Abstract
Objective
To investigate the effect of vocal fold injury location on vibratory amplitude and lateral phase difference.
Study design
Repeated measures with each excised canine larynx serving as own control.
Setting
Basic science study conducted in university laboratory.
Methods
Vocal fold vibration of excised canine larynges was recorded with a high speed camera before and after inducing vocal fold injury at one of five locations: anterior, middle, posterior, medial, or superior. Medial and superior injuries were created within the middle third of the vocal fold. Five larynges were used for each of the five injury locations. Kymography was performed at the midpoint of the vocal folds for each video. Pre- and post-injury vibratory amplitude and lateral phase difference were compared for each location.
Results
The anterior and medial injuries produced consistent decreases in vibratory amplitude. Middle and posterior injuries may slightly decrease amplitude. Superior injuries seemed to have no effect on amplitude. Anterior and medial injuries induce phase asymmetry between the right and left vocal folds. Middle injuries appeared to affect phase difference slightly, whereas posterior and superior injuries had no effect.
Conclusion
Injury to the anterior or medial portions of the vocal fold may be most likely to cause abnormal vocal fold vibration. Using caution in these locations during phonosurgery may favor superior post-operative vocal outcomes.
Keywords: vocal fold scarring, videokymography, mucosal wave, vibratory amplitude, phase difference
INTRODUCTION
The vocal fold cover includes the epithelium and superficial lamina propria (SLP), and oscillates relative to the deeper layers of the lamina propria. Disruption at this site can compromise vibratory function. In vocal fold scarring, the SLP is typically damaged, causing reduced vibratory amplitude, phase asymmetry, reduced mucosal wave propagation, and air escape, potentially leading to dysphonia.1-5 Vocal misuse, smoking, inflammation, laser treatment, radiotherapy, trauma, and phonosurgery can cause scarring, potentially decreasing patient quality of life. For example, it is especially hazardous to use CO2 laser treatments on the free edge of the vocal fold as it can cause collateral damage to the lamina propria.1
A limited number of studies have quantitatively investigated vibratory parameters in vocal fold scarring using animal models and reported reduced vibratory amplitude6-7 and glottal area.7 Using a finite element model, Berry et al. investigated the effects of scar location on phonation threshold pressure (PTP), frequency, and acoustic intensity.8 Scars within 2 mm of the superior-medial junction caused the greatest increase in PTP. Additionally, scars on the medial surface had a greater impact than those on the superior surface. Preserving the medial edge, therefore, may lead to good vocal outcomes. Although Berry et al. simulated the effects of scar location, a physical experiment investigating this has not been performed.
The severity of scar-induced dysphonia is related to scar location and magnitude.9 We investigated this relationship to discover if certain locations cause different degrees of vibratory dysfunction. Scarring was modeled at five locations (superior, medial, anterior, middle, and posterior) and effects on vibratory amplitude and lateral phase difference were evaluated. The right vocal fold was injured and the left served as a control. We hypothesized that injury to the medial, anterior, or middle vocal fold would decrease ipsilateral vibratory amplitude and result in greater phase asymmetry between the right and left vocal folds compared to controls. As current treatment of vocal fold scar is limited, prophylaxis is critical. Identifying which areas of the vocal fold are most susceptible to injury-induced vibratory changes may be valuable to phonosurgeons when determining the optimal approach to excise a vocal fold mass.
METHODS
Larynges
This study was exempt from review by the University of Wisconsin Institutional Animal Care and Use Committee.
Twenty-five canine larynges were harvested post-mortem from mongrel dogs, none of which were sacrificed for this study. No laryngeal disorders or head and neck injuries were present. Larynges were quick-frozen, stored in 0.9% saline at −12°C, and thawed at room temperature (20°C) before experimentation. Five larynges were used for each injury group (superior, medial, anterior, middle, and posterior; Figure 1). Laryngeal dissections were carried out according to the procedure of Jiang and Titze.10 The epiglottis, hyoid bone, and thyroid cartilage superior to the vocal folds were removed to facilitate visualization. Three-pronged micrometers inserted into the arytenoid cartilages permitted vocal fold adduction. Approximately 5 cm of the trachea was left intact and fixed to the apparatus.
