Abstract
The aromatic prenyltransferase dimethylallyltryptophan synthase in Claviceps purpurea catalyzes the normal prenylation of tryptophan at C4 of the indole nucleus in the first committed step of ergot alkaloid biosynthesis. 4-Methyltryptophan is a competitive inhibitor of the enzyme that has been used in kinetic studies. Upon investigation of background activity during incubations of 4-methyltryptophan with dimethylallyl diphosphate, we found that the analogue was an alternate substrate, which gave four products. The structures of three of these compounds were established by 1H NMR and 2D NMR studies and revealed that dimethylallyltryptophan synthase catalyzed both normal and reverse prenylation at C3 of the indole ring and normal prenylation of N1. Similarly, 4-methoxytryptophan was an alternate substrate, giving normal prenylation at C5 as the major product. 4-Aminotryptophan, another alternate substrate, gave normal prenylation at C5 and C7. The ability of dimethylallyltryptophan synthase to prenylate at five different sites on the indole nucleus, with normal and reverse prenylation at one of the sites, is consistent with a dissociative electrophilic alkylation of the indole ring where orientation of the substrates within the active site and substituent electronic effects determine the position and type of prenylation. These results suggest a common mechanism for prenylation of tryptophan by all of the members of the structurally related dimethylallyltryptophan synthase family.
Introduction
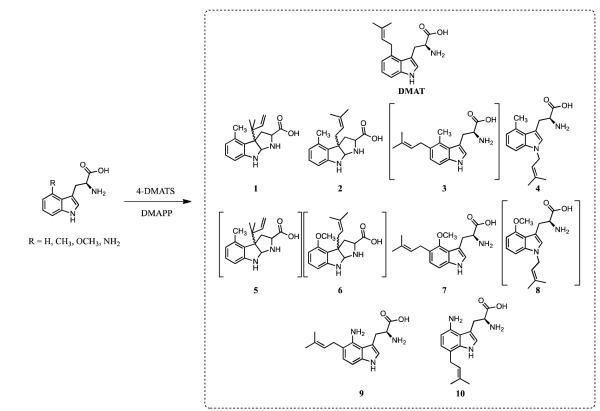
Prenylated indole alkaloids constitute a large family of chemically diverse biologically active natural products.1-3 Biosynthetically, the carbon skeletons of these compounds are constructed from prenyl diphosphates and tryptophan or derivatives of tryptophan. Well-known examples include the ergot alkaloids, the brevianamides, epiamauromine, and verruculogen (Figure 1). Collectively a family of enzymes, the dimethylallyl tryptophan synthases (DMATS) catalyze alkylation of the indole ring in tryptophan or tryptophan-containing dipeptides at positions N1, C2, C3, C4, C5, C6, or C7 by dimethylallyl diphosphate (DMAPP) to give the different naturally occurring carbon skeletons.4-11 The dimethylallyl moiety can be attached at C1’ (“normal” prenylation) or C3’ (“reverse” prenylation), further increasing structural diversity. For wild type DMATSs both normal and reverse prenylation is seen at N14,12 and at C2.5,13 Reverse prenylation at C3, in either the α- or β-configuration, is catalyzed by the DMATSs AnaPT6 and CdpC3PT,7 respectively. Very recently, FtmPT1 (a normal C2 prenyltransferase) was shown to catalyze normal prenylation at C3 of tryptophan-containing cyclic dipeptides.14 Only normal prenylation is seen at C4,8,15 C5,9 and C6.10 7-DMATS catalyzes normal prenylation at C7 of l-tryptophan11 and MpnD catalyzes reverse prenylation at C7 of an indolactam.16
Figure 1.
Indole ring numbering, possible prenylation orientations, and representative structures of prenylated indole alkaloids.
4-DMATS catalyzes the normal prenylation of tryptophan at C4 by DMAPP as the first committed step in ergot alkaloid biosynthesis.17 The enzyme was the first indole prenyltransferase to be purified, characterized, and later available from expression clones and has been studied from Claviceps purpurea8,18,19 and Aspergillus fumigatus.15,20-22 4-DMATS (FgaPT2) from A. fumigatus consists of a 10-stranded antiparallel β-barrel surrounded by α-helices.22 This “ABBA PT” fold has been reported for five other prenyltransferases - FtmPT123 and CdpNPT,24 normal C2 and reverse C3 indole prenyltransferases, respectively; NphB (formerly Orf2),25 CloQ,26 and EpzP,27 non-indole aromatic prenyltransferases. BLAST alignments with 4-DMATS from C. purpurea and other DMATSs report the following sequence identities: 56% with FgaPT2 from A. fumigatus, 35% with FtmPT1 from A. fumigatus, 35% with CdpC3PT from Neosartorya fischeri, 31% with CdpNPT from A. fumigatus, 47% with 5-DMATS from A. clavatus, 23% with IptA from Streptomyces sp. SN-593, and 28% with 7-DMATS from A. fumigatus.28 A recent phylogenetic analysis illustrates the relationships between aromatic and indole prenyltransferases.10
The mechanism proposed for prenylation by DMATSs is a dissociative electrophilic alkylation of the indole ring by DMAPP where cleavage of the carbon-oxygen bond in DMAPP gives a dimethylallyl cation-PPi ion pair, with subsequent alkylation of the indole moiety, followed by loss of a proton to give the prenylated product.19,29 In the case of 4-DMATS, regiospecific alkylation at C4 of the indole ring by C1’ of the allylic cation generates an arenium intermediate, which rearomatizes by deprotonation at C4 to produce dimethylallyl tryptophan (DMAT). The crystal structure of FgaPT2 from A. fumigatus in complex with tryptophan and dimethylallyl S-thiolodiphosphate, a non-hydrolyzable DMAPP analogue,30 revealed two active site amino acids thought to be important for catalysis.22 A hydrogen bond between Glu89 and the indole N-H likely increases the electron density in the indole ring. Lys174 is situated near C4 and is a likely candidate for removing the C4 proton during rearomatization. Recently, Luk et. al reported that the K174A mutant of FgaPT2 gave a C3 reverse-prenylated hexahydropyrroloindole as the major product in addition to a small amount of 4-DMAT.31 The authors suggest the initial alkylation is a reverse prenylation at C3 of the indole ring, followed by a Cope rearrangement, a mechanism originally proposed by Wenkert and Silwa,32 to give the normal C4 prenylated arenium intermediate followed by deprotonation facilitated by Lys174 to produce 4-DMAT. Although there is no direct experimental evidence for the Cope rearrangement in a 3-substituted indole, Schwarzer et. al reported a related homo-Cope rearrangement at room temperature driven by relief of cyclopropyl ring strain.33
4-Methyltryptophan has been used as a competitive dead-end analogue of tryptophan in kinetic studies of 4-DMATS.34 The substitution of the hydrogen atom at the C4 by a methyl group should block alkylation at that position. During inhibition studies with 4-DMATS from C. purpurea, we utilized 4-methyl-d,l-tryptophan as a competitive substrate inhibitor and found a consistently high background of activity. We subsequently discovered that 4-methyltryptophan is an alternate substrate for 4-DMATS and we now report studies with the analogue that provide insights about the mechanism of the reaction.
