Abstract
Medication non-adherence is a key clinical concern in bipolar disorder across the lifespan. Cognitive deficits in older adults with bipolar disorder may hinder medication management ability, which, in turn, may lead to non-adherence. Using an innovative performance-based measure of medication management ability, the Medication Management Ability Assessment (MMAA), we compared performance of 29 middle-aged older community dwelling outpatients with bipolar disorder who were clinically stable (mean age=61, sd=11 years; rage 45 to 86) to 59 normal control subjects (NCs) and 219 outpatients with schizophrenia. The MMAA is a role-play task that simulates a medication regimen likely to be encountered by older adults. Within the bipolar group, we examined the relationships of MMAA scores to demographic, psychiatric symptoms severity, and the Dementia Rating Scale scores. The bipolar group made 2.8 times the errors on the MMAA than NCs (Bipolar group =6.2 (sd=5.5) vs. NCs=2.2 (sd=2.5), and did not significantly differ from the schizophrenia group in errors on the MMAA. Errors in the bipolar group were more likely to be in taking too few medications as in taking too many. Within the bipolar group, a significant correlation was seen between MMAA scores and DRS Total Score, but not with age, education, BPRS, HAM-D, number of psychiatric medications, or medical conditions. Among DRS subscales, the Memory Subscale correlated most strongly with MMAA errors. This small cross-sectional study suggests that deficits in medication management ability may be present in later-life bipolar disorder. Neurocognitive deficits may be important in understanding problems with unintentional non-adherence.
Keywords: Bipolar disorder, aging, older adults, medication management ability, medication adherence, memory
Introduction
Empirical research on medication adherence in bipolar disorder has focused on intentional non-compliance, such as lack of insight into the need for medications, desire to avoid medication side-effects, and other beliefs or attitudes about the psychotropic medications(1). However, cognitive changes and other factors associated with aging may place older patients with bipolar disorder at risk for unintentional non-adherence.
A key concept in regard to unintentional non-adherence is medication management ability, which is the capacity to carry out a prescribed medication regimen. Effective self-management of medications requires a series of cognitive processes, involving language/comprehension, prospective memory, verbal episodic memory, and attention/working memory and other executive functions (2).
Neuropsychological deficits among older patients with bipolar disorder may be worse than those seen in young adult patients (3–5). Although it is unclear whether any such age-related decline reflects the additive effect of normal age-related cognitive changes, it is suggested that accumulation of affective episodes may result in greater neurocognitive impairment (6). Older patients are also prone to higher numbers of co-morbid medical conditions, and the parallel polypharmacy; thus, are more likely to have complex medication management regimens while having fewer cognitive resources (7–9).
Despite clinical relevance, there is a dearth of empirical information on the frequency, magnitude, and causes of impairment in medication management ability among older patients with bipolar disorder. However, several structured performance-based measures of medication management ability have been developed and successfully applied in research on patients with HIV-infection (10) or schizophrenia (11). They involve a simulated medication management task, i.e., the patient is presented with a set of pill bottles, and is asked to orchestrate a hypothetical regimen by following the labels (11). The Medication Management Ability Assessment (MMAA), developed at our Research Center, is the only measure designed for use with middle-age and older patients with serious mental illness (11, 12). It has been validated in older outpatients with schizophrenia (11, 13), showing high correspondence with self-report and other measures of actual medication adherence (11).
