Abstract
The ability to generate intentional behavior is undeniably at the core of what makes us acting subjects. Intentional actions consist of at least 2 components (Brass M, Haggard P. 2008. The what, when, whether model of intentional action. Neuroscientist. 14:319–325.): choosing an appropriate behavior (what) and selecting the moment of execution (when). The aim of this study was to identify differing and overlapping neural networks underlying the “what” and “when” of intentional movement initiation. While scanned with functional magnetic resonance imaging, 35 healthy subjects performed self-initiated and reactive, that is, internally and externally triggered movements of the right or left index finger in 3 experimental conditions: 1) “Free Choice” (free timing: when/choice of hand: what), 2) “Timed Choice” (external timing/choice of hand: what), and 3) “No Choice” (external timing/cued hand). The what-component specifically employed the presupplementary motor area (SMA) and dorsal premotor cortex bilaterally. The when-network consisted of superior SMA together with insula and Area 44 bilaterally as well as bilateral anterior putamen, globus pallidus, and left cerebellum subcortically. These 2 components recruited different networks, pointing to a partially distinct neuronal realization of the relating functions. Finally, the more intentional components were involved, the higher was activity in the anterior midcingulate cortex, which highlighted its role in intentional initiation of behavior.
Keywords: anterior midcingulate cortex, fMRI, free movement timing, intentional motor control, movement selection
Introduction
Since the discovery of the “Bereitschaftspotential” preceding self-initiated movements by Kornhuber and Deecke (1965), neuronal activity relating to intentional movement generation has been a vital field of research. Based on recent findings, Brass and Haggard (2008) proposed a heuristic framework for the investigation of intentional action that distinguishes 3 major components: 1) a component related to the decision about which action to execute (“what”-component), 2) a component about when to execute an action (“when”-component), and 3) the decision about whether or not to execute an action (“whether”-component). In the experimental context, however, we face the contradiction between freedom of choice as experimental condition and the empirical dictum of maximized control over conditions. Thus, in an empirical context, we only may consider partly free decisions. A common strategy to examine the 3 components individually is to compare predetermined reactions with actions of a certain degree of freedom (what or when) or movement execution with inhibition of movement execution (whether; Haggard 2008). A second problem consists in the difficulty to reliably operationalize all 3 intentional components in the same experiment, which is necessary to account for possible interdependencies between components. It seems especially difficult to integrate the whether-component together with the other 2 (what and when) because in case of a decision against movement execution, there is no behavior to directly relate to. In that case, we have to rely on introspections of the subject about the what- and the when-component at the same time, which entail known problems related to subjective reports (e.g., inaccuracy of retrospection). Therefore, in the current study, we focused on the what and when of self-initiated movements.
Typically, intentional action is operationalized either as the choice between predefined movements (what) or as the selection of a time point (when) to execute an action. Two decades ago, the dorsolateral prefrontal cortex (DLPFC) and the SMA were associated with the free choice between responses (Frith et al. 1991; Playford et al. 1992) in experiments using positron emission tomography. Jahanshahi et al. (1995) examined brain function during cued and non-cued rhythmic button presses and found that the right DLPFC significantly differentiated self-initiated from externally triggered movements. Using the same paradigm with irregular timing, Jenkins et al. (2000) found additional activation in left DLPFC, pre-SMA, and the anterior midcingulate cortex (aMCC; Palomero-Gallagher et al. 2009; Shackman et al. 2011). Varying movement frequency and complexity in a similar functional magnetic resonance imaging (fMRI) experiment, Deiber et al. (1999) observed that self-initiated movements induced stronger activation specifically in pre-SMA and aMCC while movement sequences increased activity in the SMA proper. Lau, Rogers, Ramnani, et al. (2004) found only pre-SMA activity tightly associated with the free choice of a target, while DLFPC activity matched a “specified target”-condition. Whereas SMA is related to movement performance (Nachev et al. 2008), the DLPFC probably contributes to attentional or working memory processes rather than to preparation and initiation of the actual motion in self-initiated movements (Wiese et al. 2005, 2006). Taken together, the pre-SMA and the aMCC seem to represent neural correlates of intentional movement selection and action initiation.
Recent attempts to disentangle the what and when components of intentional actions described above, suggested the interplay of different neuroanatomically dissociable subfunctions in voluntary action control (Mueller et al. 2007; Krieghoff et al. 2009). Mueller et al. (2007) deployed a paradigm demanding to press 1 of 2 buttons. The choice of a movement to execute could be made either internally or was determined by a visual cue. Importantly, the timing was prespecified (though not directly cued) in both conditions. In particular, movements had to be performed syncopated, that is, executed rhythmically at the midpoint between sets of visual pacing stimuli every 1.2 s. The results indicated that movement selection (what) is associated with activity of the aMCC. The further conclusion about the when-component, however, was based on indirect evidence. The authors reasoned the pre-SMA to be linked to movement timing or initiation because it is activated in both conditions requiring syncopated movement pacing. It has been shown before that the pre-SMA plays a role in this mode of movement initiation, as it is reliably activated when externally timed movements are not executed synchronized with the rhythmic cue (Mayville et al. 2002; Jantzen et al. 2004). Yet, the pre-SMA was repeatedly found to be involved in various aspects of selecting an action (what), such as the choice of a specific response (Lau et al. 2004; van Eimeren et al. 2006) or the initiation of different action sets, for example, sets of action–selection rules, as necessary for task switching (Rushworth et al. 2004). Krieghoff et al. (2009) combined the selection of the left or right hand to move (what) with the decision between 2 auditory cued time points for movement execution (when) in one paradigm to dissociate internal movement selection and timing. After an instruction cue indicating an internal or external what or when decision and a variable delay, 4 tones were presented with interstimulus intervals (ISIs) of 1 s. Subjects had to choose between the third and the fourth tone to execute either a cued or non-cued movement. The results indicated an involvement of the aMCC in movement selection and of the paramedian frontal cortex anterior and dorsal to pre-SMA in action timing. The analysis, however, was focused on instruction-related neural activity, that is, activity related to the cue indicating the internally specified response to be made shortly, assuming that both choices (what and when) are always made immediately (within 1 s) after cue presentation. This, however, represents a strong assumption. Moreover, due to this approach, the results may pertain more to activity due to the preparation for a decision that is about to be taken shortly rather than to the decision itself. Alternatively, as timing was not free but a choice between 2 possible time points, subjects may have chosen a cue which, however, is different from actual free timing of movement initiation. This consideration together with the fact that the inference was based on a post hoc signal strength analysis with a rather liberal threshold, considerably weakens the dissociation of the what- and when-component in this study.
The aim of the present study was to address the described shortcomings and thereby robustly compare the what- and when-component of intentional movement initiation. We examined 2 major aspects of intentional actions, namely internally triggered movement selection and initiation, by combining the free choice of the executed movements (what) with a free timing of movement execution (when) in the same paradigm. While maintaining direct comparability of self-initiated and reactive movements, we intended to delineate the nodes of possibly differing neural networks underlying the free choice of a movement and of when to perform it. We hypothesized both the pre-SMA and the aMCC to be involved in self-initiated movements and especially aimed to clarify whether there is a differential involvement of these 2 neuroanatomically dissociable brain areas in the selection and timing of movements. Furthermore, we hypothesized the basal ganglia to be particularly activated during internal timing of actions, which is suggested by previous work (Cunnington et al. 2002; Debaere et al. 2003; Francois-Brosseau et al. 2009) and by behavioral symptoms of basal ganglia damage in Parkinson’s disease (O'Boyle et al. 1996).
Materials and Methods
Subjects
We examined 35 healthy volunteers (age range 21–62 years, mean age 35.9 ± 12.4 standard deviation [SD] years; 17 females) without any record of neurological or psychiatric disorders and normal or corrected-to-normal vision. All subjects gave informed written consent to the study protocol, which had been approved by the local ethic committee of the RWTH Aachen University. Right-hand dominance of the participants was established by means of the Edinburgh handedness inventory (Oldfield 1971).