Experimental
4-Methyl-l-tryptophan
In a nitrogen atmosphere, 4-methylindole (160 mg, 1.22 mmol) and l-serine (250 mg, 2.38 mmol) were incubated at 80 °C with 7.5 mg of recombinant tryptophan synthase from the hyperthermophilic bacterium Thermotoga maritime35 in 25 mL of Buffer TS (100 mM K2HPO4, pH 7.5. containing 180 mM KCl, 120 μM PLP and 2% DMSO for 89 h. After incubation, the reaction mixture was cooled to rt and the enzyme was removed by filtration (Millipore Centricon, 10,000 molecular weight cutoff (MWCO)). The filtrate was concentrated in vacuo, the resulting residue was purified by silica gel flash chromatography using a gradient of 0 – 20 % H2O in ACN to give 175 mg (66%) of an off-white solid; Rf 0.65 (ACN/H2O 4:1); 1H NMR (300 MHz, D2O/NaOD) δ 2.19 (s, 3H), 2.44 (dd, J = 8.1, 14.4 Hz, 1H), 2.83 (dd, J = 5.4, 14.4 Hz, 1H), 2.96 (dd, J = 5.7, 7.8 Hz, 1H), 6.38 (d, J = 7.5 Hz, 1H), 6.61 (t, J = 7.8 Hz, 1H), 6.69 (s, 1H), 6.86 (d, J = 8.1 Hz, 1H); UV λmax 219.1, 270.0, 278.4 (shoulder), 289.1 nm (shoulder); MS-ESI m/z 219.2 [M + H]+; HRMS-ESI TOF m/z [M + H]+ calcd for C12H15N2O2 219.1134, found 219.1136.
4-Methoxy-l-tryptophan
4-Methoxyindole (160 mg, 1.09 mmol) was incubated with 8 mg of tryptophan synthase for 24 h as described above. Column chromatography yielded 120 mg (47%) of a light yellow solid; Rf 0.44 (ACN:H2O 4:1); 1H NMR (500 MHz, D2O) δ 3.23 (dd, J = 8.5, 14.5 Hz 1H), 3.67 (dd, J = 4.5, 14.5 Hz, 1H), 4.01 (s, 3H), 4.14 (dd, J = 4.5, 8.5 Hz, 1H), 6.70 (d, J = 7.0 Hz, 1H), 7.17 (d, J = 8.0 Hz, 1H), 7.18 (s, 1H), 7.22 (t, J = 7.5 Hz); 13C NMR (125 MHz, D2O) δ 28.0, 55.5, 56.5, 100.1, 105.7, 107.7, 116.6, 123.3, 124.4, 138.3, 153.8, 174.8; UV λmax 217.9, 265.3, 279.6, 289.1 nm; MS-ESI m/z 257.1 [M + Na]+, 273.1 [M + K]+; HRMS-ESI TOF m/z [M + H]+ calcd for C12H14N2O3Na 257.0902, found 257.0903.
4-Amino-l-tryptophan
4-Aminoindole (160 mg, 1.21 mmol) was incubated with 2 mg tryptophan synthase for 16 h as described above. Column chromatography yielded 220 mg (83%) as a gray solid; Rf 0.34 (ACN:H2O 4:1); 1H NMR (500 MHz, D2O) δ 3.38 (dd, J = 8.0, 16.0 Hz, 1H), 3.62 (dd, J = 5.0, 16.0 Hz, 1H), 4.02 (dd, J = 5.0, 8.0 Hz, 1H), 6.57 (t, J = 4.5 Hz, 1H), 7.08-7.10 (m, 2H), 7.20 (s, 1H); UV λmax 221.5, 267.7 (shoulder), 272.4, 283.1 nm (shoulder); MS-ESI m/z 220.1 [M + Na]+, 242.2 [M + Na]+, 258.1 [M + K]+; HRMS-ESI TOF m/z [M + H]+ calcd for C11H14N3O2 220.1086, found 220.1078.
Production and purification of 4-DMATS
A production strain for soluble 4-DMATS was constructed by cotransforming E. coli BL21 Star™ (DE3) with pHDMAT and pGroESL36 according to the manufacturer’s instructions. E. coli BL21 Star™ (DE3)/pHDMAT/pGroESL was incubated in 1 L of LB medium containing 35 μg/mL kanamycin and 34 μg/mL chloramphenicol at 37 °C with shaking at 225 rpm until an OD600 of 0.6 was reached. The culture was then cooled to 20 °C and protein expression was induced with IPTG to a final concentration of 0.4 mM. After 18 h, cells were harvested by centrifugation (4,000 × g, 20 min, 4 °C). Pelleted cells were resuspended in lysis buffer (50 mM NaH2PO4, pH 8.0, containing 300 mM NaCl, 10 mM imidazole, 1 mg/mL of lysozyme and 10 μg/mL of RNase A). After incubation at 0 °C for 30 min, the cell suspension was sonicated for 6 × 10 sec at 4 °C. The lysate was clarified by centrifugation (10,000 × g, 20 min, 4 °C). 4-DMATS was purified by nickel affinity chromatography using a gradient elution 0 – 100% of lysis buffer/elution buffer (50 mM NaH2PO4, pH 8.0, containing 300 mM NaCl and 500 mM imidazole). The purified protein was dialyzed against 2 × 4 L of dialysis buffer (20 mM Tris-HCl, pH 8.0, containing 1 mM βME, and then against 4 L of dialysis buffer containing 20% glycerol. The protein was stored at −80 °C until use.