In the present study, we examined performance on the MMAA among 29 middle aged and older adults with bipolar disorder and compared them to 54 age-matched neuropsychiatrically healthy comparison subjects (NCs) and 219 outpatients with schizophrenia. Within the bipolar group, we assessed the correlates of MMAA performance to demographic characteristics, medical comorbidity, number psychotropic medications, psychiatric symptoms, and cognitive functioning, as measured with the Dementia Rating Scale. We also assessed the relative strength of these independent variables on MMAA scores, as well as within the specific subscales of the DRS. We hypothesized that, on average, medication management capacity would be impaired among bipolar patients compared to NCs, but better than the schizophrenia group. However, given the degree of heterogeneity in level of cognitive and functional impairment in bipolar disorder (5), we also anticipated a wide-range of medication management ability would be present. Further, in light of the apparent cognitive-dependency of medication management (2), we hypothesized that cognitive functioning would be the strongest predictor of MMAA performance. Finally, we predicted that three domains of cognitive functioning (DRS Attention, Initiation/Perseveration, and Memory) would relate to MMAA scores
Materials and Methods
Participants
This report is based on analyses of data from several studies on later life psychotic disorders housed within the National Institute of Mental Health (NIMH) - funded Advanced Center for Interventions and Services Research at the University of California, San Diego (UCSD) between April 1998 and August 2001. The participants with bipolar disorder or schizophrenia were recruited through UCSD’s outpatient psychiatric clinics, the VA hospital, and board and care facilities in San Diego. NCs were volunteers recruited through local advertisements. All the participants provided written informed consent prior to participating. The UCSD Human Research Protections Program reviewed and approved the original protocols. Some of the data from the participants have been included in prior reports (5, 14), however data on the MMAA in patients with bipolar disorder have not been published.
We included all participants with DSM-IV diagnoses of bipolar disorder I or II who had been administered the MMAA (n=29). We included all participants who were NCs (n=54) or with schizophrenia (n=219) who had been administered the MMAA whose ages were within the range of that of the bipolar group. All participants were screened with a medical history questionnaire and laboratory and physical examinations to exclude the following: 1) history of major neurological disorders or head trauma, 2) or DSM-IV diagnosis of dementia, 3) current inpatient treatment for medical or psychiatric disorder, and 4) diagnosis of current substance abuse or dependence. Diagnoses were made with administration of the Structured Clinical Interview for the DSM-IV by trained psychology or psychiatry fellows, and confirmed during a subsequent consensus meeting.
All but one bipolar participant (diagnosed with Bipolar II) were diagnosed with Bipolar I. Current medication data were available on all but 1 bipolar participants. A total of 35% were taking an anti-convulsant (carbemazapine, valproic acid, other anticonvulsant) and 29% were taking lithium. In addition, 72% were taking antipsychotic medications, of which 75% were atypical antipsychotics and 25% typical antipsychotics. Medication data was missing for 8 participants with schizophrenia. A total of 98% were taking an antipsychotic, of which 79% were atypical and 21% were typical antipsychotics. The higher than expected proportion of typical antipsychotic medications is because some of the data were collected prior to the widespread use of atypical antipsychotics
Measures
Demographic and Clinical Information
Data were collected by clinical interview with a trained research assistant on age, gender, ethnicity, living situation, education, number of current psychotropic medications (up to 4) and self-reported ICD-10 medical illnesses (up to 6).
Medication Management Ability Assessment (MMAA)
The MMAA involves a role-play in which the assessor describes each of four medications with their respective dosages and instructions. Approximately 30 minutes later participants are asked to describe how they would take their regimen of medications throughout the day (e.g., at breakfast I would take…). They are able to refer to the bottle labels at all times, and with each dose the participant physically hands over the “pills” to the examiner. Errors are recorded as the number over or under the number possible correct (out of 21 “doses”). Details for the reliability and validity of the MMAA have been published previously (11).
Cognitive Assessment
Severity of cognitive deficits was evaluated with the Mattis Dementia Rating Scale (DRS) (15). Scores range from 0 to 144, with lower scores indicating greater impairment. Subscales include attention, initiation/perseveration, construction, conceptualization, and memory. Prior research has shown the DRS is sensitive to cognitive impairments associated with bipolar disorder (3).
Clinical Evaluation
Severity of psychopathologic symptoms were evaluated with the Hamilton Depression Rating Scale (HAM-D) (16), and the Positive and Negative Syndrome Scale (17). To assess manic symptoms, from the PANSS, we extracted a four item “Excitement” subscale (grandiosity, impulsiveness, hostility, and excitability), which has been previously shown to correspond to established measure of manic symptoms (18). The research assistants who administered and scored these scales were kept unaware of the participant’s diagnostic status (to the extent possible) and DRS performance during administration and scoring.