Experimental Protocol
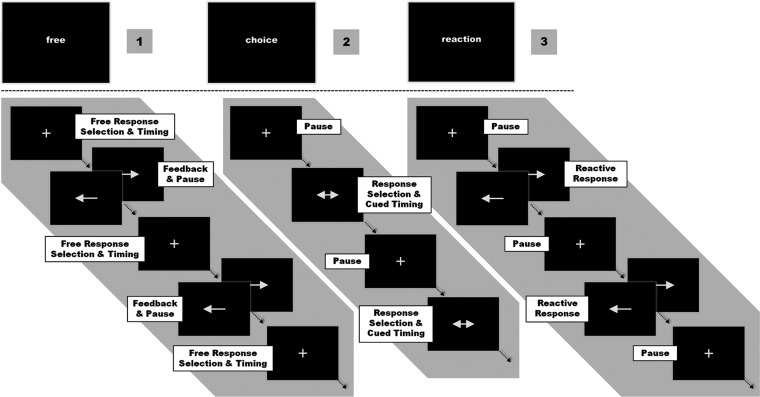
The experimental task consisted of unilateral button presses performed with the right or left index finger under 3 different conditions: 1) a free choice of button presses with the left or right hand at a self-chosen point in time (what and when), 2) a Timed choice task, when the time of movement was cued by a visual stimulus but the hand to be moved was chosen by the subject (what), or 3) a no choice task when laterality and time of movement were cued by a visual stimulus (reaction). Responses were recorded using MRI-compatible response pads (LumiTouch, Burnaby, Canada). All visual stimuli were presented using the “Presentation” software package (Version 14.1; Neurobehavioral Systems Inc., Albany, CA) and were displayed on a custom-built shielded thin film transistor screen at the rear end of the scanner visible via a mirror mounted on the head coil (14° × 8° viewing angle). In the experiment, task blocks of 60 s duration were periodically alternated with rest periods of black screen presentation for 15 s serving as implicit “baseline.” Each task block was introduced by a one-word instruction presented for 1.5 s, which informed the subject which of the 3 conditions had to be performed in the upcoming block. All cues consisted of white arrows presented on a black screen in the central field of view. A fixation cross in the middle of the screen indicated an ongoing task in each of the 3 conditions (Fig. 1).
Figure 1.
1) [free] Choice, 2) Timed [choice], and 3) No Choice = [reaction]. Conditions were pseudorandomized in blocks of (1-3-2) or (1-2-3). Randomized ISIs and laterality of the beginning “Free”-condition determined the response cues in both the following “Choice”- and “No Choice”-conditions.
Free Choice—Self-Timed Movement Selection and Execution (Free Choice of Hand/Free Timing)
In the “Free”-condition, the movements were entirely self-initiated. The subjects were instructed to press 1 of the 2 buttons at any self-chosen time. Every response was immediately followed by a 3.5 s visual feedback consisting of an arrow pointing to the side of the button press. During the feedback, no further responses were allowed to prevent sequential finger tapping and to separate the events for the statistical analysis. When training the subjects, they were explicitly instructed to vary the ISIs as well as the hand used in order to prevent rhythmic responses or any kind of movement routine. The time intervals between single responses were recorded online and subsequently used as ISIs for the visual cued responses in the other 2 conditions. Likewise, the frequency of right and left button presses was fed back as visual cues triggering a lateralized response in the “No Choice”-condition.
Timed Choice—Movement Choice at a Cued Time Point (Free Choice of Hand/External Timing)
In the “Timed”-condition, stimuli consisted of arrows pointing to both sides presented for 3.5 s. The task was to respond as fast as possible by pressing either the left or the right button. Subjects were free in choosing the side of response but should vary between left- and right-sided responses. The ISIs and thus the number of button presses from the preceding Free-condition were presented in a random sequence to assure comparability of motor responses timing between conditions.
No Choice—Lateralized Reaction (External Cue for Hand/External Timing)
In contrast to the “Timed Choice”-condition, responses in the No Choice-condition were fully predetermined by the visual cue. Subjects had to react as fast as possible to a single-headed arrow pointing to the left or right by pressing the corresponding button. Like in the Timed-condition, ISIs and lateralization of responses were matched to the preceding Free-condition.
In summary, each ISI generated by a subject in the Free-condition was subsequently used to trigger one response both in the subsequent Timed- and No Choice-condition. By randomizing ISIs in the Timed-condition and ISIs and number of left and right responses (independently) in the No Choice-condition, anticipation confounds with respect to cue sequences were avoided, while comparability across conditions was preserved. For each condition, 8 blocks were presented in alternating sequences of either 1 (Free)—2 (Choice)—3 (Reactive) or 1-3-2 in a pseudorandomized order. The sequences were spread evenly across the experiment session to minimize any potential confounds due to order effects. The whole experiment lasted approximately 33 min. We did not introduce a factorial 2 × 2 design with each the what- (hand) and when-component (timing) manipulated independently because this would have compromised the close comparability between conditions. The missing when-condition with free timing and cued hand would for instance have produced an additional set of ISIs. Also the visual input would not have been correlated with movements in that condition. This would have made a parallelization of timing parameters and visual input across conditions impossible.
Behavioral Data Analysis
Behavioral performance assessed during the fMRI experiment was analyzed offline using MATLAB (Mathworks, Natick, MA). The number of left and right button presses in the Free- and the Timed-condition across subjects were compared by means of paired t-tests using a statistical threshold of P < 0.05. Likewise, mean reaction times for correct responses were compared in the Timed- and the No Choice-condition using a paired t-test.
Functional Magnetic Resonance Imaging
Eight hundred and ninety two volumes were acquired on a Siemens Trio 3-T whole-body scanner (Erlangen, Germany) using a blood oxygen level–dependent (BOLD) contrast sensitive imaging sequence (gradient echo planar imaging [EPI], time repetition = 2.2 s, time echo = 30 ms, flip angle = 90°, in plane resolution = 3.1 × 3.1 mm, 36 axial slices, 3.1 mm thickness) covering the whole brain. Image acquisition was preceded by 4 dummy images allowing for saturation in contrast. These images were discarded from further processing. The remaining 888 EPI images were analyzed using the SPM5 software package (www.fil.ion.ucl.ac.uk/spm). Images were first corrected for head movement by affine registration using a two-pass procedure, by which images were initially realigned to the first image and subsequently to the mean of the realigned images. After realignment, the mean EPI image for each subject was spatially normalized using the “unified segmentation” approach (Ashburner and Friston 2005). The resulting parameters of a discrete cosine transform, which defined the deformation field necessary to warp the subjects data into the space of the Montreal Neurological Institute (MNI) tissue probability maps, were applied to the individual EPI volumes and resampled at 2 × 2 × 2 mm3 voxel size. The normalized images were spatially smoothed using an 8 mm full-width at half-maximum Gaussian kernel to meet the statistical requirements of the general linear model (GLM) and to compensate for residual intersubject variations in brain anatomy.
Statistical Analysis
The fMRI data were analyzed using the GLM as implemented in SPM5. Each response (button press) was modeled as an individual event for the left and the right hand in the 3 experimental conditions. The event-related input functions were then convolved with a canonical hemodynamic response function and its first-order temporal derivative to yield the final regressors. Including the temporal derivatives of the task regressors into the design has been shown to increase sensitivity and specificity of the GLM by accommodating deviations of the BOLD timecourse from its canonical form (Josephs and Henson 1999; Henson et al. 2001). Parameter estimates were subsequently calculated for each voxel using weighted least squares to provide maximum likelihood estimators based on the temporal autocorrelation of the data (Kiebel and Holmes 2003). The first regressor for both hands in all 3 conditions represented the 6 simple main effects against the implicit baseline for every subject. These 6 individual first-level contrasts were then fed into a second-level group-analysis using an analysis of variance (ANOVA) employing a random effects model (Penny and Holmes 2003). We allowed for violations of sphericity by modeling nonindependence across images from the same subject and allowing unequal variances between conditions and subjects as implemented in SPM5.