Kinetic Studies
All kinetic assays were performed in 50 mM Tris-HCl buffer, pH 8.0, containing 4 mM MgCl2 and 50 μM 14C-DMAPP (10 μCi/μmol), in a total volume of 100 μL. Each reaction was incubated at 30 °C and quenched by heating at 100 °C for 30 s. After centrifugation, 10 μL of reaction mixture were spotted and developed by RP-C8 TLC using a solvent system of 4:6 25 mM NH4HCO3/methanol. The developed TLC plates were then imaged on a storage phosphor screen (Molecular Dynamics), scanned by a Typhoon 8600 Variable Mode Imager (GE Healthcare), and the data were visualized and processed with ImageQuant 5.2. Background radioactivity was determined from incubations without the tryptophan substrate. Each kinetic assay was performed in duplicate or triplicate. For l-tryptophan, the assay contained 50 nM 4-DMATS and l-tryptophan concentrations of 3, 10, 20, 30 and 60 μM, with a 5 min incubation. For the tryptophan analogues, the assay contained 500 nM 4-DMATS with 20 min incubations. For 4-methyltryptophan, final concentrations were 30, 100, 300 and 600 μM; for 4-methoxytryptophan, 30, 100, 300, 600, 1000 and 3000 μM; and for 4-aminotryptophan, 3, 10, 20, 30, 60, and 100 μM.
Product studies
Incubations were in 50 mM Tris-HCl buffer, pH 8.0, containing 10 mM MgCl2, 8.8 mM DMAPP, 20 mM tryptophan analogue and 15 μM 4-DMATS in a total volume of 5 mL. Glycerol present in the 4-DMATS dialysis buffer was removed by repeated dialysis/centrifugation using an Amicon Ultra 10 kDa MWCO filter. The reaction mixture was incubated at 30 °C for 18-42 h. 4-DMATS was removed by MWCO filtration, samples were concentrated by lyophilization, and the products were purified by HPLC on a Waters 2690 Separation Module using a Microsorb MV™ C18 5 μm column at a flow rate of 1 mL/min with isocratic elution using ACN:H2O (25/75). Products were detected with a Waters 996 photodiode array detector with simultaneous monitoring at 206, 225 and 254 nm. Product ratios were calculated by integrating the area under each product peak visualized at 214 nm (4-methyl/4-methoxy) or 225 nm (4-amino).
Structure of 4-substituted-dimethylallyltryptophan products
After purification, each product was lyophilized, dissolved in D2O (D, 99.9%), lyophilized again, dissolved in D2O (D, 99.96%) (Cambridge Isotope Laboratories, Inc.; Andover, MA) and placed in a Norell 3 mm NMR tube (Sigma). NMR experiments were recorded on an INOVA 600 NMR spectrometer equipped with a HCN cryogenic probe. Unless otherwise noted, the following NMR experiments were performed: 1D 1H, COSY 1H-1H, TOCSY 1H-1H, ROESY 1H-1H, HSQC or HMQC 1H-13C, and HMBC 1H-13C. All spectra were processed with MestReNova 7.1.
Results
Kinetic Studies
Michaelis-Menten kinetic parameters for tryptophan and its 4-substituted analogues are listed in Table 1. Rates (kcat) and Michaelis constants (KM) were determined from a non-linear regression fit of initial velocities versus concentration by GraFit 5.0.11. KM = 19.5 μM for tryptophan, which compared favorably with the previously reported value.19 KM = 16.7 μM for 4-aminotryptophan was comparable to the normal substrate, while those for the 4-methyl- and 4-methoxy analogues, 88.5 and 250.6 μM, respectively, were substantially higher. Values of kcat for the tryptophan analogues ranged between 0.42 and 1.28 s−1, substantially smaller than kcat = 59.7 s−1 for tryptophan. The catalytic efficiencies (kcat/KM) for the analogues were less than 1% of the normal reaction.
Table 1.
Kinetic parameters for 4-DMATS with tryptophan and 4-substituted analogues
| KM (μM) | kcat (s−1) | kcat / KM (s−1 M−1) |
Relative Activitya (%) |
|
|---|---|---|---|---|
| TRP | 20 ± 5 | 60 ± 6 | 3.1 × 106 | 100 |
| 4-CH3-TRP | 89 ± 16 | 0.75 ± 0.04 | 8.5 × 103 | 0.28 |
| 4-OCH3-TRP | 250 ± 43 | 1.3 ± 0.1 | 5.1 × 103 | 0.17 |
| 4-NH2-TRP | 17 ± 3 | 0.42 ± 0.02 | 2.5 × 104 | 0.81 |
Relative Activity is defined as the percent kcat / KM compared to TRP
Product Studies
In preliminary work with C. purpurea 4-DMATS (dmaW), we used racemic 4-methyltryptophan as a substrate. However, in a study of the A. fumigatus enzyme (FgaPT2) d-tryptophan was also a substrate, although at a rate of only 1.8% that of the l-enantiomer.15 Thus, we decided to use enantiomerically pure 4-methyl-l-tryptophan in our study. The substrate was synthesized from 4-methylindole and l-serine upon incubation with the PLP-dependent tryptophan synthase β subunit.35 The same procedure was used to convert 4-methoxyindole and 4-aminoindole to 4-methoxy-l-tryptophan and 4-amino-l-tryptophan, respectively. All of the NMR data matched previously reported data.37-39
4-Methyltryptophan
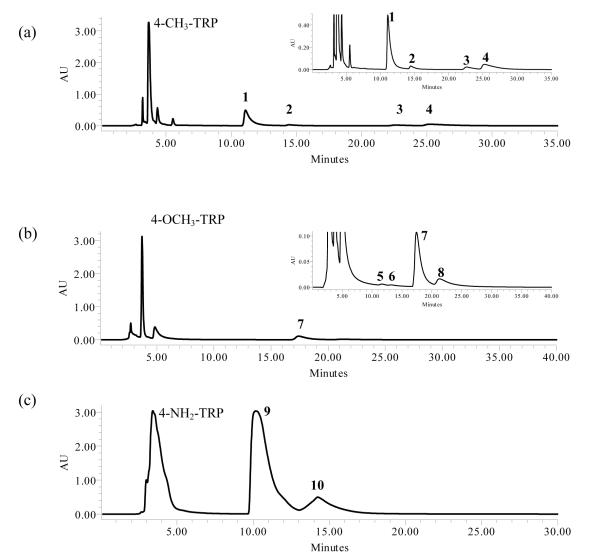
Incubation of 4-methyl-l-tryptophan and DMAPP with 4-DMATS gave four products (see Figure 2a). The UV spectra of compounds 1 (11.4 min, 44%) and 2 (15.1 min, 5%) had peaks at 209.7, 242.8, 293.8 nm and 206.2, 239.2, 289.1, 293.8 nm, respectively, suggesting that the indole chromophore (λmax ≈ 278 nm) had been disrupted to give an indoline-like structure.40 Compounds 3 (24.6 min, 7% and 4 (26.9 min, 44%) had peaks at 223.8, 272.4 nm and 225.0, 276.0, 290.3 nm, respectively, suggesting that they contained intact indole chromophores. Chromatography of the reaction mixture from 4-methyl-l-tryptophan on a C18 RP-HPLC column with an acidic solvent system (1% TFA) resulted in complete loss of 1, while the ratios for compounds 2, 3 and 4 did not change (data not shown). HRMS analysis of the four products gave molecular ions that were 68 Da higher than the corresponding peaks for 4-methyl-l-tryptophan or its sodium adduct, indicating the addition of one isoprene unit.