Statistical Analysis
We compared the three groups on demographic, clinical, and MMAA variables via univariate ANOVAs for continuous variables and chi-square tests for categorical variables, and we conducted pairwise comparisons with Bonferroni corrections, controlling for age and gender. Within the bipolar group we examined Pearson correlations with the Number of Pill Errors on MMAA (range 0 to 21) and the continuous variables. Finally, to compare obtained correlations between the different DRS subscales and MMAA scores, we calculated t-values for differences in correlations in a dependant sample, based on the formula provided by Cohen et al. (19). For correlational analyses, the p-value set to 0.01 to mitigate Type 1 error.
Results
Comparison of the demographic characteristics, psychopathology rating scale scores, and cognitive functioning scores among the three groups is provided in Table 1. Among the three groups, the NCs were the oldest (66.0 years (sd=11.0)), BD participants were intermediate (60.7 years (10.9)), and the SC group the youngest (sd=52.8 years (6.1) F(2,296)=101.3, p=0.010. The patient groups had a lower proportion of women. All subsequent analyses controlled for age and gender. The NCs and bipolar groups had a higher mean level of educational attainment than the schizophrenia group, and both patient groups were more likely to reside in a supported housing. The bipolar group was intermediate between the schizophrenia and NCs in terms of psychotic symptomatology (PANSS). Global cognitive functioning (DRS Total) among bipolar patients was intermediate between that of schizophrenia patients and NCs(5). The patient groups did not differ in depressive symptom severity (HAM-D) or manic symptoms (PANSS ‘Excitement’ subscale, both with higher scores than the NCs. The mean level of psychiatric symptomatology was in the mild range on both the BPRS and HAM-D.
Table 1.
Demographic, Psychiatric, and Neurocognitive Characteristics of the NC and Bipolar Groups
| Normal Comparison Subjects (NCs) | Bipolar Disorder Group (BD) | Schizophrenia Group (SC) | F(df) or X2 (df) | p-value | Post-Hoc1 | |
|---|---|---|---|---|---|---|
| N=54 | N=29 | N= 219 | ||||
| Mean (SD) | Mean (SD) | Mean (SD) | ||||
| Age (years) | 66.0 (11.0) | 60.7 (10.9) | 52.8 (6.1) | F(2,296)=101.3 | 0.010 | SC<BD<NC |
| Education (years) | 13.4 (2.3) | 13.9 (3.2) | 12.0 (2.6) | F(2,291)=6.1 | 0.091 | -------- |
| Gender (% Female) | 64.8% | 37.9% | 33.3% | X2 (2)=18.0 | <0.001 | -------- |
| Ethnicity (% Caucasian) | 68.5% | 89.7% | 58.9% | X2 (2)=11.2 | 0.004 | -------- |
| Living Situation (% fully independent) | 100.0% | 44.8% | 25.8% | X2(2)=98.6 | <0.001 | -------- |
| Number of Medical Diagnoses | 1.8 (1.8) | 1.1 (1.1) | 0.08 (0.43) | F(2,295)=19.5 | 0.027 | SC<BD=NC |
| Age of Onset | -------- | 27.0 (12.3) | 24.8 (10.4) | F(2,203)=61.5 | <0.001 | -------- |
| Number of Psychotropic Medications | -------- | 2.2 (1.1) | 2.1 (1.2) | F(2,243)=0.397 | 0.611 | -------- |
| Dementia Rating Scale (DRS) Total | 139.7 (3.6) | 131.9 (9.7) | 125.9 (12.7) | F(2,233)=33.4 | 0.006 | SC<BD<NC |
| HAM-D Total Score | 2.8 (2.7) | 7.3 (5.5) | 9.3 (6.4) | F(2,266)=59.8 | <0.001 | NC<BD=SC |
| PANSS Overall | 34.9 (4.4) | 48.9 (11.8) | 57.6 (15.4) | F(2,295)=53.5 | 0.001 | NC<BD<SC |
| PANSS Excitement Subscale | 4.8 (1.1) | 6.8 (2.9) | 6.2 (2.4) | F(2,295)=13.7 | 0.003 | NC<SC=BD |
