 1.43 | 98.16 | 0.155 | -0.42 | 0.38 | -1.13 | 91.56 | 0.260 |
| Age*Time | |||||||||||||||
| Post-treatment | -0.01 | 0.12 | -0.10 | 92.84 | 0.918 | -0.04 | 0.08 | -0.58 | 98.40 | 0.560 | 0.02 | 0.02 | 0.81 | 91.66 | 0.422 |
| 3 Month Follow-up | 0.23 | 0.11 | 1.99 | 92.34 | 0.050 | -0.16 | 0.07 | -2.20 | 97.94 | 0.030 | 0.04 | 0.02 | 2.00 | 91.19 | 0.049 |
| Group*Time | |||||||||||||||
| Post-treatment | 3.12 | 2.31 | 1.35 | 92.21 | 0.181 | -4.57 | 1.44 | -3.18 | 97.68 | 0.002 | 0.52 | 0.38 | 1.36 | 9.94 | 0.176 |
| 3 Month Follow-up | 6.57 | 2.21 | 2.97 | 92.15 | 0.004 | -3.23 | 1.42 | -2.28 | 97.51 | 0.025 | 1.15 | 0.36 | 3.17 | 9.87 | 0.002 |
Note. Significant findings are indicated in bold font. CCT = Compensatory Cognitive Training; EE = Effect Estimate; HVLT-R = Hopkins Verbal Learning Test-Revised; MIST = Memory for Intentions Screening Test; PANSS = Positive and Negative Syndrome Scale; QOLI = Quality of Life Interview; SE = Standard Error; UPSA = UCSD Performance-Based Skills Assessment
Figure 1.
Mean of Outcomes by Group
Treatment Effects on Targeted Cognitive Domains
Compared to participants receiving SP, those in the CCT group demonstrated improvement in attention at three-month follow-up (p=0.049). The CCT group differentially improved in verbal memory at both post-treatment and three-month follow-up (ps≤0.039). Group differences at the three-month follow-up approached significance for prospective memory (p=0.05), with the CCT group showing more improvement than the SP group.
Treatment Effects on Functional Capacity
Compared with those in the SP group, participants in the CCT intervention improved significantly more in functional capacity (UPSA) at the three-month follow-up time point (p=0.004).
Treatment Effects on Other Secondary Outcomes
There were also CCT-associated improvements in negative symptoms at post-treatment and three-month follow-up (ps≤0.025) and in subjective quality of life at the three-month follow-up (p=0.002).
Treatment Effects on Self-Reported Cognitive Problems and Strategy Use
CCT participants reported significantly fewer cognitive problems than did SP participants at post-treatment (EE =-0.24, SE=0.10, p=0.020), and those in the CCT group reported using more cognitive strategies than did SP participants at both post-treatment (EE =0.47, SE=0.13, p<0.001) and follow-up (EE =0.40, SE=0.20, p=0.002).
All other group differences in outcomes at post-treatment and follow-up were non-significant (ps≥0.158), and the group by time effects did not vary by age for any outcomes (ps≥0.121). Effect sizes comparing the groups’ change scores at post-treatment and three-month follow-up for all outcome measures are presented in Table 4.
Table 4.