Figure 2.
HPLC chromatograms of preparative enzymatic assays for isolation and purification. (a) Incubation of 4-CH3-Trp and DMAPP with DMATS, (b) incubation of 4-OCH3-Trp and DMAPP with DMATS, (c) incubation of 4-NH2-Trp and DMAPP
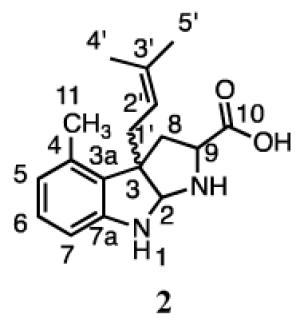
The structures of the products were determined from their 1H and 13C NMR spectra (Table 2). The dimethylallyl moiety in compound 1 was attached in a reverse orientation as evidenced by the distinctive signals for the three vinyl protons at 5.85 ppm (dd, J = 10.8, 17.4 Hz), 4.95 ppm (dd, J = 0.6, 10.2 Hz), and 4.94 ppm (dd, J = 0.6, 17.4 Hz), and two methyl groups at 0.93 and 0.85 ppm. The HMBC spectrum showed correlations between the methyl peaks of the prenyl moiety and C3’ (44.7 ppm) of the isoprene unit and C3 (68.1 ppm) in the indoline moiety. Aromatic protons at C5 (6.61 ppm), C6 (7.00 ppm), and C7 (6.48 ppm) support prenylation at C3 of the indole ring. In addition, the peak for the proton at C2 (5.06 ppm) is near reported values for C2 diamine protons in tricyclic C3-prenylated tryptophan derivatives.24,31 Collectively, these data confirm that 1 is formed by a reverse prenylation at the C3 position of 4-methyltryptophan, followed by cyclization to give a hexahydropyrroloindole (HHPI) structure.
Table 2.
1H and 13C NMR data of isolated enzymatic products including structures
| Structure/ Compound |
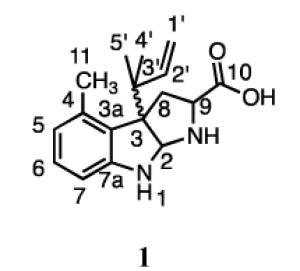
1 |
2 |
4 |
|||
| Position | δH, multi., J | δ C | δH, multi., J | δ C | δH, multi., J | δ C |
| 1 | exc. | - | exc. | - | - | - |
| 2 | 5.06, s | 83.3 | 4.84, s | 84.2 | 7.07, s | 130.6 |
| 3 | - | 68.1 | - | 61.1 | - | 112.0 |
| 3a | - | 138.9 | - | 137.7 | - | 128.2 |
| 4 | - | 130.9 | - | 133.7 | - | 133.8 |
| 5 | 6.61, d, 7.8 | 125.8 | 6.57, d, 7.8 | 124.5 | 6.80, d, 7.2 | 123.3 |
| 6 | 7.00, t, 7.8 | 131.5 | 6.94, t, 7.8 | 130.8 | 7.03, dd, 7.2, 8.4 | 124.4 |
| 7 | 6.48, d, 7.8 | 110.8 | 6.45, d, 7.8 | 110.5 | 7.19, d, 8.4 | 110.7 |
| 7a | - | 152.7 | - | 152.2 | - | 138.9 |
| 8 | 2.72, dd, 7.8, 13.8 | 38.0 | 2.51, dd, 6.6, 12.6 | 44.2 | 3.42, dd, 4.8, 15.0 | 32.4 |
| 2.34, dd, 8.4, 13.8 | - | 2.00, dd, 8.4, 12.6 | - | 2.98, dd, 9.0, 15.0 | - | |
| 9 | 3.49, t, 7.8 | 63.5 | 3.40, m | 63.4 | 3.61, dd, 4.8, 9.0 | 59.6 |
| 10 | - | 179.5 | - | n.f. | - | n.f. |
| 11 | 2.29, s | 23.1 | 2.22, s | 20.0 | 2.58, s | 22.2 |
| 1′ | 4.95, dd, 0.6, 10.2 | 116.2 | 2.55, dd, 6.6, 15.0 | 37.4 | 4.60, d, 6.6 | 46.3 |
| 4.94, dd, 0.6, 17.4 | - | 2.34, dd, 8.4, 15.0 | - | - | - | |
| 2′ | 5.85, dd, 10.8, 17.4 | 147.6 | 4.77, t, 7.2 | 121.7 | 5.24, t, 6.6 | 121.9 |
| 3′ | - | 44.7 | - | 138.6 | - | 140.4 |
| 4′ | 0.93, s | 26.1 | 1.47, s | 19.8 | 1.70, s | 19.8 |
| 5′ | 0.85, s | 25.7 | 1.46, s | 27.4 | 1.60, s | 27.2 |
| Structure/ Compound |
7 |
9 |
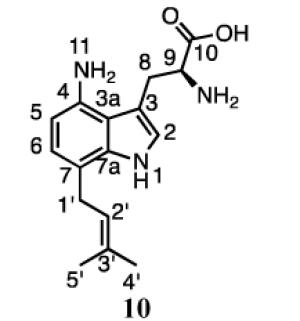
10 |
|||
| Position | δH, multi., J | δ C | δH, multi., J | δ C | δH, multi., J | δ C |
| 1 | exc. | - | exc. | - | exc. | - |
| 2 | 7.10, s | 127.8 | 7.02, s | 127.3 | 7.05, s | 126.6 |
| 3 | - | n.f. | - | 109.9 | - | 111.3 |
| 3a | - | n.f. | - | 120.0 | - | 119.9 |
| 4 | - | n.f. | - | 138.4 | - | 139.9 |
| 5 | - | n.f. | - | n.f. | 6.36, d, 7.2 | 110.1 |
| 6 | 6.98, d, 8.4 | 126.8 | 6.87, d, 8.4 | 127.0 | 6.75, d, 7.2 | 124.1 |
| 7 | 7.15, d, 8.4 | 111.7 | 6.89, d, 8.4 | 107.0 | - | 120.3 |
| 7a | - | n.f. | - | 139.4 | - | 138.9 |
| 8 | 3.41-3.44, m | n.f. | 3.42-3.47, m | 31.0 | 3.34-3.40, m | 31.4 |
| 3.06, dd, 9.0, 15.0 | - | 3.17, dd, 8.4, 15.6 | - | 3.15, dd, 7.2, 15.0 | - | |
| 9 | 3.87, dd, 4.8, 9.0 | n.f. | 3.77-3.82, m | 58.7 | 3.68-3.72, m | 58.9 |
| 10 | - | n.f. | - | n.f. | - | n.f. |
| 11 | 3.79, s | 65.1 | exc. | - | exc. | - |
| 1′ | 3.35, d, 7.2 | 29.7 | 3.24, d, 7.2 | 31.9 | 3.35, d, 7.8 | 31.2 |