Figure 1.
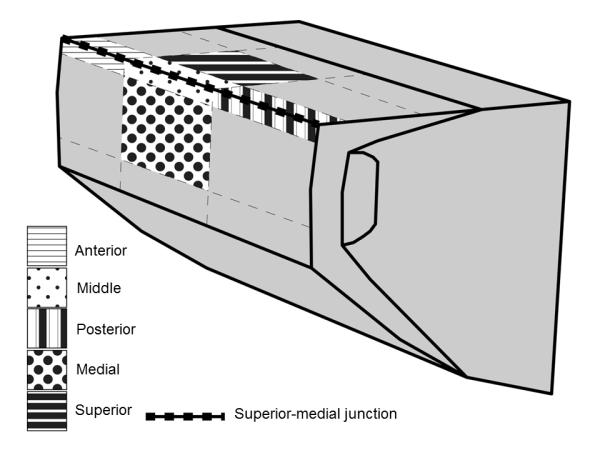
The locations of induced vocal fold injuries displayed in a 3-D diagram.
Experimental setup
Experiments were performed in a sound-attenuated room using the excised larynx bench apparatus (Figure 2). The larynx was mounted to a pipe linked to a pseudolung and the trachea was stabilized with a hose clamp. Two ConchaTherm III heater-humidifiers (Fisher and Paykel Healthcare, Inc., Laguna Hills, California) in series were used to maintain a stable subglottal airflow in terms of both temperature (35-38°C) and humidity (95-100% relative humidity). Throughout the experiment, 0.9% saline was applied to the vocal folds to maintain hydration and avoid alterations in physiological or biomechanical properties.
Figure 2.
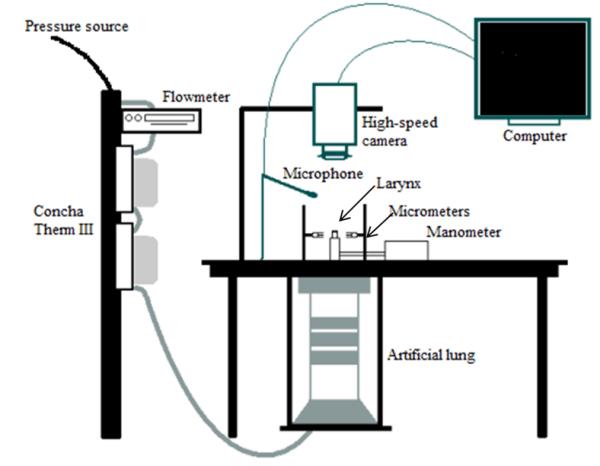
The excised larynx bench apparatus.
A high-speed digital camera (Fastcam-ultima APX, Photron USA, Inc., San Diego, California) recorded vocal fold vibration at a subglottal pressure of 20 cmH2O. The resolution was 256×512 pixels, with a recording rate of 4000 frames per second. We believe 20 cmH2O is an appropriate pressure to use in comparing injured and uninjured vocal folds because it is sufficiently high for both to phonate, but low enough to avoid chaotic vibrations, which could confound analysis.
Once stable phonation was achieved, two video recordings were collected with one second between recordings. Each recording was 0.192 seconds in duration, or 768 frames. Immediately after recording vibration of the uninjured vocal folds, the larynx was removed from the apparatus. Saline was injected into the right vocal fold with a 26 gauge needle to separate the epithelium and SLP from the intermediate and deep layers of the lamina propria. This ensured that only the mucosa and superficial lamina propria were affected; care was taken not to violate deeper structures. As injected saline is resorbed within seconds, the injection itself had no effect on vocal fold vibration during trials. An injury was created at the location of interest on the right vocal fold by removing the epithelium and SLP with microforceps and microscissors. The location of injury along the vocal fold was determined using a digital caliper. Vocal fold length was measured as the distance from the vocal process to the anterior commissure. This value was divided by three, which became the length assigned for the anterior, middle, and posterior segments. Each injury was created in its corresponding area covering one-third of the vocal fold length. In the anterior, posterior, and middle groups, approximately one-third of the vocal fold length at the respective location was injured at the superior-medial junction, as referenced in Berry et al.8 In the superior and medial groups, the middle one-third of the vocal fold was injured on the superior and medial surfaces, respectively. The total area affected by the injury covered a length of one-third the vocal fold with a width of 2-3 millimeters. Injury locations are displayed in Figure 1. After injury, the larynx was remounted on the apparatus. Two videos of the injured vocal fold were recorded following the aforementioned recording procedure. For every injury condition, the “uninjured-record-injured-record” sequence was used.