Bonferroni correction applied to pairwise comparisons; Group comparisons (except for age) control for age and gender; HAM-D: Hamilton Depression Rating Scale; PANSS: Positive and Negative Syndrome Scale; MMAA: Medication Management Ability Assessment
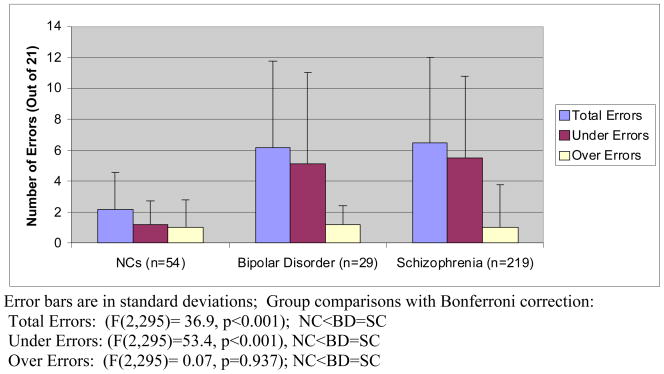
The mean number of errors in the bipolar group was 2.8 times higher than that in the NCs group, and not statistically different from that in the schizophrenia group (Figure 1) [(F(2,295)=36.9, p<0.001); pairwise NC<BD=SC]. A total of 83 % of the bipolar group made at least two errors and 52% made at least five errors out of 21 correct responses. In contrast, 50% of the NCs made at least two errors, and only 17% made 5 or more. A total of 78% of the schizophrenia made at least two errors, and 53% made more than 5. The bipolar group was significantly more likely than the NCs to make errors in taking too few medications (F(2,295)=53.4, p<0.001; NC<BD=SC), but groups did not differ in the number of errors in taking too many medications [(F(2,295)=0.066, p=0.937).
Figure 1.
Performance on the Medication Management Ability Assessment (MMAA) in NCs, Bipolar Disorder, and Schizophrenia Groups
Within the bipolar group, the only significant correlation with MMAA scores was the DRS Total Score (r=0.554, p=0.003) (Table 2). Older age was positively, but non-significantly, related to MMAA errors (r=0.329, p=0.082). For categorical variables, we found no differences in MMAA errors by gender (F(1,27)=1.69, p=0.204), ethnicity (F(1,27)=2.09, p=0.159), or living situation (F(1,27)=2.09, p=0.159). None of the other continuous variables (age of onset, duration of illness, HAM-D, PANSS Overall, PANSS ‘Excitement’, number of psychotropic medications) correlated significantly with MMAA errors. Among DRS subscales, the only significant correlate at the p<0.01 level of MMAA errors was the Memory subscale (r−0.521, p=0.006), with correlations for the other subscales as follows: Attention (r=−0.297, p=0.141), Initiation/Perseveration (r= −0.346, p=0.84), Construction (r=−0.411, p=0.037), and Conceptualization (r=−0.427, p=0.029). The correlation between Memory and MMAA scores was not significantly different from the other correlations, with the largest obtained t-value for the Attention subscale (t=1.75<critical value=2.06).
Discussion
Medication management ability is a vital aspect of staying adherent to medication regimens. As hypothesized, we found significantly worse performance on a performance-based measure of medication management ability in bipolar disorder relative to NCs. Participants with bipolar disorder made three times as many errors as the NCs group, and errors on the MMAA were seen in taking too few pills rather than too many. Despite relatively better cognitive and symptom profiles than patients with schizophrenia, performance on the MMAA between the two groups did not differ. Among patients with bipolar disorder, the most potent predictor of MMAA scores was, as hypothesized, cognitive impairment. However, there were no other significant associations. The Memory subscale of the DRS significantly predicted MMAA scores, but Initiation/Perseveration and Attention did not. Future study will be needed to understand how specific cognitive abilities in bipolar disorder map on to medication management. Our data suggests that interventions to enhance medication adherence in later-life bipolar disorder may benefit from including compensatory strategies (e.g., medication reminders and calendars) to counteract memory deficits.