Simple main effects of each task (vs. the resting baseline) as well as comparisons between experimental factors were tested by applying appropriate linear contrasts to the ANOVA parameter estimates. Conjoint main effects were tested by means of a conjunction analysis using the minimum statistics approach (Nichols et al. 2005). The resulting SPM(T) maps were then thresholded at P < 0.05 conducting a family-wise error (FWE) correction on the cluster-level (cluster forming threshold at voxel level P < 0.001; extend threshold: k = 313 voxels; Worsley et al. 1996). Anatomical assignment of the resulting activation clusters was achieved using the cytoarchitectonic maximum probability maps implemented in the SPM Anatomy toolbox (www.fz-juelich.de/ime/spm_anatomy_toolbox, V1.6; Eickhoff et al. 2005, 2007; Eickhoff, Heim, et al. 2006), which relies on previous studies that provided details about cytoarchitecture and intersubject variability of brain areas, such as Broca's Area (Brodmann area [BA]44, BA45; Amunts et al. 1999, 2004), premotor cortex (BA6; Geyer 2004), primary motor cortex (4a, 4p; Geyer et al. 1996), primary somatosensory areas (3a, 3b, 1, 2; Geyer et al. 1999, 2000; Grefkes et al. 2001), secondary somatosensory areas (OP1–4; Eickhoff, Amunts, et al. 2006; Eickhoff, Schleicher, et al. 2006), intraparietal sulcus (hIP1-3; Choi et al. 2006; Scheperjans, Eickhoff, et al. 2008; Scheperjans, Hermann, et al. 2008), superior parietal areas (7A, 7PC; Scheperjans, Eickhoff, et al. 2008; Scheperjans, Hermann, et al. 2008]), inferior parietal areas (PFop, PFt, PF, PFm, PFcm, PGa; Caspers et al. 2006, 2008), extrastriate visual areas (V3v, V4, V5/hOc3v, hOc4v, hOC5; Malikovic et al. 2007; Rottschy et al. 2007), and the cerebellum (Diedrichsen et al. 2009).
Results
Behavioral Data
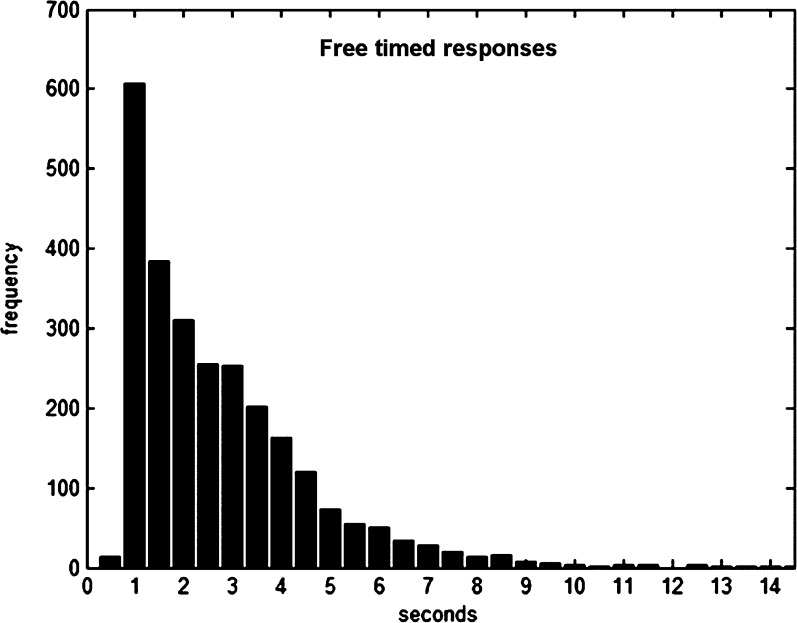
In the Free- and the Timed-condition, participants conducted a balanced proportion of right and left button presses (Free: R 42.8 ± 9.2/L 42.4 ± 10.6, P = 0.41, R 51.25%; Timed: R 41.8 ± 9.2/L 41.3 ± 9.7, P = 0.40, R 50.77). Intervals between feedback offset and self-initiated responses were in average 2.4 s [SD: 1.66 s] and featured a strongly skewed normal distribution (cf. Fig. 2 + Supplementary Fig. S1). Response times in the Timed-condition (mean (M): 412 ms, SD: 124 ms) were not different from reaction times in the No Choice-condition (M: 436 ms, SD: 77 ms; P = 0.203). The error rate in the No Choice-condition was on average 1.51% (SD: 1.87%) and did not differ between button presses with the right or left hand (P = 0.17).
Figure 2.
Response time distribution of ∼3000 responses after feedback offset in the “Free”-condition.
Imaging Data—Movement-Related Neural Activity
Dominant right hand movements contrasted to the left hand independent of condition (Rall ∩ [Rfree > Lfree] ∩ [Rtimed > Ltimed] ∩ [Rno > Lno]; Fig. 3) revealed one cluster of activation in the contralateral primary motor (anatomical labeling: Areas 4a and 4p) and somatosensory cortices along the postcentral gyrus (Areas 3b, 3a, 1, 2) and a second in the ipsilateral cerebellum (Lobule V and VI). As expected, responses of the left nondominant hand (Lall ∩ [Lfree > Rfree] ∩ [Ltimed > Rtimed] ∩ [Lno > Rno]) produced a virtually mirror-reversed pattern of activity including an additional activation cluster in the right parietal operculum (OP 1) and the adjacent posterior insula cortex (Ig2).
Figure 3.
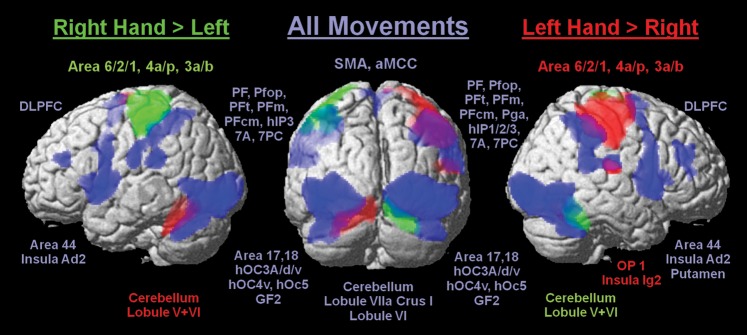
Significant BOLD signal increases in all 3 conditions due to movements of the right hand (green), left hand (red), and both hands (blue) relative to baseline with cytoarchitectonic informed anatomical labeling (P < 0.05, cluster level FWE).
In order to identify regions that were constantly active throughout all conditions, that is, areas involved in performing hand movements independently of movement side and mode of movement initiation (core motor areas), a conjunction analysis over all conditions, that is, all 6 regressors, was performed. This analysis revealed a widespread bilateral network consisting of striate (V1/Area 17), extrastriate visual (V2/Area 18; V3/hOC3A, hOC3d, and hOC3v; V4/, hOC4v; V5/hOc5), fusiform gyrus (GF2), somatosensory (Area 2, OP 1 and OP 4) cortices, SMA (Area 6), posterior MCC, area 44, insula (extending into putamen on the right), cerebellum (Lobule VIIa Crus I, Lobule VI), middle frontal gyrus (DLPFC), and inferior (IPL/Area PFop, PFt, PF, PFm, PFcm, and right PGa) together with superior parietal lobule (SPL/Area 7A and 7PC) extending into intraparietal sulcus (IPS/Area hlP3 and right hlP1, hlP2).
Imaging Data—Movement Selection Network
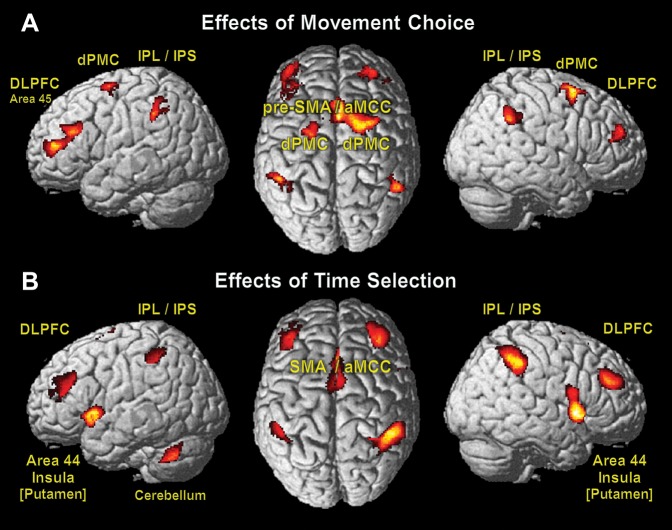
To precisely trace each effect of interest (what- or when-component), we conducted an analysis of all simple main effects between conditions (for MNI coordinates of significant activations, see Supplementary Material) and subsequently computed conjunctions of all contrasts including the specific effect of interest. Although this approach is statistically more conservative than using the main effects only, the mere difference was smaller activation clusters in the more complex conjunctions. Neural effects of the selection to move the left or right hand (what) were localized by contrasting activation in those conditions where the hand to be moved could be freely chosen by the subjects, to those where the hand was visually cued ([Timed > No Choice] ∩ [Free > No Choice]; Fig. 4A). This analysis revealed increased activation in medial frontal cortex in a region comprising the pre-SMA extending into aMCC. Bilateral activation was observed in the dorsal premotor (dPMC, Area 6) and the dorsolateral prefrontal cortices (DLPFC: middle frontal gyrus expanding to left Area 45). Bilateral activation was also found in the inferior parietal lobules (IPL/left Area PF, right Area PFm) extending into anterior intraparietal sulcus on the left (IPS/left Areas hIP1 and hIP2). There was no significant effect of movement laterality, which was specific to the Timed-condition only (right hand: [Rtimed > Ltimed] ∩ [Timed > No Choice]; left hand: [Ltimed > Rtimed] ∩ [Timed > No Choice]).