According to Berry et al.8, the superior-medial junction is most sensitive to presence of scar. Therefore, anterior, posterior, and middle injuries were made at this junction. Because superficial scars within the first few millimeters of depth are thought to have a more adverse impact on vocal fold vibration than deep scars,8 only superficial injuries were created.
Histology
Samples were processed to paraffin and sectioned. The injured side was embedded to provide a sagittal view of the injured vocal fold. The block was positioned so that a complete section of vocal fold, including the injury, was visible. Three slides (each with a thickness of 5 μm) were saved for hematoxylin and eosin staining. Sections were taken 150 μm apart. Images were created using a Nikon Eclipse E600 microscope (Nikon, Mellville, NY).
Data analysis
A custom MATLAB program (version 7.2.0.232 (R2006a), The MathWorks, Inc., Natick, Massachusetts) was used to perform digital kymography and curve fitting. A kymogram was created after the line scan was positioned perpendicular to the glottal axis at the midpoint along the length of the vocal fold. The glottal edges were extracted and curve-fitted for each kymogram. Left-upper (LU) and right-upper (RU) vocal fold lips were each fitted to a sine wave and either compared with each other for a certain group or compared individually between the control and injury groups.
As outlined in Krausert et al.,11 vibratory motion was modeled with a sine wave where Yα(t) is the position of vocal fold lip α at time t. α=1,2 correspond to the left-upper and right-upper vocal fold lips, respectively (Figure 3).
where n, the order number of mucosal wave vibration, ranges from 1 to 8. A0 is the initial offset amplitude, and is a measure of vertical displacement in a selected frame. This measurement is automatically calculated by the MATLAB program, but is not important for this study. A1 represents sine wave amplitude and provides information on the degree of vocal fold displacement between the open and closed phases of the glottal cycle. Though not reported in this study, f is sine wave frequency, or the frequency of mucosal wave vibration. t represents time. φ is phase number, and is determined by the horizontal position of the wave in the frame. If frequency is constant, subtracting the phase numbers of the waves results in phase difference (Figure 3). The phase difference between the right and left vocal folds is called lateral phase difference, and provides information on right-left vibrational phase symmetry. In this experiment, we evaluated the phase difference between the upper lips of the right and left vocal folds.
Figure 3.

Sample kymograms from this study. The kymogram on the left is from a control trial, while the kymogram on the right is from a medial injury trial. In both images, “A” represents a line connecting the sinusoidal wave peak of the left vocal fold to the sinusoidal wave trough of the right vocal fold. In the control sample, these local extrema align. In the injury sample, these extrema do not align, leading to asymmetrical vibration (“C” = degree of asymmetrical shift). In both images, “B” represents the lateral phase difference. In symmetrical vibration (left), this is equal to pi radians. In the injury sample (right), an asymmetrical shift (“C”) has led to decreased lateral phase difference.
As in Krausert et al.,11 to avoid the inclusion of negative values, we found phase difference by taking the absolute value of the difference between the individual wave numbers, which range from 0 to 2π radians. If this value was greater than π, we subtracted the value from 2π. This ensured that phase difference would always be between 0 and π radians. As the two sine waves are mirror images of each other (representing the right and left vocal folds which move away from each other during opening and towards each other during closure), the two curves (and thus, vocal fold vibrations) are symmetric when the phase difference is π radians, such that the trough of one wave (representing left vocal fold) touches the crest of the other (representing right vocal fold) (Figure 3, left). During asymmetric vibration, these points on the curve do not touch and there is asymmetry, with a value between 0 and π radians (Figure 3, right). Peak-to-peak amplitude, or vertical distance between the crest and trough, is two times larger than the measured amplitude of a sine wave. Therefore, this recorded value was doubled to relate to vibratory amplitude, which is also equal to the maximum lateral displacement observed from a medial position of the vocal fold lip (Figure 3).