Effect Sizes for Group Differences in Change Scores at Post-treatment and Three-month Follow-up
| Post-treatment minus baseline change score | Three-month follow-up minus baseline change score | |
|---|---|---|
| Targeted cognitive domains | ||
| Prospective Memory (MIST total) | 0.09 | 0.53 |
| Attention (maximum forward Digit Span) | 0.10 | 0.24 |
| Verbal Learning (HVLT-R recall total) | 0.03 | 0.27 |
| Verbal Memory (HVLT-R % retained) | 0.53 | 0.38 |
| Executive Functioning (WCST total) | 0.23 | 0.34 |
| Non-targeted cognitive domains | ||
| Processing Speed (Digit Symbol total) | -0.05 | 0.02 |
| Working Memory (LNS total) | -0.13 | 0.03 |
| Verbal Fluency (COWAT total) | 0.11 | 0.06 |
| Functional Capacity (UPSA) | 0.61 | 0.72 |
| Social Skills Performance (SSPA) | 0.06 | 0.14 |
| Negative Symptoms (PANSS) | 0.92 | 0.43 |
| Positive Symptoms (PANSS) | 0.03 | 0.27 |
| Depressive Symptoms (HAM-D) | 0.14 | 0.19 |
| Subjective Quality of Life (QOLI) | 0.53 | 0.81 |
| CPSA Cognitive Problems | 0.88 | 0.46 |
| CPSA Cognitive Strategies | 0.85 | 0.84 |
Note. All effect sizes have been presented such that a positive effect size denotes differential improvement in the Compensatory Cognitive Training group compared with the standard pharmacotherapy group. COWAT = Controlled Oral Word Association Test; CPSA = Cognitive Problems and Strategies Assessment; HAM-D = Hamilton Depression Rating Scale; HVLT-R = Hopkins Verbal Learning Test-Revised; LNS = Letter-Number Sequencing; MIST = Memory for Intentions Screening Test; PANSS = Positive and Negative Syndrome Scale; QOLI = Quality of Life Interview; SSPA = Social Skills Performance Assessment; UPSA = UCSD Performance-Based Skills Assessment; WCST = Wisconsin Card Sorting Test
Sensitivity Analysis
The following variables were found to have significant outliers: HVLT-R percent retained, Digit Symbol total correct, Letter-Number sequencing total correct, PANSS negative symptoms, HAM-D, UPSA, and CPSA cognitive problems. Sensitivity analyses were run excluding the outliers for each of the above variables. When outliers were removed, the group difference was no longer significant at the post-treatment time point for verbal memory (p=0.078); however the group difference at follow-up remained significant (EE = -11.49, SE = 5.17, p=0.029). The group difference in self-reported cognitive problems at post-treatment also became non-significant (p=0.070) when the outliers were removed. The findings for all of the other outcomes tested did not change.
Discussion
Our results showed that, compared with participants receiving SP alone, those who received group CCT plus SP for 12 weeks demonstrated improvements in some targeted areas of cognition (attention and memory; p=0.05 trend for prospective memory), functional capacity as measured by the UPSA, negative symptom severity, and subjective quality of life. Effect sizes associated with significant cognitive improvements ranged from small to medium (.24-.53). However, the effect sizes associated with significant improvement in functional capacity (.61) negative symptom severity (.92), and subjective quality of life (.81), were larger. It may be that CCT and similar interventions have small-to-moderate cognitive effects that can, in turn, yield larger effects in more distal functional outcomes, or it may be that CCT had effects on symptoms or functioning that were independent of cognitive improvement. Many of the effect sizes associated with CCT exceeded the average effect size benchmarks in published trials to date (i.e., .45 for cognition, .42 for psychosocial functioning, and .18 for symptoms, according to the recent meta-analysis by Wykes and colleagues4), despite CCT being a briefer than average intervention (24 hours vs. 32 hours).4
Importantly, some effect sizes increased from post-treatment to three-month follow-up (i.e., those for prospective memory, attention, learning, executive functioning, functional capacity, and subjective quality of life), which may result from continued strategy practice during the follow-up period. However, the improvements in negative symptom severity and self-rated cognitive problems were smaller at three-month follow-up than at post-treatment. Improvements in negative symptom severity following cognitive remediation are not unusual6,40-41, but little is known about the time course of such improvements. In the case of CCT, it is possible that group participation had a salutary effect on negative symptoms, but the effect was attenuated during the follow-up period. Similarly, participation in CCT may have heightened participants’ awareness of cognitive problems, but such awareness may have diminished during the follow-up period. Finally, although CCT's effects on attention and subjective quality of life showed continued improvement at three-month follow-up, the significance of these effects may have been partially attributable to declining scores in the SP group.