Figure 4.
Significant effects of movement choice (what; A) and time selection (when; B) with macroanatomic labels (P < 0.05, cluster level FWE).
The reverse contrast testing for areas with increased activity in the No Choice-condition compared with both Free and Timed did not yield significant results. Testing the conditions individually, only the No Choice- against the Timed-condition revealed bilaterally enhanced activity at the temporooccipital junction including V5 (Area hOC5).
Imaging Data—Movement Timing Network
The free determination of the point in time when to execute a particular movement was the exclusive feature of the Free-condition. To dissociate the neural effects of internal timing from the effects of movement choice, we contrasted the Free- against the Timed-condition in conjunction with the Free- against the No Choice-condition ([Free > Timed] ∩ [Free > No Choice]; Fig. 4B). Effects of timing selection independent from the used hand were bilaterally found in superior parts of the SMA (Area 6) and the aMCC. Bilateral involvement was also significant for area 44 including anterior insula, anterior putamen, globus pallidus, and DLPFC (middle frontal gyrus). The parietal cortex showed enhanced activity in IPS and IPL (Areas hIP2 and PF), which was more pronounced in the right hemisphere (Areas hIP1, hIP3, PFm) extending into the superior parietal lobule (SPL/right Area 7PC). Unilateral activation due to movement timing was present in the left cerebellum (Lobule VIIa Crus I and Lobule VI).
The reverse contrast (Timed > Free), however, did not yield any significant neuronal activation.
Imaging Data—Comparison of Movement Selection and Timing
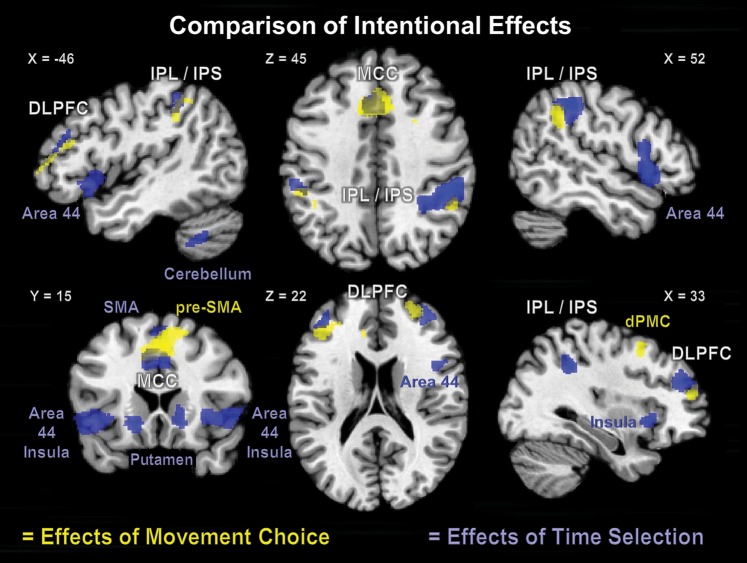
The comparison of activation patterns associated with movement selection (what) and those for internal timing of movement execution (when) revealed that both factors engaged the aMCC, the IPL/IPS, and the DLPFC in both hemispheres (Fig. 5). For movement selection, the contribution of IPL/IPS was rather symmetrical between the hemispheres, while for movement timing, IPL/IPS activation appeared to have a tendency of right hemispherical lateralization. In contrast, within the DLPFC, movement selection showed a left hemispheric dominance, while internal timing seemed distributed quite similar. Furthermore, prefrontal activation relating to timing selection was located more superior and posterior to activation due to movement selection. A conjunction analysis between movement selection and timing ([Free > Timed] ∩ [Timed > No Choice] ∩ [Free > No Choice]) revealed a common focus of activity in the aMCC. A closer look on the 6 parameter estimates (right and left hand in each condition = 2 × 3; Fig. 6) at the peak voxel of the aMCC demonstrated that the activation of the aMCC was proportional to the “intentional load” represented by the number of selection components (what/when) necessary for movement initiation. That is, while reactive movements did not evoke any additional activation in the aMCC compared with baseline, the selection of hand in the Timed-condition evoked a significant neuronal response, which even increased significantly when the selection of execution timing was additionally required in the Free-condition. Only the aMCC was activated in this highly specific manner, that is, only the aMCC featured the specific profile of activity indicating a key role in internally specified (generated) actions: (Free > Timed > Reactive = Baseline). To test whether other regions were involved solely in the internal selection of movements or timing, the effect of reactive movements (No Choice-condition vs. baseline; P < 0.05, cluster level FWE) was used as an exclusive mask for the effects of movement selection and of movement timing. This analysis thus aimed at revealing regions showing an effect of movement or timing choice while not showing activity related to reactive movements. (Supplementary Fig. S2). The masked what’-contrast (movement selection) revealed that pre-SMA and bilateral dPMC were exclusively activated in relation to the internal selection of movements but not by reactive movements as were parts of bilateral DLPFC and of left IPL/IPS (IPL/Area PF; IPS/Area hIP1 and hIP2). Masking the when-contrast (timing selection) showed activation in bilateral anterior putamen and globus pallidus as well as parts of left DLPFC and an inferior aMCC in movement timing, but no significant activation evoked by reactive movements.
Figure 5.
Comparison of intentional effects with regions of activation due to movement choice (what in yellow) and time selection (when in blue) marked with macroanatomic labels (P < 0.05, cluster level FWE). Those regions that feature conjoint activation of “what” and “when” are labeled in using white fond.
Figure 6.
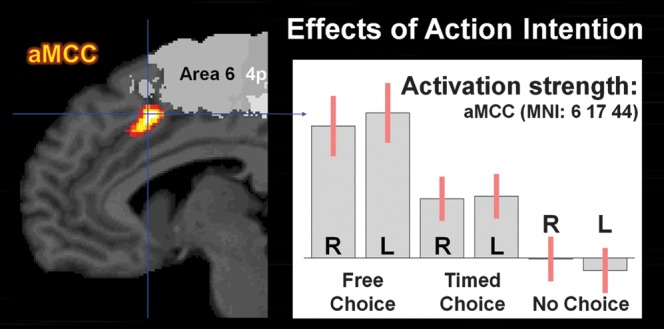
Parameter estimates of the aMCC for right (R) and left (L) hand movements in 3 experimental conditions (confidence intervals in red). Neuronal activity was increased in the “Timed”- (what) and even higher in the “Free”-conditions (what + when) compared with the “No Choice”-condition.
Discussion
In this study, we manipulated movement selection and timing within the same paradigm introducing for the first time actual free determination of a time point for movement initiation in addition to the free choice of which movement to enact. More specifically, in our free condition, subjects had to choose between a left and a right hand movement to be initiated on a not-cued point in time but rather spontaneously, that is, self-initiated. Importantly, the current design allowed us to ensure a high degree of comparability between the conditions, as we controlled for visual stimulation (by introducing the feedback in the Free-condition) and used the (randomized) timing and response parameters from the self-initiated condition for the subsequent reactive and forced-choice blocks. Finally, we applied an event-related design with the trigger set on the movements to be certain to effectively analyze neural activity related to internally specified movement initiation. By focusing on spontaneous movement initiation and parceling out activity due to visual stimulation and movement execution, the current study allowed to specifically isolate the what and when components of internally specified movements in an ecologically valid paradigm.
The choice of “what to do” evoked robust activity in the pre-SMA extending into the aMCC, along with bilateral dPMC, which are all involved in movement selection and execution (Haggard 2008). The choice of when to act reliably increased neural activity in the aMCC, together with bilateral area 44, anterior insula, SMA, putamen, globus pallidus, and left cerebellum, all associated with internal timing and sequencing of movements (Wiener et al. 2010). Both selection and timing of movements engaged adjacent regions in the parietal and prefrontal cortices frequently associated with spatial attention and behavioral planning (Corbetta and Shulman 2002). The key finding of this study is that the aMCC was the only region that featured increasing activity with more intentional components during movement initiation. Thereby, we provide additional evidence for a crucial contribution of the aMCC to intentional motor control (Paus 2001).