To calibrate distance and allow for measurement in millimeters rather than pixels, two sutures were attached to the thyroid cartilage, and the distance between them was measured prior to mounting the larynx. The sutures are seen in the video and a line can be drawn between them. The previously measured distance in millimeters is then converted into pixels. Each pixel is assigned a distance in millimeters, and sine wave amplitude can be converted to millimeters.
Statistical analysis
Paired t-tests were performed to compare amplitude and phase difference between control and injury conditions and amplitude between right and left vocal folds to study right-left symmetry. If data did not meet assumptions of parametric testing, Wilcoxon signed rank tests were performed. A significance level of α=0.05 was used.
One-way analysis of variance (ANOVA) was performed to compare percent change in amplitude and phase difference between control and injury conditions across injury positions. Percent change was evaluated to control for inter-group variability. If data did not meet assumptions of parametric testing, ANOVA on ranks was performed. A significance level of α=0.05 was used with Holm-Sidak corrections for the significance levels of pairwise comparisons.
RESULTS
After injury, kymograms did not display evidence of chaos or higher order vibrations. Specifically, no subharmonics, period tripling or quadrupling, or chaos were observed. It was more difficult to sustain vocal fold oscillation following injury, but the consistent input subglottal pressure of 20 cmH2O was adequate across trials.
The anterior and medial groups showed significantly lower right-upper amplitude compared to pre-injury trials. No significant differences were noted between the left-upper amplitudes. The middle, posterior, and superior groups showed no significant differences in either right-upper or left-upper amplitudes compared to pre-injury controls. Summary data and statistical analyses are provided in Table 1.
Table 1.
Summary data and results of statistical analyses for amplitude in control and injured conditions.
| Injury location | p-value | ||
|---|---|---|---|
| Control | Anterior | ||
| RU | 1.817 (0.188) | 1.021 (0.132) | <0.001* |
| LU | 0.786 (0.093) | 0.776 (0.077) | 0.264 |
| p-value | <0.001* | <0.001* | |
|
| |||
| Control | Medial | ||
| RU | 1.036 (0.230) | 0.622 (0.170) | 0.033* |
| LU | 1.009 (0.158) | 1.085 (0.300) | 0.687 |
| p-value | 0.557 | 0.002* | |
|
| |||
| Control | Middle | ||
| RU | 1.006 (0.425) | 0.880 (0.405) | 0.202 |
| LU | 1.095 (0.284) | 0.956 (0.295) | 0.066 |
| p-value | 1.000 | 0.076 | |
|
| |||
| Control | Posterior | ||
| RU | 0.669 (0.071) | 0.665 (0.148) | 0.956 |
| LU | 0.728 (0.338) | 0.786 (0.190) | 0.552 |
| p-value | 0.846 | 0.036* | |
|
| |||
| Control | Superior | ||
| RU | 1.212 (0.531) | 1.141 (0.376) | 0.625 |
| LU | 1.406 (0.745) | 1.295 (0.370) | 0.601 |
| p-value | 0.052 | 0.191 | |
Values are presented as mean (standard deviation) with units of millimeters.
= significant p-value.
No control group showed significant differences between right-upper and left-upper amplitudes except the one associated with the anterior group, which had significantly lower left-upper amplitude. Groups showing significantly lower right-upper vocal fold lip amplitude than left-upper were medial and posterior. The superior and middle groups showed no significant differences between the right and left-upper vocal fold lip amplitudes, although the decrease in right-upper amplitude in the middle group approached significance (p=0.076). In the anterior group, the left-upper vocal fold lip showed significantly lower amplitude than right-upper.