Although our initial results are promising, this study has limitations, including a small sample size and a relatively short follow-up period. We also had a significant dropout rate, and have presented results related to predictors of dropout in a separate publication42 (briefly, we found that study completers had more formal education and lower daily doses of antipsychotic medications than did dropouts no CCT exposure, and there were no significant differences between participants who completed CT and those who began CT but later dropped out). Because CCT is a novel intervention and our primary research question concerned its efficacy, we did not use an active control condition that matched CCT for therapist time or group involvement; our results should be considered preliminary until they are replicated in a larger sample in a study using an active control condition. Although we do not believe that the effects of CCT on objective neuropsychological and performance-based functioning tests administered by blinded raters to be attributable to non-specific therapeutic factors, such factors could have affected self-report measures (e.g., quality of life). A new study of CCT using a robust control group is now underway. Although CCT participants reported using the strategies they were taught, we did not collect data on homework completion, nor did we have an objective measure of strategy use in real-world settings. We did not correct for alpha inflation due to our small sample size, and it is possible that some of our results reflect Type I error. On the other hand, our pilot study was adequately powered (.80) to detect large (d=.8) effect sizes, and the consistency of the findings supports the conclusion regarding significant benefits of CCT in this population. Also, although CCT was delivered in clinics that offered psychosocial rehabilitation opportunities, not all clients participated in rehabilitation activities, and as a research-based group, CCT was not well-integrated with other treatment or rehabilitation options. Previous meta-analyses4,43 have shown that cognitive remediation is more effective when integrated within a broader psychiatric rehabilitation program, such as one that includes supported employment.44-45 It is possible that the effects of CCT could have been greater if this had been possible within the context of this study.
In summary, these preliminary results lead us to recommend further research on CCT and similar interventions for people with psychosis. Future measurement of motivation and insight regarding neurocognitive impairment could result in better clinical tailoring of cognitive remediation interventions to specific individuals.46-48 Just as some restorative cognitive remediation approaches have shown effects on brain structure, function, and biomarkers such as brain-derived neurotrophic factor49-51, it is possible that the behavior changes resulting from compensatory cognitive remediation interventions could result in observable brain changes, which should be measured in future investigations.
Supplementary Material
Clinical Points
Compensatory Cognitive Training is a brief, manualized, low-tech intervention aimed at improving cognitive impairment and everyday functioning in abilities in people with schizophrenia.
Our results showed that Compensatory Cognitive Training led to improvements in attention, memory, functional capacity, negative symptom severity, and patient-rated quality of life.
Acknowledgments
Funding for this study was provided by grants from the National Alliance for Research on Schizophrenia and Depression, Great Neck, NY, USA, and by the National Institute of Mental Health (R01MH080150, P30MH080002, and R34MH93453).
Appendix. Cognitive Problems and Strategies Assessment
Please read the subject each item and record the response by placing a check in the appropriate box.
Say, “First I'm going to ask you about problems some people have with their thinking and memory. Tell me how frequently each one is a problem for you, using this scale.” Show the subject the scale (detach the back page).