The What of Self-Initiated Movements
The decision of what to do, that is, the free selection of a left or right index finger flexion recruited pre-SMA including aMCC together with dPMC. Chouinard and Paus (2006, 2010) pointed out the importance of the dPMC in response selection. As demonstrated in numerous previous studies, pre-SMA and aMCC feature increases of activity during internal selection and initiation of movements (Deiber et al. 1999; Cunnington et al. 2002, 2003; Lau, Rogers, Haggard, et al. 2004; Cunnington 2005; van Eimeren et al. 2006). Lau, Rogers, Ramnani, et al. (2004) and Lau et al. (2006) showed that the free selection of responses is tightly associated with the pre-SMA, whereas response conflicts triggered activity increase especially in the aMCC. On the other hand, Nachev et al. (2007) demonstrated that pre-SMA injury can lead to a selective deficit in the ability to inhibit a response. Likewise, a recent meta-analysis by Swick et al. (2011) highlighted the role of the pre-SMA for response inhibition in both STOP-Signal and GO/ NO-GO tasks. Hence, there is strong evidence for inhibition of behavior rather than selection as an essential function of the pre-SMA. Yet, following the argument of Mostofsky and Simmonds (2008) and Simmonds et al. (2008), response inhibition can be conceptionalized as selection to withhold a specific response, that is, selective movement inhibition (Coxon et al. 2009). Consequently, inhibition and selection can be seen as 2 sides of the same coin (Mostofsky and Simmonds 2008). Well in line is the predominant role of the pre-SMA in resolving response competition (Ullsperger and von Cramon 2001; Lau et al. 2006) when the selection of one response and the inhibition of another are simultaneously required to yield coherent behavior. Thus, the pre-SMA seems to fulfill a gating function in intentional motor control by inhibiting stimulus-driven reactive behavior as well as triggering non-cued movements, as for instance necessary in asynchronous (syncopated) movement pacing to rhythmic cues (Mueller et al. 2007). Consequently, our data suggest that the pre-SMA is specifically associated with the what-component of self-initiated movements conceived as selective behavior in contrast to stimulus-driven reactive behavior, that is, selective motor initiation.
The When of Self-Initiated Movements
The decision when to act, that is, the free timing of a finger flexion, yielded activity increase in aMCC, superior SMA, and left cerebellum as well as bilateral involvement of area 44 extending to anterior insula, putamen, and globus pallidus. The interpretation of this result is limited in so far that the free timing of movement initiation (when) was assessed only conjointly with the free hand choice (what). Hence, some aspects of free movement timing may not be captured by the current subtraction design. Instead, the conducted experiment was especially designed to maximize comparability between conditions and thereby between what and when of self-initiated movements. Importantly, studies specifically examining the free timing of predefined movements previously demonstrated the association of aMCC and SMA activity with self-paced movement initiation (Ball et al. 1999; Deiber et al. 1999; Jenkins et al. 2000). The SMA, however, is also involved in externally triggered movements (Romo and Schultz 1987; Thaler et al. 1988; Picard and Strick 2003; Grefkes et al. 2008) and mediated by the type of movement (Deiber et al. 1999; van Eimeren et al. 2006; Bortoletto and Cunnington 2010).
Following Lewis and Miall (2003), intentional movement timing may be scaled in subsecond and in suprasecond intervals relating to more spontaneous (automatic) and more cognitively controlled timing, respectively. Both timing processes are jointly engaged in intentional movement initiation. In a voxelwise meta-analysis accounting for 45 imaging experiments, Wiener et al. (2010) found the SMA and the right area 44 as part of a core network mediating timing in the brain. Unsurprisingly, the speech dominant left area 44 seemed restricted to subsecond perceptual timing (Wiener et al. 2010). Regarding manual control as demanded in this study, area 44 is involved in execution timing, that is, delay of hand postures (Makuuchi 2005) and in response selection and inhibition on base of internal representations (Kan and Thompson-Schill 2004; Zhang et al. 2004). In the same meta-analysis, putamen, globus pallidus, and cerebellum were consistently implicated in rather automated subsecond timing, whereas bilateral insula demonstrated significant contribution to more cognitive suprasecond timing (Wiener et al. 2010). In self-initiated movements, the execution of nonroutine movement pattern was demonstrated to specifically activate bilateral putamen (Francois-Brosseau et al. 2009) and globus pallidus (Jankowski et al. 2009). Also in line with our results, lobule VII crus I of the left cerebellum was found to be especially sensitive to timing in the context of interval coding (Harrington et al. 2004). Furthermore, the anterior insula is thought to play an essential role in evaluating the consequences of intentional action (Brass and Haggard 2010). Taken together, our results reflect previous findings, associating area 44, and anterior insula with more cognitive internal timing of actions and SMA, basal ganglia, and left cerebellum with rather automatic timing and coordination of movement execution (Witt et al. 2008). As hypothesized, parts of the basal ganglia, in particular, bilateral anterior putamen and globus pallidus are involved in intentional movement timing in contrast to cued timing. In line with our hypothesis, decreased control of motor timing in Parkinson's disease may be explained at least partly by impaired activation of putamen, SMA, right insula, and aMCC (Playford et al. 1992; Jahanshahi et al. 1995) as well as by decreased functional connectivity of left putamen and right insula with the pre-SMA (Wu et al. 2011).
Intentional Movement Initiation
The aMCC was sensitive to what and when decisions in self-initiated movements and showed additive effects when both were combined. In particular, the aMCC (MNI coordinates: x = −3, y = 18, z = 42) featured not only increased activity for internal movement selection (what) over reactive movements but even higher levels of activation for additional internal timing of movement execution (when). This additive effect of what and when provides strong evidence for the interdependence of both components on the neuronal level, as suggested by Krieghoff et al. (2009). This characteristic is well in line with the current view of the aMCC as a brain area crucially involved in various cognitive control functions (cf. Shackman et al. 2011). On one hand, this area accounts for conflict processing, that is, conflict monitoring (Botvinick et al. 2004; Carter and van Veen 2007) and conflict anticipation (Murtha et al. 1996; Brown and Braver 2005). Furthermore, the aMCC is essentially involved in higher order cognitive processes, such as reward-guided action selection (Bush et al. 2002; Rushworth et al. 2004; Walton et al. 2004) and the implementation of task sets (Dosenbach et al. 2006, 2007). Recently, Aarts et al. (2008) provided evidence for a more fundamental role of the aMCC in anticipatory control, that is, preparatory activity reflecting control adjustments in relation to an upcoming task, independent of anticipated conflict or error likelihood. Our findings that the very simple task of choosing a finger to flex and the moment to do so (without any anticipation of conflict or reward) likewise evoked robust activation in the aMCC supports this view. Furthermore, anticipation should not be any factor in the Free-condition as there is no upcoming event but rather the intentional self-specified decision to perform a movement. We would thus conclude that the role of the aMCC may not necessarily be related to anticipatory control, even though there is always the implicit expectation of sensory consequences in intentional action (Fink et al. 1999). We would thus interpret our findings as evidence for the view (Paus 2001) that the aMCC is situated in a strategic position to regulate the interaction between high-level cognition and motor control, which is also supported by the current knowledge on the structural and functional organization of the primate anterior cingulate cortex (Hoshi et al. 2005). Overall, our results thus strongly suggest a key role of the aMCC in intentional motor control. Its putative function as a hub for the implementation of intentions into actions in turn may provide the foundation for other cognitive functions frequently associated with this area.
Behavioral Planning
“Willed action” is typically related to the PFC (Frith et al. 1991; Hyder et al. 1997) as it was suggested by most of the studies on intentional action mentioned above. Studies in nonhuman primates showed that lateral PFC is primarily involved in behavioral planning and less in the specification of motor aspects of behavior (Tanji et al. 2007). Likewise, the lateral PFC in humans preferentially contributes to attentional and working memory processes involved in the preparation rather than the initiation of the actual movements (Wiese et al. 2005, 2006). Using electroencephalography and fMRI in one study, Bortoletto and Cunnington (2010) directly demonstrated that the lateral PFC plays an important role in determining the timing for movement initiation 1 s prior to self-initiated movements. In their comprehensive review, Tanji and Hoshi (2008) presented evidence for a functional heterogeneity within the lateral PFC. Generally speaking, the ventrolateral part is associated with “first-order” executive processes, such as active retrieval and selection of information, whereas the DLPFC is more involved in “higher order” executive functions, such as monitoring, integration, and manipulation of information. In our study, the what and when of intentional action initiation recruited mainly the DLPFC, which is in line with the concept of intentional actions being rooted in those higher order executive functions. However, no convergence of what and when of self-initiated movements was found within the DLPFC further supporting the notion of a functional heterogeneous DLPFC.