Anterior and medial groups showed a significant decrease in lateral phase difference. Posterior and superior groups showed no significant differences compared to their respective control, while the middle injury group demonstrated a change which approached, but did not reach, significance (p=0.103). Summary data and statistical analyses are provided in Table 2.
Table 2.
Summary data and results of statistical analyses for lateral phase difference in control and injured conditions.
| Injury location | p-value | ||
|---|---|---|---|
| Control | Anterior | ||
| RU-LU | 2.254 (0.177) | 1.243 (0.243) | <0.001* |
|
| |||
| Control | Medial | ||
| RU-LU | 2.495 (0.084) | 2.155 (0.280) | 0.038* |
|
| |||
| Control | Middle | ||
| RU-LU | 3.014 (0.-6-) | 2.848 (0.128) | 0.103 |
|
| |||
| Control | Posterior | ||
| RU-LU | 2.799 (0.343) | 2.746 (0.328) | 0.805 |
|
| |||
| Control | Superior | ||
| RU-LU | 2.694 (0.245251 | 2.668 (0.177) | 0.688 |
Values are presented as mean (standard deviation) with units of radians.
= significant p-value.
The change in right vocal fold amplitude was significantly different across injury positions (p=0.006) (table 3). The only significant differences on pairwise comparisons were between the anterior and superior (p=0.003, α=0.005) and anterior and posterior (p=0.005, α=0.006). The change in left vocal fold amplitude was not significant across groups (p=0.555). The change in phase difference was significantly different (p<0.001), with the anterior group demonstrating a larger change compared to the other four positions.
Table 3.
Summary data and results of analysis of variance (ANOVA) evaluating percent change in parameters across injured conditions.
| Parameter | Anterior | Medial | Middle | Posterior | Superior | p-value |
|---|---|---|---|---|---|---|
| LU amplitude | −1.07 (1.93) | 11.50 (42.26) | −7.46 (5.15) | 15.50 (27.38) | 2.66 (29.54) | 0.555 |
| RU amplitude | −43.90 (2.00) | −38.20 (19.24) | −11.10 (19.54) | −0.33 (20.47) | 4.05 (34.69) | 0.006* |
| Phase difference | −44.8 (10.39) | −13.70 (10.01) | −6.12 (5.28) | −0.59 (18.21) | −0.69 (5.27) | <0.001* |
Values are presented as mean (standard deviation) with units of percent.
= significant p-value.
DISCUSSION
We hypothesized that injury to all areas except posterior and superior would decrease ipsilateral vibratory amplitude. Most of the data agree with this hypothesis; however, a significant difference was not found for middle injury (p=0.202). Amplitude decreased from 1.006±0.425 to 0.880±0.405, but relatively large intra-group variability and a modest sample size masked any potential difference on hypothesis testing. Injury to the middle vocal fold also caused an unexpected significant decrease in left-upper lip amplitude compared to pre-injury controls. Injury to the right vocal fold may impact the left as well, though to a lesser degree. Additionally, interruption of vibrational symmetry can affect contralateral vibratory amplitude. This phenomenon was observed in our previous study investigating vocal fold injury using multiple videokymographic line scan positions.11 Though not specifically evaluated, reductions in vibratory amplitude of the contralateral vocal fold were observed at all line scan positions. Additional investigations further evaluating the impact of scarring location on biomechanical parameters such as tension or stiffness may clarify these issues.
We expected right-upper vocal fold lip amplitude to be significantly lower than left-upper in all injury conditions except posterior and superior. The data were contrary to our hypothesis in the anterior and middle injury location groups. It is likely that the middle group failed to reach significance due to a modest sample size (p=0.076). The posterior group showed significantly lower right amplitude than left, suggesting the posterior vocal fold may be vulnerable to injury. However, the anterior and medial positions are still likely more vulnerable, as demonstrated by more consistent effects on amplitude and lateral phase difference representative of injury. Effects of injury to the anterior vocal fold were the most dramatic of any group, as shown by ANOVA. The anterior group exhibited significantly larger right-upper lip amplitude compared to the left; however, this finding must be interpreted with caution because its control group shows an even greater difference between the means of the left and right amplitudes. Also, because the amplitude of the right-upper vocal fold lip decreased significantly after anterior injury, it is likely that the injury still caused the expected effect. It is possible some of the larynges in this group were physiologically abnormal or the arytenoids were medialized asymmetrically on the bench apparatus. Though visual inspection is performed to exclude any larynges with gross pathology prior to experimentation and to ensure symmetric vocal fold approximation, it is possible that larynges with defects not visible to the eye were included or subtle asymmetry was present. Also of note, the control group associated with the superior group exhibited near significantly lower right amplitude than left (p=0.052). This could be caused by physiological abnormalities or asymmetric vocal fold approximation, as with the control for the anterior group.