| 2′ | 5.25, t, 7.2 | 126.2 | 5.19, t, 7.2 | 125.0 | 5.31, t, 7.8 | 124.2 |
| 3′ | - | 136.3 | - | 136.8 | - | 137.3 |
| 4′ | 1.67, s | 19.8 | 1.66, s | 19.6 | 1.62, s | 19.4 |
| 5′ | 1.60, s | 27.5 | 1.61, s | 27.4 | 1.61, s | 27.3 |
δH and δC in ppm
J in Hz
n.f. = not found
exc. = exchanged
Compound 2 also contains an HHPI moiety formed by normal prenylation at C3 of 4-methyltryptophan, as confirmed by the presence of proton signals for methylene protons at C1’ (2.34 (dd, J = 8.4, 15.0 Hz) and 2.55 ppm (dd, J = 6.6, 15.0 Hz)), the proton at C2’ (4.77 ppm, app t, J ≈ 7.2 Hz), and the methyl groups at 1.47 and 1.46 ppm. Cross peaks were also seen between a proton at C1’ (2.34 ppm) and C3 (61.1 ppm) and between the proton at C2 (4.84 ppm) and C1’ (37.4 ppm) in the HMBC spectrum. In addition, signals were seen for the aromatic protons at C5 (6.57 ppm), C6 (6.94 ppm), and C7 (6.45 ppm). Thus, 4-DMATS catalyzes the normal and reverse prenylation at the C3 of 4-methyltryptophan.
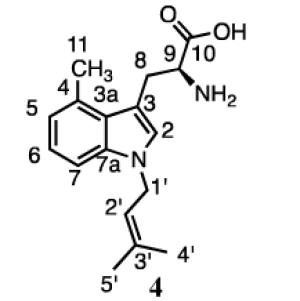
The 1H spectrum of 4 had peaks for the protons at C1’ (4.60 ppm, d, J = 6.6 Hz) and C2’ (5.24 ppm, t, J = 6.6 Hz) and the methyl groups at C3’ (1.70 and 1.60 ppm) for normal prenylation. Signals for aromatic protons at C5 (6.80 ppm), C6 (7.03 ppm), and C7 (7.19 ppm) indicated that prenylation had occurred in the pyrrole ring. The chemical shifts of C1’ (46.3 ppm) and the directly attached protons (4.60 ppm), along with cross peaks between the C1’ protons and C2 (130.6 ppm) in the HMBC spectrum indicate that normal prenylation occurred at the N1 position on the indole ring. The UV spectrum and the resonance at 7.07 ppm for the proton at C2 indicated that the indole ring was intact. A negative ion fast atom bombardment mass spectroscopy spectrum of fully exchanged product D in deuterated glycerol and D2O verified indole ring nitrogen substitution (Figure S43). Unfortunately, we were unable to obtain sufficient quantities of 3 to establish its structure, although as mentioned earlier, the indole ring appeared to be intact. Co-injection experiments (Figure S44) with 3 and two products we had previously isolated and characterized, normal 7-dimethylallyl-4-methyl-l-tryptophan and reverse N-dimethylallyl-4-methyl-l-tryptophan (unpublished results) excluded these structures as candidates for 3.
4-Methoxytryptophan
As described for 4-methyltryptophan, incubation of 4-methoxy-l-tryptophan with 4-DMATS gave products 5 (11.8 min, 5%), 6 (13.3 min, 7%), 7 (17.5 min, 71%), and 8 (21.4 min, 17%) (see Figure 2b). HRMS analysis indicated that the molecular ions of the compounds were 68 Da higher than 4-methoxytryptophan and its sodium adduct, as expected for monoprenylation. UV spectra for 5 and 6 suggested that the indole moiety had been disrupted, while those for 7 and 8 indicated that the indole nucleus was intact.
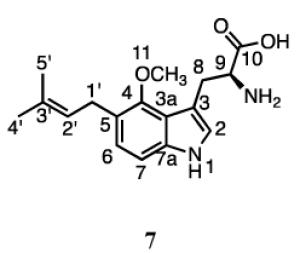
The 1H spectrum of 7 had peaks for the protons at C1’ (3.35 ppm, d, J = 7.2 Hz) and C2’ (5.25 ppm, t, J = 7.2 Hz) and the methyl groups at C3’ (1.67 and 1.60 ppm) for normal prenylation. Signals for the proton at C2 (7.10, s) and two other aromatic protons ((7.15 ppm, d, J = 8.4 Hz) and (6.98 ppm, d, J = 8.4 Hz)) indicated that prenylation had occurred at either the 5 or 7 position of the indole ring. Although we were unable to unambiguously determine the prenylation site from this data, we previously isolated and characterized normal 7-dimethylallyl-4-methoxy-l-tryptophan (unpublished data). A comparison of UV spectra and NMR chemical shifts for both compounds confirm they are distinct products implying 7 is prenylated at C5. Thus, 4-DMATS catalyzes a normal prenylation at C5 of 4-methoxytryptophan.