There are a number of study limitations. The small sample size may have lacked power to detect certain associations (e.g., age). The sample was also comprised of stably treated bipolar I outpatients with mild levels of psychiatric symptoms. Research with more severely ill patients would be needed to gauge the effect of symptoms of medication management ability, and these results may not apply to other sub-groups of patients with bipolar disorder, including bipolar disorder II or bipolar disorder NOS. We lacked a measure of manic symptoms in this sample, such as the Young Mania Rating Scale(20), and cannot rule out that manic symptoms impaired performance. However, none of the bipolar participants were inpatients and their PANSS excitement scores were not elevated beyond that found in the schizophrenia group as would be expected among people in manic states. We lacked a measure of medication adherence in this sample, and therefore we cannot be certain that MMAA performance correlates with real-world adherence to psychotropic or non-psychotropic medications among people with bipolar disorder. However, the MMAA has been previously associated with actual antipsychotic adherence as measured by pharmacy record in a sample with schizophrenia(11).
Despite these limitations, our findings suggest several potentially important aspects of medication management in later-life bipolar disorder. There were a large number of errors by patients with bipolar disorder. Despite their relatively better cognitive and psychiatric symptom severity profiles in this study compared to patients with schizophrenia, patients with bipolar disorder showed a similar pattern of performance on the MMAA. The most common type of error among bipolar patients (along with patients with schizophrenia) was in taking too few medications. This study suggests that, despite somewhat better clinical state among older patients with bipolar disorder, impairments in medication management ability is similar between later life bipolar disorder and schizophrenia. This corresponds with previous study by Bartels and colleagues, indicating a similar degree of impairment in community living skills in patients with either bipolar disorder or schizophrenia, despite some differences in symptoms (21).
Our findings have potential implications for understanding unintentional non-adherence among older patients with bipolar disorder. In mixed-age patients with bipolar disorder, the rate of non-adherence is around 40 to 50%(22), but there may be better self-reported and pharmacy record assessed adherence in older age groups (23). It may be that rates of intentional non-adherence decrease with older age alongside greater insight and acceptance of one’s diagnosis (24). However, our findings suggest that older adults may be at risk for unintentional non-adherence due to diminished capacity to manage medications. Pharmacy record measures of adherence may not detect these kinds of unintentional medication errors, and patients may not be aware of difficulties in medication management on self-report measures. Future research should examine correspondence between the MMAA and self-report, pharmacy record, and pill count measures of actual adherence among older adults.
As with Jeste et al (13) in their sample of patients with schizophrenia, we found strong association between the Dementia Rating Scale and MMAA scores. There is mounting evidence that neuropsychological performance is impaired in bipolar disorder(6), longer duration of illness may relate to worsening deficits (6), and substantial proportion of older adults with bipolar disorder show signs of cognitive impairment (3). Contrary to our hypotheses, the pattern of relationships between neurocognitive deficits and MMAA scores appeared general rather than specific. Although the memory subscale had the highest correlation with the MMAA, this relationship was not significantly stronger than that found between any of the other DRS subscale scores.
In addition to our limited sample size, one reason to be cautious in overinterpreting the lack of evidence of differential cognitive effects on medication management is the nature of the DRS itself. The DRS is relatively simple and brief, and can be administered to patients with a wide range of functioning. However, the DRS may be suboptimal as a means of identifying the effects of different cognitive abilities on medication management. For instance, prospective memory, or the ability to “remember to remember”(25), is a cognitive domain that entails planning and metacognition, which are apart from the initiation/perseveration subscale of the DRS, which taps into disinhibition-related aspects of executive function. Future research with more sensitive neuropsychological instruments should investigate which domains of cognitive functioning map on to the tasks involved in medication management.