| Problems with Thinking and Memory | ||||
|---|---|---|---|---|
| Rarely/Never (0) | Sometimes (1) | Often (2) | Always (3) | |
| 1. I have difficulty remembering to do things that I have scheduled. | ||||
| 2. I forget to go to doctor's appointments. | ||||
| 3. I have difficulty remembering to take medications. | ||||
| 4. I forget to do housework or chores. | ||||
| 5. I have difficulty remembering to take a bath or shower. | ||||
| 6. I forget whether I've taken my medication. | ||||
| 7. I have trouble remembering events that are coming up in the next few weeks. | ||||
| 8. I forget people's names. | ||||
| 9. I have trouble remembering the names of my medications. | ||||
| 10. I forget my medication dosages. | ||||
| 11. I have difficulty memorizing things that I need to know. | ||||
| 12. I forget details from conversations. | ||||
| 13. I have problems with memory retrieval (I know the information is in my brain, but I just can't seem to get it out). | ||||
| 14. I have trouble learning new information. | ||||
| 15. I lose things like my keys, glasses, or wallet. | ||||
| 16. If I have a lot of things to do, I have trouble knowing which thing to do first. | ||||
| 17. My living space is a mess because I have trouble getting organized with my chores. | ||||
| 18. I run out of medication because I have not planned ahead to get my medication. | ||||
| 19. I have trouble staying focused during conversations. | ||||
| 20. I get distracted by other things when I am talking with someone. | ||||
| 21. I have trouble staying focused while I work on a task. | ||||
| 22. I get distracted by other things when I am working on a project. | ||||
| 23. When I have a conversation, I get off track instead of staying on the topic. | ||||
| 24. When I don't understand what someone is saying, I just pretend that I do understand. | ||||
| 25. I have trouble understanding what to do when someone gives me instructions. | ||||
| 26. I have trouble solving problems. | ||||
| 27. My thinking gets stuck in a rut. | ||||
| 28. When I need to solve a problem, I try one solution, and if it doesn't work, I give up. | ||||
| 29. There is only one way to solve a problem. | ||||
| 30. If I'm solving a problem and my solution is not working, I keep trying the same strategy until it works. | ||||
Say, “Now I'm going to ask you about strategies some people use to help with their thinking and memory. Tell me how frequently you use each one, using the same scale.”
| Memory and Thinking Strategies | ||||
|---|---|---|---|---|
| Rarely/Never (0) | Sometimes (1) | Often (2) | Always (3) | |
| 1. I use a calendar regularly to schedule and remember appointments and activities. | ||||
| 2. I check a calendar every day to see what I have scheduled that day. | ||||
| 3. Once a week or so, I look at my calendar and make a plan for the week. | ||||
| 4. I keep a written list of things I need to do. | ||||
| 5. I keep a written list of appointments I need to go to. | ||||
| 6. I remember to do certain things by pairing them up with other things that I do on a regular basis (e.g., remember to clean out the refrigerator every time I come home with groceries). | ||||
| 7. I remember where things are by putting them in the same place all the time. | ||||
| 8. If I need to remember something, I write it down somewhere. | ||||
| 9. I place reminders for myself where I am sure to see them. | ||||
| 10. I remember things by creating visual pictures in my mind. | ||||
| 11. I take notes on things I want to learn and remember. | ||||
| 12. If I want to remember something I've just heard, I repeat it to myself over and over. | ||||
| 13. I remember things by linking new information to information I already know. | ||||
| 14. I use acronyms to remember things. | ||||
| 15. I put things I have to remember into categories. | ||||
| 16. I use rhymes to remember things. | ||||
| 17. If I want to learn something, I study it over and over until I know it by heart. | ||||
| 18. I repeat back what I hear to make sure I've understood things people tell me. | ||||
| 19. I make eye contact with someone who is talking to help me understand what is being said. | ||||
| 20. To stay focused, I talk to myself while I'm working on a task. | ||||
| 21. If I don't understand something that someone says, I ask the person questions about it until I am sure I understand. | ||||
| 22. I usually stick to a daily schedule. | ||||
| 23. My living space is organized so there is a place for everything, and everything is in its place. | ||||
| 24. I use brainstorming to help me solve problems. | ||||
| 25. I use a problem-solving method to help me solve problems. | ||||
| 26. When I am solving a problem, I talk myself through it, step by step. | ||||
| 27. I test out my ideas to see if they are accurate. | ||||
| 28. I test out ideas by gathering “pro” and “con” evidence. | ||||
| 29. When I am working on something, I monitor myself to see how I'm doing. | ||||
| 30. When I'm having trouble solving a problem, I switch to a different strategy. | ||||
Footnotes
An early version of this work was presented at the Schizophrenia International Research Society annual meeting, April 10-14, 2010, Florence, Italy.