4-Aminotryptophan
As described for 4-methyl and 4-methoxytryptophan, incubation of 4-amino-l-tryptophan with 4-DMATS gave products 9 (10.2 min, 82%) and 1×0 (14.2 min, 18%) (see Figure 2c). HRMS analysis indicated that the molecular ions of the compounds were 68 Da higher than 4-aminotryptophan as expected for monoprenylation. The UV spectra for 9 and 10 suggested that the indole nucleus was intact.
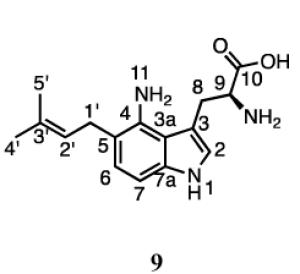
The dimethylallyl moiety in compound 9 was attached in a normal orientation as evidenced by the distinctive signals for the methylene protons at C1’ (3.24 ppm, d, J = 7.2), the proton at C2’ (5.19 ppm, t, J = 7.2 Hz), and the methyl groups at 1.66 and 1.61 ppm. Signals for the proton at C2 (7.02, s) and two other aromatic protons ((6.89 ppm, d, J = 8.4 Hz) and (6.87 ppm, d, J = 8.4 Hz)) indicated that prenylation had occurred at either the 5 or 7 position of the indole ring. The ROESY spectrum showed correlations between the methylene protons of the prenyl moiety and the aromatic proton at C6 (6.87 ppm). The HMBC spectrum showed correlations between the methylene protons of the prenyl moiety and C3a (120.0 ppm), C4 (138.4 ppm), and C6 (127.0 ppm). Collectively, these data confirm that 9 is formed by a normal prenylation at the C5 position of 4-aminotryptophan.
Compound 10 also contains normal prenylation as confirmed by the presence of proton signals for methylene protons at C1’ (3.35, d, J = 7.8), the proton at C2’ (5.31 ppm, t, J = 7.8), and the methyl groups at 1.62 and 1.61 ppm. Signals for the proton at C2 (7.05, s) and two other aromatic protons ((6.75 ppm, d, J = 7.2 Hz) and (6.36 ppm, d, J = 7.2 Hz)) indicated that prenylation had occurred at either the 5 or 7 position of the indole ring. The COSY spectrum showed correlations between the methylene protons of the prenyl moiety and the aromatic proton at C6 (6.75 ppm). The HMBC spectrum showed correlations between the methylene protons of the prenyl moiety and C7 (120.3 ppm) and C7a (138.9 ppm), as well as a correlation between the aromatic proton at C6 and C1’ (31.2 ppm). Collectively, these data confirm that 10 is formed by a normal prenylation at the C7 position of 4-aminotryptophan. Thus, 4-DMATS catalyzes the normal prenylation at C5 and C7 of 4-aminotryptophan.
Discussion
The 4-DMATS subgroup is an important member of the dimethylallyltryptophan synthase superfamily. These enzymes, including 4-DMATS from C. purpurea8 and FgaPT2 from A. fumigatus,15 catalyze normal prenylation at C4 in the indole ring of l-tryptophan as the first pathway specific step in the biosynthesis of ergot alkaloids.41 Although the natural substrate is an unsubstituted tryptophan, both 4-DMATS and FgaPT2 catalyze C4 prenylation of tryptophan analogues with substituents on the indole ring as well as the amino acid chain.19,20 The methyl group at C4 in 4-methyltryptophan blocks prenylation at that site and the compound is an inhibitor of the normal reaction.34 During studies in our laboratory with 4-DMATS using 4-methyltryptophan as a competitive inhibitor against tryptophan, we detected a higher than expected background in our assays and discovered what appeared to be prenylation of 4-methyltryptophan.
Incubation of 4-methyltryptophan with 4-DMATS gave four prenylation products, two monoprenylated indoline derivatives and two monoprenylated indoles. The major product was a hexahydropyrroloindole reverse prenylated at C3, accompanied by a smaller amount of the normal prenylated isomer, both of which eluted before the putative indoles. The more abundant indole isomer (4) was normally prenylated at N1. Unfortunately, we were unable to obtain a sufficient quantity of 3, the less abundant isomer for NMR spectroscopy. Co-injection of 3 with reverse N-dimethylallyl-4-methyl-l-tryptophan and normal 7-dimethylallyl-4-methyl-l-tryptophan excluded both of these structures.
Incubation with 4-methoxytryptophan also gave four products. The elution and UV profiles for compounds 5 - 8 were similar to those of the prenylated 4-methyltryptophan derivatives. Normally prenylated 5-dimethylallyl-4-methoxytryptophan, whose elution profile was similar to 3 was the major product and the only compound to be obtained in sufficient quantity to be fully characterized. Given the similar HPLC retention times and UV profiles for the four products from 4-methyl- and 4-methoxytryptophan, it is likely that 5 and 6 are indolines normal and reverse prenylated at C3 and 8 is a normal prenylated indole, most likely at N1 or C7. 4-DMATS also catalyzes the normal prenylation of 4-aminotryptophan at the C5 and C7 positions of the indole nucleus. Thus, 4-DMATS catalyzes normal or reverse prenylation of tryptophan and C4-substituted analogues at five different sites on the indole ring, N1, C3, C4, C5, and C7, depending on the substituent (Figures 3 and 4).
Figure 3.
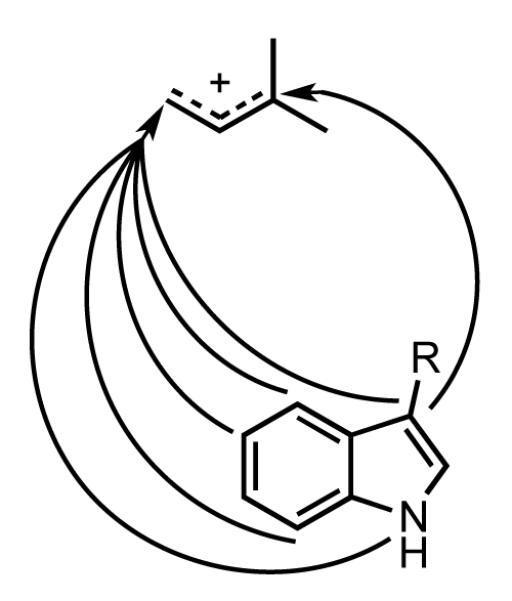
Sites for alkylation of 4-methyltryptophan by 4-DMATS.
Figure 4.
Characterized and proposed (bracketed) products from incubation of tryptophan analogues and DMAPP with 4-DMATS.