It was somewhat surprising that neither HAM-D nor BPRS scores correlated with MMAA performance. Our sample had a relatively mild level of symptoms, but not all were euthymic. It may be that medication management ability is more ‘trait-like’ in bipolar disorder. There were no other significant relationships with the MMAA, although older age approached significance. This may stem from the marked heterogeneity in later life bipolar disorder, as the mean and standard deviation in the number of errors on the MMAA were roughly equivalent.
The primary clinical implication is that, in addition to adherence, medication management ability may be an important area to assess in later life bipolar patients. Given the high degree of medical comorbidity among older patients with bipolar disorder(9) along with the combination strategies used in treating bipolar illness, most people have a large number medications. Clinicians may need to ask about how patients organize their medications, and what sorts of strategies they use to stay adherent. The MMAA is a relatively brief (15 minute) measure of medication management ability that could be useful in a clinical setting. Psychosocial interventions developed to enhance medication adherence in younger adults with bipolar disorder are largely targeted toward enhancing motivation to take medications(26). Adaptation of psychosocial intervention in later life bipolar disorder should incorporate training in compensatory strategies to make medication taking more automatic – such as pill boxes, forming implementations intentions, and routinizing medication taking through pairing with daily events. We have previously developed and evaluated such an intervention in a pilot study, described elsewhere(27). Lastly, research on the optimal treatment and clinical course of later life bipolar disorder is needed, particularly how functional disability can be addressed in this often vulnerable group of patients.
Footnotes
Disclosures of Conflict of Interest: None of the authors have any disclosures of interest to report
References
- 1.Scott J. Using Health Belief Models to understand the efficacy-effectiveness gap for mood stabilizer treatments. Neuropsychobiology. 2002;46 (Suppl 1):13–5. doi: 10.1159/000068022. [DOI] [PubMed] [Google Scholar]
- 2.Brown SC, Park DC. Theoretical models of cognitive aging and implications for translational research in medicine. Gerontologist. 2003;43(Spec No 1):57–67. doi: 10.1093/geront/43.suppl_1.57. [DOI] [PubMed] [Google Scholar]
- 3.Gildengers AG, Butters MA, Seligman K, et al. Cognitive functioning in late-life bipolar disorder. Am J Psychiatry. 2004;161(4):736–8. doi: 10.1176/appi.ajp.161.4.736. [DOI] [PubMed] [Google Scholar]
- 4.Young RC, Murphy CF, Heo M, et al. Cognitive impairment in bipolar disorder in old age: literature review and findings in manic patients. J Affect Disord. 2006;92(1):125–31. doi: 10.1016/j.jad.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 5.Depp C, Moore DJ, Sitzer DI, et al. Neurocognitive impairment among middle-aged and eldelry adults with bipolar disorder: Comparison to schizophrenia and normal comparison subjects. Journal of Affective Disorders. doi: 10.1016/j.jad.2006.11.022. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson LJ, Ferrier IN. Evolution of cognitive impairment in bipolar disorder: a systematic review of cross-sectional evidence. Bipolar Disord. 2006;8(2):103–16. doi: 10.1111/j.1399-5618.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- 7.Gildengers A, Butters M, Seligman K, et al. Cognitive functioning in late-life bipolar disorder. American Journal of Psychiatry. 2004;161(4):736–738. doi: 10.1176/appi.ajp.161.4.736. [DOI] [PubMed] [Google Scholar]
- 8.Depp C, Jeste DV. Bipolar disorder in older adults: A critical review. Bipolar Disord. 2004;6(5):343–367. doi: 10.1111/j.1399-5618.2004.00139.x. [DOI] [PubMed] [Google Scholar]