We also hypothesized that lateral phase difference would be significantly lower in all injury groups compared to controls, except for superior and posterior injuries. The results largely coincide with this hypothesis, though the difference in the middle injury group did not reach significance (p=0.103).
This study has two limitations. First, a relatively small sample size was used. Though trends were observed, it is possible that meaningful differences were not observed due to low power. Second, the scar model is not a true representation of human vocal fold scar. Ex vivo canine vocal folds were injured, and no tissue remodeling or scarring responses were possible. While an in vivo tissue response is ideal for studying vocal fold scarring, it would be difficult to create repeatable injuries in specific locations with live canines. This scar model has value because, as in a true scar response, the layered structure of the epithelium and the superficial lamina propria was interrupted (figure 4), and these layers are important for normal vibration.12-14 In addition, canine vocal folds are considered a good model for both the layered structure of the lamina propria and the vibratory properties observed in humans.15 Importantly, while we cannot determine how vibratory parameters may change over time as scars mature, we can determine how violations of the lamina propria in different areas of the vocal fold affect vibratory function.
Figure 4.
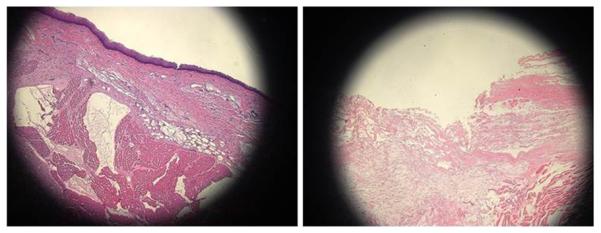
Sagittal section of vocal fold before (left) and after (right) injury involving the epithelium and superficial lamina propria. A 26 gauge needle was used to inject saline, elevating the superficial lamina propria. Magnification: 10X; stain: hematoxylin and eosin.
Data relating vocal fold injury location to vibratory properties may have value for clinicians, especially phonosurgeons excising vocal fold masses. Based on the results of this study, it appears that procedures on the anterior and medial surfaces carry the highest risk. This potential risk should be acknowledged and other options such as voice therapy should be considered if possible, especially in cases where the surgical site would be on the anterior or medial surface. If the lesion is on the anterior or medial surface and surgery is necessary, surgeons may consider using prophylactic treatments perioperatively such as those outlined by Bless et al.9 Although treatments such as corticosteroids, hyaluronic acid, and mesenchymal stem cells have not all been investigated in humans, they may reduce surgically-induced inflammation and scarring in the future. Additionally, future studies with larger sample sizes should investigate the effect of injury in the middle third of the vocal fold near the superior-medial junction. A statistically significant effect on vibration was not observed here, but a modest sample size may have prevented the difference from reaching statistical significance. Therefore, future studies may demonstrate that the middle third of the vocal fold, in addition to the anterior and medial surfaces, is a location at which the integrity of the layered structure is crucial.
Acknowledgments
The authors thank Satoshi Kinoshita for his valuable help with the histological components of this study. This research was supported by NIH grant numbers R01 DC008850 and R01 DC05522 from the National Institute on Deafness and Other Communication Disorders.