All authors report no competing interests.
Clinical trial registry name: Cognitive Training for Patients with Schizophrenia (NCT01521026; URL: http://clinicaltrials.gov/ct2/show/NCT01521026?term=twamley&rank=2).
References
- 1.Dixon LB, Dickerson F, Bellack AS, et al. The 2009 schizophrenia PORT psychosocial treatment recommendations and summary statements. Schizophr Bull. 2010;36:48–70. doi: 10.1093/schbul/sbp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the “right stuff”? Schizophr Bull. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- 3.Koren D, Seidman LJ, Goldsmith M, Harvey PD. Real-world cognitive – and metacognitive – dysfunction in schizophrenia: a new approach for measuring (and remediating) more “right stuff”. Schizophr Bull. 2006;32:310–326. doi: 10.1093/schbul/sbj035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168:472–485. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]
- 5.Kurtz MM, Seltzer JC, Shagan DS, Thime WR, Wexler BE. Computer-assisted cognitive remediation in schizophrenia: what is the active ingredient? Schizophr Res. 2007;89:251–260. doi: 10.1016/j.schres.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Velligan DI, Bow-Thomas CC, Huntzinger C, et al. Randomized controlled trial of the use of compensatory strategies to enhance adaptive functioning in outpatients with schizophrenia. Am J Psychiatry. 2000;157:1317–1323. doi: 10.1176/appi.ajp.157.8.1317. [DOI] [PubMed] [Google Scholar]
- 7.McGurk SR, Meltzer HY. The role of cognition in vocational functioning in schizophrenia. Schizophr Res. 2000;45:175–184. doi: 10.1016/s0920-9964(99)00198-x. [DOI] [PubMed] [Google Scholar]
- 8.Spaulding WD, Fleming SK, Reed D, et al. Cognitive functioning in schizophrenia: implications for psychiatric rehabilitation. Schizophr Bull. 1999;25:275–289. doi: 10.1093/oxfordjournals.schbul.a033378. [DOI] [PubMed] [Google Scholar]
- 9.Twamley EW, Woods SP, Zurhellen CH, et al. Neuropsychological substrates and everyday functioning implications of prospective memory impairment in schizophrenia. Schizophr Res. 2008;106:42–49. doi: 10.1016/j.schres.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butters MA, Soety EM, Glisky EL. Memory rehabilitation. In: Snyder PJ, Nussbaum PD, editors. Clinical Neuropsychology. American Psychological Association; Washington, DC: 1998. pp. 450–466. [Google Scholar]
- 11.Clare L, McKenna PJ, Mortimer AM, Baddeley AD. Memory in schizophrenia: What is impaired and what is preserved? Neuropsychologia. 1993;31:1225–1241. doi: 10.1016/0028-3932(93)90070-g. [DOI] [PubMed] [Google Scholar]
- 12.Kéri S, Juhász A, Rimanóczy A, et al. Habit learning and the genetics of the dopamine D3 receptor: evidence from patients with schizophrenia and healthy controls. Behav Neurosci. 2005;119:687–693. doi: 10.1037/0735-7044.119.3.687. [DOI] [PubMed] [Google Scholar]
- 13.Thakkar KN, Park S. Impaired passive maintenance and spared manipulation of internal representations in patients with schizophrenia [published online ahead of print December 31, 2010]. Schizophr Bull. doi: 10.1093/schbul/sbq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bayley PJ, Frascino JC, Squire LR. Robust habit learning in the absence of awareness and independent of the medial temporal lobe. Nature. 2005;436:550–553. doi: 10.1038/nature03857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellack AS, Mueser KT, Gingerich S, Agresta J. Social skills training for schizophrenia: a step-by-step guide. 2nd ed. The Guilford Press; New York, NY: 2004. [Google Scholar]
- 16.Meichenbaum D, Cameron R. Training schizophrenics to talk to themselves: A means of developing attentional controls. Behav Ther. 1973;4:515–534. [Google Scholar]