Movement Intentions and Motor Awareness
Intentional movement initiation reliably activates the inferior parietal cortex (cf. Deiber et al. 1999; Jenkins et al. 2000), which seems to be a critical node for the representation of actions and intentions to act (Tunik et al. 2007). In our study, movement selection (what) involved bilateral dPMC and IPS, which conjointly are known as the dorsal attention network (Fox et al. 2006; Corbetta et al. 2008) and are closely linked to control of hand movements (Filimon 2010) and motor imagery (Lorey et al. 2011). Recently, Gallivan et al. (2011) showed that specific movement intentions can be predicted by the spatial activity patterns in these areas. Moreover, although lesions in the inferior parietal cortex do not entail difficulties initiating voluntary actions, they seem to impair awareness of movement intentions (Sirigu et al. 2004). Conversely, direct electrical stimulation of the IPL triggered the strong intention to move a body part and with increased stimulation intensity led to illusory movement awareness (Desmurget et al. 2009). Stimulation on the dPMC, on the other hand, evoked movements without movement intention or motor awareness. Thus, in context of intentional action, the IPL/IPS seem to contribute to movement intention and motor awareness, whereas the dPMC is closer to movement execution.
A Medial and a Lateral Premotor System
Over 2 decades ago, Goldberg (1985) distinguished 2 separate premotor systems based on phylogenetic characteristics, structural connectivity pattern, and functional properties of the areas involved. A medial system consisting of SMA and basal ganglia was associated with internal movement generation. In contrast, external movement generation was associated with a lateral premotor system consisting of the lateral premotor cortex and the cerebellum. In our study, we focused on internal movement generation and found both the medial and the lateral system involved in this process. In particular, we observed rostral aspects of the bilateral dPMC activity in the internal selection (what) of movements in comparison with external (reactive) movement generation. The cerebellum (Lobule VIIa Crus I, Lobule VI) as the subcortical part of the lateral system was involved in both internal and external movement generation with the left hemisphere showing increased activity for internal timing (when). Likewise, the SMA proper as center of this medial system was involved in both internal and external generation of movements, while its superior aspect moreover increased activity with internal movement timing (when). Furthermore, while absent in external movement generation, the pre-SMA was involved in internal selection (what), whereas the basal ganglia, namely globus pallidus and anterior putamen, were exclusively activated by internal movement timing (when). In contrast, only aMCC was additively recruited by internal movement selection (what) and timing (when) without any activity during the generation of reactive movements. Taken together, our study thus adds evidence for 2 essential modifications of the Goldberg model. First, the lateral system is not exclusively involved in external movement generation but seems to be rather linked to movement selection (what) per se. Second, as proposed earlier (cf. Haggard 2008), the medial premotor system consists of the pre-SMA together with anterior putamen and globus pallidus subcortically. Possibly mediated by aMCC, this medial system seems to play a key role in internal movement generation especially if both what and when of a movement are internally specified.
Conclusion
In this study, we examined intentional movement initiation and directly demonstrated the essential involvement of the aMCC both in internal selection (what) and timing (when) of movements. The pre-SMA is specifically associated with selective motor initiation (what), in which the dPMC seems to account for movement execution. Internal timing (when) relies crucially on bilateral anterior putamen and globus pallidus, which together with the pre-SMA are known as the medial premotor system. Moreover, intentional movement timing seems to rely on a well-distributed timing network comprised of bilateral area 44 and anterior insula for cognitive time processing and SMA, basal ganglia, and cerebellum related to more automated timing of movement execution. In internal movement generation, IPL/ IPS are closely related to movement intention and motor awareness. Finally, we provide additional evidence for a fundamental role of the aMCC in initiating and implementing intentional motor control and thereby translating intentions into actions.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
Human Brain Project/Neuroinformatics Research (National Institute of Biomedical Imaging and Bioengineering, National Institute of Neurological Disorders and Stroke, and National Institute of Mental Health to K.A. and K.Z.); Human Brain Project (grant R01-MH074457-01A1 to S.B.E.); and Helmholtz Initiative on SystemsBiology (Human Brain Model; K.Z. and S.B.E.).
Supplementary Material
Acknowledgments
Conflict of Interest : None declared.
References
- Aarts E, Roelofs A, van Turennout M. Anticipatory activity in anterior cingulate cortex can be independent of conflict and error likelihood. J Neurosci. 2008;28:4671–4678. doi: 10.1523/JNEUROSCI.4400-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HBM, Zilles K. Broca's region revisited: cytoarchitecture and intersubject variability. J Comp Neurol. 1999;412:319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Amunts K, Weiss PH, Mohlberg H, Pieperhoff P, Eickhoff S, Gurd JM, Marshall JC, Shah NJ, Fink GR, Zilles K. Analysis of neural mechanisms underlying verbal fluency in cytoarchitectonically defined stereotaxic space—the roles of Brodmann areas 44 and 45. Neuroimage. 2004;22:42–56. doi: 10.1016/j.neuroimage.2003.12.031. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Ball T, Schreiber A, Feige B, Wagner M, Lucking CH, Kristeva-Feige R. The role of higher-order motor areas in voluntary movement as revealed by high-resolution EEG and fMRI. Neuroimage. 1999;10:682–694. doi: 10.1006/nimg.1999.0507. [DOI] [PubMed] [Google Scholar]
- Bortoletto M, Cunnington R. Motor timing and motor sequencing contribute differently to the preparation for voluntary movement. Neuroimage. 2010;49:3338–3348. doi: 10.1016/j.neuroimage.2009.11.048. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brass M, Haggard P. The what, when, whether model of intentional action. Neuroscientist. 2008;14:319–325. doi: 10.1177/1073858408317417. [DOI] [PubMed] [Google Scholar]
- Brass M, Haggard P. The hidden side of intentional action: the role of the anterior insular cortex. Brain Struct Funct. 2010;214:603–610. doi: 10.1007/s00429-010-0269-6. [DOI] [PubMed] [Google Scholar]
- Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307:1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci U S A. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn Affect Behav Neurosci. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Caspers S, Eickhoff SB, Geyer S, Scheperjans F, Mohlberg H, Zilles K, Amunts K. The human inferior parietal lobule in stereotaxic space. Brain Struct Funct. 2008;212:481–495. doi: 10.1007/s00429-008-0195-z. [DOI] [PubMed] [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K. The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. Neuroimage. 2006;33:430–448. doi: 10.1016/j.neuroimage.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Zilles K, Mohlberg H, Schleicher A, Fink GR, Armstrong E, Amunts K. Cytoarchitectonic identification and probabilistic mapping of two distinct areas within the anterior ventral bank of the human intraparietal sulcus. J Comp Neurol. 2006;495:53–69. doi: 10.1002/cne.20849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard PA, Paus T. The primary motor and premotor areas of the human cerebral cortex. Neuroscientist. 2006;12:143–152. doi: 10.1177/1073858405284255. [DOI] [PubMed] [Google Scholar]
- Chouinard PA, Paus T. What have we learned from “perturbing” the human cortical motor system with transcranial magnetic stimulation? Front Hum Neurosci. 2010;4:173. doi: 10.3389/fnhum.2010.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Coxon JP, Stinear CM, Byblow WD. Stop and go: the neural basis of selective movement prevention. J Cogn Neurosci. 2009;21:1193–1203. doi: 10.1162/jocn.2009.21081. [DOI] [PubMed] [Google Scholar]