- 9.Kilbourne AM. The burden of general medical conditions in patients with bipolar disorder. Curr Psychiatry Rep. 2005;7(6):471–7. doi: 10.1007/s11920-005-0069-5. [DOI] [PubMed] [Google Scholar]
- 10.Albert SM, Flater SR, Clouse R, et al. Medication management skill in HIV: I. Evidence for adaptation of medication management strategies in people with cognitive impairment. II. Evidence for a pervasive lay model of medication efficacy. AIDS Behav. 2003;7(3):329–38. doi: 10.1023/a:1025404105378. [DOI] [PubMed] [Google Scholar]
- 11.Patterson TL, Lacro J, McKibbin CL, et al. Medication management ability assessment: results from a performance-based measure in older outpatients with schizophrenia. J Clin Psychopharmacol. 2002;22(1):11–9. doi: 10.1097/00004714-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Moore DJ, Palmer BW, Patterson TL, et al. A review of performance-based measures of functional living skills. J Psychiatr Res. 2007;41(1–2):97–118. doi: 10.1016/j.jpsychires.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Jeste SD, Patterson TL, Palmer BW, et al. Cognitive predictors of medication adherence among middle-aged and older outpatients with schizophrenia. Schizophr Res. 2003;63(1–2):49–58. doi: 10.1016/s0920-9964(02)00314-6. [DOI] [PubMed] [Google Scholar]
- 14.Heaton RK, Gladsjo JA, Palmer BW, et al. Stability and course of neuropsychological deficits in schizophrenia. Arch Gen Psychiatry. 2001;58(1):24–32. doi: 10.1001/archpsyc.58.1.24. [DOI] [PubMed] [Google Scholar]
- 15.Mattis S. Dementia Rating Scale (DRS) Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- 16.Hamilton M. Development of a rating scale for primary depressive illness. British Journal of Psychiatry. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 17.Kay S, Opler L, Lindenmayer J. Reliability and validity of the Positive and Negative Syndrome Scale for schizophrenia. Schizophrenia Bulletin. 1988;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 18.Lindenmayer J, Brown E, Baker R, et al. An excitement subscale of the Positive and Negative Syndrome Scale. Schizophrnenia Research. 2004;68(2–3):331–337. doi: 10.1016/S0920-9964(03)00087-2. [DOI] [PubMed] [Google Scholar]
- 19.Cohen J, Cohen P, West SG, et al. Applied multiple regression/correlation analysis for the behavioral sciences. 6. London: Erlbaum; 2003. [Google Scholar]
- 20.Young RC, Biggs J, Ziegler V, et al. A rating scale for mania: Reliability, validity, and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 21.Bartels S, Mueser K, Miles K. A comparative study of elderly patients with schizophrenia and bipolar disorder in nursing homes and the community. Schizophrenia Research. 1997;27:181–190. doi: 10.1016/S0920-9964(97)00080-7. [DOI] [PubMed] [Google Scholar]
- 22.Lingam R, Scott J. Treatment non-adherence in affective disorders. Acta Psychiatr Scand. 2002;105(3):164–72. doi: 10.1034/j.1600-0447.2002.1r084.x. [DOI] [PubMed] [Google Scholar]
- 23.Sajatovic M, Blow FC, Kales HC, et al. Age comparison of treatment adherence with antipsychotic medications among individuals with bipolar disorder. Int J Geriatr Psychiatry. 2007 doi: 10.1002/gps.1777. [DOI] [PubMed] [Google Scholar]
- 24.Greenhouse WJ, Meyer B, Johnson SL. Coping and medication adherence in bipolar disorder. J Affect Disord. 2000;59(3):237–41. doi: 10.1016/s0165-0327(99)00152-4. [DOI] [PubMed] [Google Scholar]
- 25.Insel K, Morrow D, Brewer B, et al. Executive Function, Working Memory, and Medication Adherence Among Older Adults. J Gerontol B Psychol Sci Soc Sci. 2006;61(2):102–107. doi: 10.1093/geronb/61.2.p102. [DOI] [PubMed] [Google Scholar]
- 26.Sajatovic M, Davies M, Hrouda DR. Enhancement of treatment adherence among patients with bipolar disorder. Psychiatr Serv. 2004;55(3):264–9. doi: 10.1176/appi.ps.55.3.264. [DOI] [PubMed] [Google Scholar]
- 27.Depp C, Patterson TL, Lebowitz B, et al. Medication adherence skills training in middle aged and elderly adults with bipolar disorder: Development and pilot study. Bipolar Disord. doi: 10.1111/j.1399-5618.2007.00397.x. In Press. [DOI] [PubMed] [Google Scholar]