- 17.Delahunty A, Morice R. A training programme for the remediation of cognitive deficits in schizophrenia. NSW, Department of Health; Albury: 1993. [Google Scholar]
- 18.Wykes T, Reeder C, Corner J, Williams C, Everitt B. The effects of neurocognitive remediation on executive processing in patients with schizophrenia. Schizophr Bull. 1999;25:291–307. doi: 10.1093/oxfordjournals.schbul.a033379. [DOI] [PubMed] [Google Scholar]
- 19.Patterson TL, Goldman S, McKibbin CL, Hughs T, Jeste DV. UCSD Performance-Based Skills Assessment: Development of a new measure of everyday functioning for severely mentally ill adults. Schizophr Bull. 2001;27:235–245. doi: 10.1093/oxfordjournals.schbul.a006870. [DOI] [PubMed] [Google Scholar]
- 20.Green MF, Schooler NR, Kern RS, et al. Evaluation of functionally meaningful measures for clinical trials of cognition enhancement in schizophrenia. Am J Psychiatry. 2011;168:400–407. doi: 10.1176/appi.ajp.2010.10030414. [DOI] [PubMed] [Google Scholar]
- 21.Twamley EW, Savla GN, Zurhellen CH, Heaton RK, Jeste DV. Development and pilot-testing of a novel compensatory cognitive training intervention for people with psychosis. Am J Psychiatr Rehabil. 2008;11:144–163. doi: 10.1080/15487760801963678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- 23.Jeste DV, Wyatt RJ. Understanding and treating tardive dyskinesia. The Guilford Press; New York, NY: 1982. [Google Scholar]
- 24.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–666. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 25.Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J Clin Exp Neuropsychol. 1991;13:933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- 26.Raskin S. Memory for Intentions Screening Test [Abstract]. J Int Neuropsychol Soc. 2004;10(suppl 1):110. [Google Scholar]
- 27.Wechsler D. Wechsler Adult Intelligence Scale – Third Edition (WAIS-III) The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- 28.Brandt J, Benedict RHB. Hopkins Verbal Learning Test – Revised (HVLT-R) Professional Manual. Psychological Assessment Resources; Lutz, FL: 2001. [Google Scholar]
- 29.Kongs SK, Thompson LL, Iverson GL, Heaton RK. Wisconsin Card Sorting Test – 64 card version (WCST-64) Psychological Assessment Resources; Odessa, FL: 2000. [Google Scholar]
- 30.Benton AL, Hamsher KS. Multilingual aphasia examination. AJA Associated; Iowa City, IA: 1989. [Google Scholar]
- 31.McClure MM, Bowie CR, Patterson TL, et al. Correlations of functional capacity and neuropsychological performance in older patients with schizophrenia: Evidence for specificity of relationships? Schizophr Res. 2007;89:330–338. doi: 10.1016/j.schres.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 32.Twamley EW, Doshi RR, Nayak GV, et al. Generalized cognitive impairments, ability to perform everyday tasks, and level of independence in community living situations of older patients with psychosis. Am J Psychiatry. 2002;159:2013–2020. doi: 10.1176/appi.ajp.159.12.2013. [DOI] [PubMed] [Google Scholar]
- 33.Mausbach BT, Bowie CR, Harvey PD, et al. Usefulness of the UCSD Performance-Based Skills Assessment (UPSA) for predicting residential independence in patients with chronic schizophrenia. J Psychiatr Res. 2008;42:320–327. doi: 10.1016/j.jpsychires.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patterson TL, Moscona S, McKibbin CL, Davidson K, Jeste DV. Social skills performance assessment among older patients with schizophrenia. Schizophr Res. 2001;48:351–360. doi: 10.1016/s0920-9964(00)00109-2. [DOI] [PubMed] [Google Scholar]