The indole ring in tryptophan is susceptible to electrophilic alkylation at N1, at C2, C5, and C7 by direct conjugation with N1 through the benzene ring, and at C4 and C6 by conjugation with N1 through the C2-C3 double bond. When C4 is blocked, 4-DMATS catalyzes alkylation at all of the activated positions, except weakly activated C6, depending on the electron-donating properties of the blocking substituent. In addition, both normal and reverse prenylation is seen at C3 for 4-methyltryptophan. Alkylation of the indole ring at sites separated by several angstroms and by normal and reverse alkylation of C3 indicates that the enzyme can bind DMAPP in different conformations. The high regioselectivity for C4 alkylation with the normal substrates suggests that binding and catalysis of a single conformation has been optimized for synthesis of 4-dimethylallyltryptophan, but when alkylation at C4 is blocked, alkylation from other conformations becomes competitive.
All of the substitutents introduced at C4 are electron-donating relative to hydrogen. For the methyl substituent (σ+ = 0.31),42 alkylation is mostly at C3, giving normal and reverse prenylated indolines, along with a smaller amount of the N1 alkylated indole. The methoxy group (σ+ = 0.78) directs alkylation predominantly to C5 giving only trace amounts of putative indolines. The amino group (σ+ = 1.3) directs alkylation exclusively to C5 and C7. Thus, when a relatively weak electron donating substituent blocks C4, the dimethylallyl cation alkylates C3 to generate normal and reverse prenylated iminium intermediates, which cyclize to the corresponding indolines. The more powerful electron-donating methoxy and amino groups direct alkylation to the ortho (C5) and para (C7) carbons, positions further activated by N1. Interestingly, the amino group in 4-aminotryptophan is not alkylated.
Our results provide strong evidence for a dissociative electrophilic alkylation of the indole nucleus by C1’, and in one instance C3’, of the dimethylallyl cation generated by heterolysis of DMAPP. 4-Dimethylallyltryptophan is the only product of the normal reaction catalyzed by 4-DMATS. When alkylation at C4 is blocked, the enzyme no longer catalyzes a regioselective reaction, but instead produces a variety of products from alkylation at the nucleophilic positions (N1, C3, C5, and C7) on the indole ring by the electrophilic dimethylallyl cation. In addition, we saw only a 3-fold variation in kcat for the methyl, methoxy, and amino tryptophans, which is most readily explained by formation of the dimethylallyl cation as the rate-limiting step, followed by alkylation of the indole nucleus. These results are consistent with previous studies with 7-methyl and 7-methoxy derivatives of tryptophan. In this case, tryptophan and the 7-substituted analogues are alkylated at C4 with only a two-fold variation in kcat.19
Recently, Luk and coworkers reported that the K174A mutant of A. fumigatus 4-DMATS catalyzes reverse prenylation of C3.31 A crystal structure of the enzyme and kinetic studies indicate that K174 is an important base, which facilitates removal of the C4 hydrogen during the electrophilic aromatic substitution reaction. They suggested that the reaction catalyzed by the wild-type enzyme proceeds in two steps, reverse alkylation at C3 followed by a Cope rearrangement and deprotonation to form the normal C4 prenylation product, as originally proposed by Wenkert and Silwa.32 The multiplicity of products we observe when C4 is blocked, including normal and reverse alkylation at C3, do not support the Cope mechanism. While a Cope rearrangement is consistent with reverse alkylation at C3 and normal alkylation at N1, it does not account for the concurrent normal prenylation at both C5 and C7. In addition, the presence of normal alkylation at C3 and the absence of a reverse N1 prenylation product suggest rearrangement from C3 to N1 is unlikely.
Conclusion
In conclusion, our work with 4-substituted analogues of tryptophan demonstrates that 4-DMATS has the innate ability to alkylate the indole ring at five different positions and by normal and reverse addition. The other reaction channels, which were revealed by blocking the normal alkylation at C4, represent five of the seven sites for alkylation of the indole nucleus by the DMATS superfamily and provide a logical rationale for evolution of members of the superfamily from a common ancestor.
Supplementary Material
Acknowledgments
We thank Reinhard Sterner and Jonathan Johnston for supplying the tmTrpB and pHDMAT vectors, respectively. We thank Jim Muller for performing mass spectroscopy and Jack Skalicky for assistance with cryoprobe NMR. This work was supported by NIH grant GM 21328.
Footnotes
Notes The authors declare no conflicts of interest.
Associated Content Additional experimental; preparation of l-tryptophan analogues using tryptophan synthase (Scheme S1); NMR spectra of 4-substituted analogues (Figures S1-S4); NMR data tables (Tables S1-S6) and NMR spectra of prenylated products (Figures S5-S40); prenylation product structures with pertinent 2D NMR correlations (Figure S41); HRMS data of prenylated products (Table S7); negative ion FABMS of compound 4 (Figures S42-S43); HPLC chromatograms of co-injection assay (Figure S44); radioTLCs and corresponding Michaelis-Menten curves of enzyme kinetics (Figures S45-S48). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).Li S-M. Nat. Prod. Rep. 2010;27:57. doi: 10.1039/b909987p. [DOI] [PubMed] [Google Scholar]