- Cunnington R. The supplementary motor area and the preparation and control of voluntary movement: studies of high-field event-related fMRI. Aust J Psychol. 2005;57:22–23. [Google Scholar]
- Cunnington R, Windischberger C, Deecke L, Moser E. The preparation and execution of self-initiated and externally-triggered movement: a study of event-related fMRI. Neuroimage. 2002;15:373–385. doi: 10.1006/nimg.2001.0976. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Deecke L, Moser E. The preparation and readiness for voluntary movement: a high-field event-related fMRI study of the Bereitschafts-BOLD response. Neuroimage. 2003;20:404–412. doi: 10.1016/s1053-8119(03)00291-x. [DOI] [PubMed] [Google Scholar]
- Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP. Internal vs external generation of movements: differential neural pathways involved in bimanual coordination performed in the presence or absence of augmented visual feedback. Neuroimage. 2003;19:764–776. doi: 10.1016/s1053-8119(03)00148-4. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Honda M, Ibanez V, Sadato N, Hallett M. Mesial motor areas in self-initiated versus externally triggered movements examined with fMRI: effect of movement type and rate. J Neurophysiol. 1999;81:3065–3077. doi: 10.1152/jn.1999.81.6.3065. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Reilly KT, Richard N, Szathmari A, Mottolese C, Sirigu A. Movement intention after parietal cortex stimulation in humans. Science. 2009;324:811–813. doi: 10.1126/science.1169896. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage. 2009;46:39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Amunts K, Mohlberg H, Zilles K. The human parietal operculum. II. Stereotaxic maps and correlation with functional imaging results. Cereb Cortex. 2006;16:268–279. doi: 10.1093/cercor/bhi106. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage. 2006;32:570–582. doi: 10.1016/j.neuroimage.2006.04.204. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, Amunts K. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 2007;36:511–521. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Schleicher A, Zilles K, Amunts K. The human parietal operculum. I. Cytoarchitectonic mapping of subdivisions. Cereb Cortex. 2006;16:254–267. doi: 10.1093/cercor/bhi105. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Filimon F. Human cortical control of hand movements: parietofrontal networks for reaching, grasping, and pointing. Neuroscientist. 2010;16:388–407. doi: 10.1177/1073858410375468. [DOI] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Halligan PW, Frith CD, Driver J, Frackowiak RS, Dolan RJ. The neural consequences of conflict between intention and the senses. Brain. 1999;122(Pt 3):497–512. doi: 10.1093/brain/122.3.497. [DOI] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois-Brosseau FE, Martinu K, Strafella AP, Petrides M, Simard F, Monchi O. Basal ganglia and frontal involvement in self-generated and externally-triggered finger movements in the dominant and non-dominant hand. Eur J Neurosci. 2009;29:1277–1286. doi: 10.1111/j.1460-9568.2009.06671.x. [DOI] [PubMed] [Google Scholar]
- Frith CD, Friston K, Liddle PF, Frackowiak RSJ. Willed action and the prefrontal cortex in man:a study with PET. Proc R Soc Lond B Biol Sci. 1991;244:241–246. doi: 10.1098/rspb.1991.0077. [DOI] [PubMed] [Google Scholar]
- Gallivan JP, McLean DA, Valyear KF, Pettypiece CE, Culham JC. Decoding action intentions from preparatory brain activity in human parieto-frontal networks. J Neurosci. 2011;31:9599–9610. doi: 10.1523/JNEUROSCI.0080-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer S. Adv Anat Embryol Cell Biol. 2004. The microstructural border between the motor and the cognitive domain in the human cerebral cortex. 174(I--VIII):1--89. [DOI] [PubMed] [Google Scholar]
- Geyer S, Ledberg A, Schleicher A, Kinomura S, Schormann T, Burgel U, Klingberg T, Larsson J, Zilles K, Roland PE. Two different areas within the primary motor cortex of man. Nature. 1996;382:805–807. doi: 10.1038/382805a0. [DOI] [PubMed] [Google Scholar]
- Geyer S, Schleicher A, Zilles K. Areas 3a, 3b, and 1 of human primary somatosensory cortex 1. Microstructural organization and interindividual variability. Neuroimage. 1999;10:63–83. doi: 10.1006/nimg.1999.0440. [DOI] [PubMed] [Google Scholar]
- Geyer S, Schormann T, Mohlerg H, Zilles K. Areas 3a, 3b, and 1 of human primary somatosensory cortex. Part 2. Spatial normalization to standard anatomical space. Neuroimage. 2000;11:684–696. doi: 10.1006/nimg.2000.0548. [DOI] [PubMed] [Google Scholar]
- Goldberg G. Supplementary motor area structure and function: review and hypotheses. Behav Brain Sci. 1985;8:567–588. [Google Scholar]
- Grefkes C, Eickhoff SB, Nowak DA, Dafotakis M, Fink GR. Dynamic intra- and interhemispheric interactions during unilateral and bilateral hand movements assessed with fMRI and DCM. Neuroimage. 2008;41:1382–1394. doi: 10.1016/j.neuroimage.2008.03.048. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Geyer S, Schormann T, Roland P, Zilles K. Human somatosensory area 2: observer-independent cytoarchitectonic mapping, interindividual variability, and population map. Neuroimage. 2001;14:617–631. doi: 10.1006/nimg.2001.0858. [DOI] [PubMed] [Google Scholar]
- Haggard P. Human volition: towards a neuroscience of will. Nat Rev Neurosci. 2008;9:934–946. doi: 10.1038/nrn2497. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Lee RR, Boyd LA, Rapcsak SZ, Knight RT. Does the representation of time depend on the cerebellum? Effect of cerebellar stroke. Brain. 2004;127:561–574. doi: 10.1093/brain/awh065. [DOI] [PubMed] [Google Scholar]
- Henson R, Rugg MD, Friston KJ. The choice of basis functions in event-related fMRI. Neuroimage. 2001;13:S149. [Google Scholar]
- Hoshi E, Sawamura H, Tanji J. Neurons in the rostral cingulate motor area monitor multiple phases of visuomotor behavior with modest parametric selectivity. J Neurophysiol. 2005;94:640–656. doi: 10.1152/jn.01201.2004. [DOI] [PubMed] [Google Scholar]
- Hyder F, Phelps EA, Wiggins CJ, Labar KS, Blamire AM, Shulman RG. “Willed action”: a functional MRI study of the human prefrontal cortex during a sensorimotor task. Proc Natl Acad Sci U S A. 1997;94:6989–6994. doi: 10.1073/pnas.94.13.6989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi M, Jenkins H, Brown RG, Marsden CD, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood-flow with PET and movement-related potentials in normal and Parkinsons-disease subjects. Brain. 1995;118:913–933. doi: 10.1093/brain/118.4.913. [DOI] [PubMed] [Google Scholar]
- Jankowski J, Scheef L, Huppe C, Boecker H. Distinct striatal regions for planning and executing novel and automated movement sequences. Neuroimage. 2009;44:1369–1379. doi: 10.1016/j.neuroimage.2008.10.059. [DOI] [PubMed] [Google Scholar]
- Jantzen KJ, Steinberg FL, Kelso JA. Brain networks underlying human timing behavior are influenced by prior context. Proc Natl Acad Sci U S A. 2004;101:6815–6820. doi: 10.1073/pnas.0401300101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins IH, Jahanshahi M, Jueptner M, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements II. The effect of movement predictability on regional cerebral blood flow. Brain. 2000;123:1216–1228. doi: 10.1093/brain/123.6.1216. [DOI] [PubMed] [Google Scholar]
- Josephs O, Henson RN. Event-related functional magnetic resonance imaging: modelling, inference and optimization. Philos Trans R Soc Lond B Biol Sci. 1999;354:1215–1228. doi: 10.1098/rstb.1999.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan IP, Thompson-Schill SL. Selection from perceptual and conceptual representations. Cogn Affect Behav Neurosci. 2004;4:466–482. doi: 10.3758/cabn.4.4.466. [DOI] [PubMed] [Google Scholar]
- Kiebel S, Holmes AP. The general linear model. In: Frackowiak RSJ, Friston KJ, Frith CD, Dolan RJ, Price CJ, Ashburner J, Penny WD, Zeki S, editors. Human brain function. 2nd ed. San Diego (CA): Academic Press; 2003. pp. 725–760. [Google Scholar]
- Kornhuber HH, Deecke L. Hirnpotentialveränderungen bei Willkürbewegungen und passiven Bewegungen des Menschen: Bereitschaftspotential und reafferente Potentiale. Pflugers Arch. 1965;284:1–17. [PubMed] [Google Scholar]