REFERENCES
- 1.Allen J. Cause of vocal fold scar. Curr Opin Otolaryngol Head Neck Surg. 2010;18:475–480. doi: 10.1097/MOO.0b013e32833fecd1. [DOI] [PubMed] [Google Scholar]
- 2.Neuenschwander MC, Sataloff RT, Abaza MM, Hawkshaw MJ, Reiter D, Spiegel JR. Management of vocal fold scar with autologous fat implantation: perceptual results. J Voice. 2001;15(2):295–304. doi: 10.1016/S0892-1997(01)00031-5. [DOI] [PubMed] [Google Scholar]
- 3.Colden D, Zeitels SM, Hillman RE, Jarboe J, Bunting G, Spanou K. Stroboscopic assessment of vocal fold keratosis and glottic cancer. Ann Otol Rhinol Laryngol. 2001;110(4):293–298. doi: 10.1177/000348940111000401. [DOI] [PubMed] [Google Scholar]
- 4.Franco RA, Dowdall JR, Bujold K, Amann C, Faquin W, Redmond RW, Kochevar IE. Photochemical repair of vocal fold microflap defects. Laryngoscope. 2011;121(6):1244–1251. doi: 10.1002/lary.21797. [DOI] [PubMed] [Google Scholar]
- 5.Svensson B, Nagubothu RS, Cedervall J, et al. Injection of human mesenchymal stem cells improves healing of scarred vocal folds: analysis using a xenograft model. Laryngoscope. 2010 Jul;120(7):1370–5. doi: 10.1002/lary.20926. [DOI] [PubMed] [Google Scholar]
- 6.Hirano S, Bless DM, Nagai H, Rousseau B, Welham NV, Montequin DW, Ford CN. Growth factor therapy for vocal fold scarring in a canine model. Ann Otol Rhinol Laryngol. 2004;113:777–785. doi: 10.1177/000348940411301002. [DOI] [PubMed] [Google Scholar]
- 7.Welham NV, Montequin DW, Tateya I, Tateya T, Choi SH, Bless DM. A rat excised larynx model of vocal fold scar. J Speech Lang Hear Res. 2009;52:1008–1020. doi: 10.1044/1092-4388(2009/08-0049). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berry DA, Reininger H, Alipour F, Bless DM, Ford CN. Influence of vocal fold scarring on phonation: predictions from a finite element model. Ann Otol Rhinol Laryngol. 2005;114(11):847–852. doi: 10.1177/000348940511401107. [DOI] [PubMed] [Google Scholar]
- 9.Bless DM, Welham NV. Characterization of vocal fold scar formation, prophylaxis, and treatment using animal models. Curr Opin Otolaryngol Head Neck Surg. 2010;18:481–486. doi: 10.1097/MOO.0b013e3283407d87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang JJ, Titze IR. A methodological study of hemilaryngeal phonation. Laryngoscope. 1993;103:872–882. doi: 10.1288/00005537-199308000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Krausert CR, Ying D, Zhang Y, Jiang JJ. Quantitative study of vibrational symmetry of injured vocal folds via digital kymography in excised canine larynges. J Speech Lang Hear Res. 2010;54(4):1022–1038. doi: 10.1044/1092-4388(2010/10-0105). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sato K, Hirano M. Age-related changes of elastic fibers in the superficial layer of the lamina propria of vocal folds. Ann Otol Rhinol Laryngol. 1997;106(1):44–48. doi: 10.1177/000348949710600109. [DOI] [PubMed] [Google Scholar]
- 13.Dailey SH, Ford CN. Surgical management of sulcus vocalis and vocal fold scarring. Otolaryngol Clin N Am. 2006;39:23–42. doi: 10.1016/j.otc.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Kim KH, Burns JA, Bernstein JJ, Maguluri GN, Park BH, de Boer JF. In vivo 3D human vocal fold imaging with polarization sensitive optical coherence tomography and a MEMS scanning catheter. Optics Express. 2010;18(14):14644–14653. doi: 10.1364/OE.18.014644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garrett CG, Coleman JR, Reinisch L. Comparative histology and vibration of the vocal folds: implications for experimental studies in microlaryngeal surgery. Laryngoscope. 2000;110:814–824. doi: 10.1097/00005537-200005000-00011. [DOI] [PubMed] [Google Scholar]