- (2).Hulvova H, Galuszka P, Frebortova J, Frebort I. Biotechnol Adv. 2012 doi: 10.1016/j.biotechadv.2012.01.005. [DOI] [PubMed] [Google Scholar]
- (3).Wallwey C, Li S-M. Nat. Prod. Rep. 2011;28:496. doi: 10.1039/c0np00060d. [DOI] [PubMed] [Google Scholar]
- (4).Grundmann A, Kuznetsova T, Afiyatullov SS, Li S-M. ChemBioChem. 2008;9:2059. doi: 10.1002/cbic.200800240. [DOI] [PubMed] [Google Scholar]
- (5).Grundmann A, Li S-M. Microbiology (Reading, U. K.) 2005;151:2199. doi: 10.1099/mic.0.27962-0. [DOI] [PubMed] [Google Scholar]
- (6).Yin W-B, Grundmann A, Cheng J, Li S-M. J. Biol. Chem. 2009;284:100. doi: 10.1074/jbc.M807606200. [DOI] [PubMed] [Google Scholar]
- (7).Yin W-B, Yu X, Xie X-L, Li S-M. Org. Biomol. Chem. 2010;8:2430. doi: 10.1039/c000587h. [DOI] [PubMed] [Google Scholar]
- (8).Gebler JC, Poulter CD. Arch. Biochem. Biophys. 1992;296:308. doi: 10.1016/0003-9861(92)90577-j. [DOI] [PubMed] [Google Scholar]
- (9).Yu X, Liu Y, Xie X, Zheng X-D, Li S-M. J. Biol. Chem. 2012;287:1371. doi: 10.1074/jbc.M111.317982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Takahashi S, Takagi H, Toyoda A, Uramoto M, Nogawa T, Ueki M, Sakaki Y, Osada H. J. Bacteriol. 2010;192:2839. doi: 10.1128/JB.01557-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Kremer A, Westrich L, Li S-M. Microbiology (Reading, U. K.) 2007;153:3409. doi: 10.1099/mic.0.2007/009019-0. [DOI] [PubMed] [Google Scholar]
- (12).Zou H-X, Xie X-L, Linne U, Zheng X-D, Li S-M. Org. Biomol. Chem. 2010;8:3037. doi: 10.1039/c002850a. [DOI] [PubMed] [Google Scholar]
- (13).Unsoeld IA, Li S-M. ChemBioChem. 2006;7:158. doi: 10.1002/cbic.200500318. [DOI] [PubMed] [Google Scholar]
- (14).Wollinsky B, Ludwig L, Xie X, Li SM. Org Biomol Chem. 2012;10:9262. doi: 10.1039/c2ob26149a. [DOI] [PubMed] [Google Scholar]
- (15).Unsoeld IA, Li S-M. Microbiology (Reading, U. K.) 2005;151:1499. doi: 10.1099/mic.0.27759-0. [DOI] [PubMed] [Google Scholar]
- (16).Ma J, Zuo D, Song Y, Wang B, Huang H, Yao Y, Li W, Zhang S, Zhang C, Ju J. ChemBioChem. 2012;13:547. doi: 10.1002/cbic.201100700. [DOI] [PubMed] [Google Scholar]
- (17).Lee S-L, Floss HG, Heinstein P. Arch. Biochem. Biophys. 1976;177:84. doi: 10.1016/0003-9861(76)90418-5. [DOI] [PubMed] [Google Scholar]
- (18).Tsai H-F, Wang H, Gebler JC, Poulter CD, Schardl CL. Biochem. Biophys. Res. Commun. 1995;216:119. doi: 10.1006/bbrc.1995.2599. [DOI] [PubMed] [Google Scholar]
- (19).Gebler JC, Woodside AB, Poulter CD. J. Am. Chem. Soc. 1992;114:7354. [Google Scholar]
- (20).Steffan N, Unsoeld IA, Li S-M. ChemBioChem. 2007;8:1298. doi: 10.1002/cbic.200700107. [DOI] [PubMed] [Google Scholar]
- (21).Luk LYP, Tanner ME. J. Am. Chem. Soc. 2009;131:13932. doi: 10.1021/ja906485u. [DOI] [PubMed] [Google Scholar]
- (22).Metzger U, Schall C, Zocher G, Unsoeld I, Stec E, Li S-M, Heide L, Stehle T. Proc. Natl. Acad. Sci. U. S. A. 2009;106:14309. doi: 10.1073/pnas.0904897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Jost M, Zocher G, Tarcz S, Matuschek M, Xie X, Li S-M, Stehle T. Journal of the American Chemical Society. 2010;132:17849. doi: 10.1021/ja106817c. [DOI] [PubMed] [Google Scholar]
- (24).Schuller JM, Zocher G, Liebhold M, Xie X, Stahl M, Li S-M, Stehle T. J. Mol. Biol. 2012;422:87. doi: 10.1016/j.jmb.2012.05.033. [DOI] [PubMed] [Google Scholar]
- (25).Kuzuyama T, Noel JP, Richard SB. Nature (London, U. K.) 2005;435:983. doi: 10.1038/nature03668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Metzger U, Keller S, Stevenson CEM, Heide L, Lawson DM. Journal of Molecular Biology. 2010;404:611. doi: 10.1016/j.jmb.2010.09.067. [DOI] [PubMed] [Google Scholar]
- (27).Zocher G, Saleh O, Heim Joel B, Herbst Dominik A, Heide L, Stehle T. PLoS One. 2012;7:e48427. doi: 10.1371/journal.pone.0048427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. J. Mol. Biol. 1990;215:403. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- (29).Qian Q, Schultz AW, Moore BS, Tanner ME. Biochemistry. 2012;51:7733. doi: 10.1021/bi3009054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Phan RM, Poulter CD. J. Org. Chem. 2001;66:6705. doi: 10.1021/jo010505n. [DOI] [PubMed] [Google Scholar]
- (31).Luk LYP, Qian Q, Tanner ME. J. Am. Chem. Soc. 2011;133:12342. doi: 10.1021/ja2034969. [DOI] [PubMed] [Google Scholar]
- (32).Wenkert E, Sliwa H. Bioorg. Chem. 1977;6:443. [Google Scholar]
- (33).Schwarzer DD, Gritsch PJ, Gaich T. Angew. Chem., Int. Ed. 2012;51:11514. doi: 10.1002/anie.201203586. [DOI] [PubMed] [Google Scholar]
- (34).Cress WA, Chayet LT, Rilling HC. J. Biol. Chem. 1981;256:10917. [PubMed] [Google Scholar]
- (35).Hettwer S, Sterner R. Journal of Biological Chemistry. 2002;277:8194. doi: 10.1074/jbc.M111541200. [DOI] [PubMed] [Google Scholar]
- (36).Goloubinoff P, Gatenby AA, Lorimer GH. Nature (London) 1989;337:44. doi: 10.1038/337044a0. [DOI] [PubMed] [Google Scholar]
- (37).Goss RJM, Newill PLA. Chem. Commun. (Cambridge, U. K.) 2006:4924. doi: 10.1039/b611929h. [DOI] [PubMed] [Google Scholar]
- (38).Winn M, Roy AD, Grueschow S, Parameswaran RS, Goss RJM. Bioorg. Med. Chem. Lett. 2008;18:4508. doi: 10.1016/j.bmcl.2008.07.053. [DOI] [PubMed] [Google Scholar]
- (39).Ma J, Yin W, Zhou H, Liao X, Cook JM. J. Org. Chem. 2009;74:264. doi: 10.1021/jo801839t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Sangster AW, Stuart KL. Chem. Rev. 1965;65:69. [Google Scholar]
- (41).Floss HG. Tetrahedron. 1976;32:873. [Google Scholar]
- (42).Brown HC, Okamoto Y. J. Am. Chem. Soc. 1958;80:4979. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.