- 35.Wallace CJ, Liberman RP, Tauber R, Wallace J. The Independent Living Skills Survey: A comprehensive measure of the community functioning of severely and persistently mentally ill individuals. Schizophr Bull. 2000;26:631–658. doi: 10.1093/oxfordjournals.schbul.a033483. [DOI] [PubMed] [Google Scholar]
- 36.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 37.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beck AT, Baruch E, Balter JM, Steer RA, Warman DM. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr Res. 2004;68:319–329. doi: 10.1016/S0920-9964(03)00189-0. [DOI] [PubMed] [Google Scholar]
- 39.Lehman AF. A quality of life interview for the chronically mentally ill. Eval Program Plann. 1988;11:51–62. [Google Scholar]
- 40.Bellucci DM, Glaberman K, Haslam N. Computer-assisted cognitive rehabilitation reduces negative symptoms in the severely mentally ill. Schizophr Res. 2003;59:225–232. doi: 10.1016/s0920-9964(01)00402-9. [DOI] [PubMed] [Google Scholar]
- 41.Eack SM, Greenwald DP, Hogarty SS, et al. Cognitive enhancement therapy for early-course schizophrenia: effects of a two-year randomized controlled trial. Psychiatr Serv. 2009;60:1468–1476. doi: 10.1176/appi.ps.60.11.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Twamley EW, Burton CZ, Vella L. Cognitive training for psychosis: Who benefits? Who stays in treatment? Schizophr Bull. 2011;37(suppl 2):S55–S62. doi: 10.1093/schbul/sbr059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007;164:1791–1802. doi: 10.1176/appi.ajp.2007.07060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGurk SR, Mueser KT, Feldman K, Wolfe R, Pascaris A. Cognitive training for supported employment: 2-3 year outcomes of a randomized controlled trial. Am J Psychiatry. 2007;164:437–441. doi: 10.1176/ajp.2007.164.3.437. [DOI] [PubMed] [Google Scholar]
- 45.Bell MD, Zito W, Greig T, Wexler BE. Neurocognitive enhancement therapy with vocational services: work outcomes at two-year follow-up. Schizophr Res. 2008;105:18–29. doi: 10.1016/j.schres.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 46.Choi J, Fiszdon JM, Medalia A. Expectancy-value theory in persistence of learning effects in schizophrenia: role of task value and perceived competency. Schizophr Bull. 2010;36:957–965. doi: 10.1093/schbul/sbq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Medalia A, Thysen J. Insight into neurocognitive dysfunction in schizophrenia. Schizophr Bull. 2008;34:1221–1230. doi: 10.1093/schbul/sbm144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burton CZ, Vella L, Twamley EW. Clinical and cognitive insight in a cognitive training intervention. Am J Psychiatr Rehabil. 2011;14:307–326. doi: 10.1080/15487768.2011.622159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wexler BE, Anderson M, Fulbright RK, Gore JC. Preliminary evidence of improved verbal working memory performance and normalization of task-related frontal lobe activation in schizophrenia following cognitive exercises. Am J Psychiatry. 2000;157:1694–1697. doi: 10.1176/appi.ajp.157.10.1694. [DOI] [PubMed] [Google Scholar]
- 50.Eack SM, Hogarty GE, Cho RY, et al. Neuroprotective effects of cognitive enhancement therapy against gray matter loss in early schizophrenia: results from a 2-year randomized controlled trial. Arch Gen Psychiatry. 2010;67:674–682. doi: 10.1001/archgenpsychiatry.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vinogradov S, Fisher M, Holland C, et al. Is serum brain-derived neurotrophic factor a biomarker for cognitive enhancement in schizophrenia? Biol Psychiatry. 2009;66:549–553. doi: 10.1016/j.biopsych.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.