- Krieghoff V, Brass M, Prinz W, Waszak F. Dissociating what and when of intentional actions. Front Hum Neurosci. 2009;3:3. doi: 10.3389/neuro.09.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau HC, Rogers RD, Haggard P, Passingham RE. Attention to intention. Science. 2004;303:1208–1210. doi: 10.1126/science.1090973. [DOI] [PubMed] [Google Scholar]
- Lau HC, Rogers RD, Passingham RE. Dissociating response selection and conflict in the medial frontal surface. Neuroimage. 2006;29:446–451. doi: 10.1016/j.neuroimage.2005.07.050. [DOI] [PubMed] [Google Scholar]
- Lau HC, Rogers RD, Ramnani N, Passingham RE. Willed action and attention to the selection of action. Neuroimage. 2004;21:1407–1415. doi: 10.1016/j.neuroimage.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Miall RC. Distinct systems for automatic and cognitively controlled time measurement: evidence from neuroimaging. Curr Opin Neurobiol. 2003;13:250–255. doi: 10.1016/s0959-4388(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Lorey B, Pilgramm S, Bischoff M, Stark R, Vaitl D, Kindermann S, Munzert J, Zentgraf K. Activation of the parieto-premotor network is associated with vivid motor imagery—a parametric fMRI study. PLoS One. 2011;6:e20368. doi: 10.1371/journal.pone.0020368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makuuchi M. Is Broca's area crucial for imitation? Cereb Cortex. 2005;15:563–570. doi: 10.1093/cercor/bhh157. [DOI] [PubMed] [Google Scholar]
- Malikovic A, Amunts K, Schleicher A, Mohlberg H, Eickhoff SB, Wilms M, Palomero-Gallagher N, Armstrong E, Zilles K. Cytoarchitectonic analysis of the human extrastriate cortex in the region of V5/MT+: a probabilistic, stereotaxic map of area h0c5. Cereb Cortex. 2007;17:562–574. doi: 10.1093/cercor/bhj181. [DOI] [PubMed] [Google Scholar]
- Mayville JM, Jantzen KJ, Fuchs A, Steinberg FL, Kelso JA. Cortical and subcortical networks underlying syncopated and synchronized coordination revealed using fMRI. Functional magnetic resonance imaging. Hum Brain Mapp. 2002;17:214–229. doi: 10.1002/hbm.10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Simmonds DJ. Response inhibition and response selection: two sides of the same coin. J Cogn Neurosci. 2008;20:751–761. doi: 10.1162/jocn.2008.20500. [DOI] [PubMed] [Google Scholar]
- Mueller VA, Brass M, Waszak F, Prinz W. The role of the preSMA and the rostral cingulate zone in internally selected actions. Neuroimage. 2007;37:1354–1361. doi: 10.1016/j.neuroimage.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Murtha S, Chertkow H, Beauregard M, Dixon R, Evans A. Anticipation causes increased blood flow to the anterior cingulate cortex. Hum Brain Mapp. 1996;4:103–112. doi: 10.1002/(SICI)1097-0193(1996)4:2<103::AID-HBM2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Nachev P, Wydell H, O'Neill K, Husain M, Kennard C. The role of the pre-supplementary motor area in the control of action. Neuroimage. 2007;36:T155–T163. doi: 10.1016/j.neuroimage.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- O'Boyle DJ, Freeman JS, Cody FW. The accuracy and precision of timing of self-paced, repetitive movements in subjects with Parkinson's disease. Brain. 1996;119(Pt 1):51–70. doi: 10.1093/brain/119.1.51. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. Assessment and analysis of handedness—Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Vogt BA, Schleicher A, Mayberg HS, Zilles K. Receptor architecture of human cingulate cortex: evaluation of the four-region neurobiological model. Hum Brain Mapp. 2009;30:2336–2355. doi: 10.1002/hbm.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Penny WD, Holmes AP. Random effects analysis. In: Frackowiak RSJ, Friston KJ, Frith CD, Dolan RJ, Price CJ, Ashburner J, Penny WD, Zeki S, editors. Human brain function. 2nd ed. San Diego (CA): Academic Press; 2003. pp. 843–850. [Google Scholar]
- Picard N, Strick PL. Activation of the supplementary motor area (SMA) during performance of visually guided movements. Cereb Cortex. 2003;13:977–986. doi: 10.1093/cercor/13.9.977. [DOI] [PubMed] [Google Scholar]
- Playford ED, Jenkins IH, Passingham RE, Nutt J, Frackowiak RS, Brooks DJ. Impaired mesial frontal and putamen activation in Parkinson's disease: a positron emission tomography study. Ann Neurol. 1992;32:151–161. doi: 10.1002/ana.410320206. [DOI] [PubMed] [Google Scholar]
- Romo R, Schultz W. Neuronal-activity preceding self-initiated or externally timed arm movements in area-6 of monkey cortex. Exp Brain Res. 1987;67:656–662. doi: 10.1007/BF00247297. [DOI] [PubMed] [Google Scholar]
- Rottschy C, Eickhoff SB, Schleicher A, Mohlberg H, Kujovic M, Zilles K, Amunts K. Ventral visual cortex in humans: cytoarchitectonic mapping of two extrastriate areas. Hum Brain Mapp. 2007;28:1045–1059. doi: 10.1002/hbm.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci. 2004;8:410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Scheperjans F, Eickhoff SB, Homke L, Mohlberg H, Hermann K, Amunts K, Zilles K. Probabilistic maps, morphometry, and variability of cytoarchitectonic areas in the human superior parietal cortex. Cereb Cortex. 2008;18:2141–2157. doi: 10.1093/cercor/bhm241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperjans F, Hermann K, Eickhoff SB, Amunts K, Schleicher A, Zilles K. Observer-independent cytoarchitectonic mapping of the human superior parietal cortex. Cereb Cortex. 2008;18:846–867. doi: 10.1093/cercor/bhm116. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirigu A, Daprati E, Ciancia S, Giraux P, Nighoghossian N, Posada A, Haggard P. Altered awareness of voluntary action after damage to the parietal cortex. Nat Neurosci. 2004;7:80–84. doi: 10.1038/nn1160. [DOI] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage. 2011;56:1655–1665. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- Tanji J, Hoshi E. Role of the lateral prefrontal cortex in executive behavioral control. Physiol Rev. 2008;88:37–57. doi: 10.1152/physrev.00014.2007. [DOI] [PubMed] [Google Scholar]
- Tanji J, Shima K, Mushiake H. Concept-based behavioral planning and the lateral prefrontal cortex. Trends Cogn Sci. 2007;11:528–534. doi: 10.1016/j.tics.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Thaler DE, Rolls ET, Passingham RE. Neuronal-activity of the supplementary motor area (SMA) during internally and externally triggered wrist movements. Neurosci Lett. 1988;93:264–269. doi: 10.1016/0304-3940(88)90093-6. [DOI] [PubMed] [Google Scholar]
- Tunik E, Rice NJ, Hamilton A, Grafton ST. Beyond grasping: representation of action in human anterior intraparietal sulcus. Neuroimage. 2007;36(Suppl 2):T77–T86. doi: 10.1016/j.neuroimage.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Subprocesses of performance monitoring: a dissociation of error processing and response competition revealed by event-related fMRI and ERPs. Neuroimage. 2001;14:1387–1401. doi: 10.1006/nimg.2001.0935. [DOI] [PubMed] [Google Scholar]
- van Eimeren T, Wolbers T, Munchau A, Buchel C, Weiller C, Siebner HR. Implementation of visuospatial cues in response selection. Neuroimage. 2006;29:286–294. doi: 10.1016/j.neuroimage.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Walton ME, Devlin JT, Rushworth MF. Interactions between decision making and performance monitoring within prefrontal cortex. Nat Neurosci. 2004;7:1259–1265. doi: 10.1038/nn1339. [DOI] [PubMed] [Google Scholar]
- Wiener M, Turkeltaub P, Coslett HB. The image of time: a voxel-wise meta-analysis. Neuroimage. 2010;49:1728–1740. doi: 10.1016/j.neuroimage.2009.09.064. [DOI] [PubMed] [Google Scholar]
- Wiese H, Stude P, Nebel K, Forsting M, de Greiff A. Prefrontal cortex activity in self-initiated movements is condition-specific, but not movement-related. Neuroimage. 2005;28:691–697. doi: 10.1016/j.neuroimage.2005.06.044. [DOI] [PubMed] [Google Scholar]
- Wiese H, Tonnes C, de Greiff A, Nebel K, Diener HC, Stude P. Self-initiated movements in chronic prefrontal traumatic brain injury: an event-related functional MR1 study. Neuroimage. 2006;30:1292–1301. doi: 10.1016/j.neuroimage.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Witt ST, Laird AR, Meyerand ME. Functional neuroimaging correlates of finger-tapping task variations: an ALE meta-analysis. Neuroimage. 2008;42:343–356. doi: 10.1016/j.neuroimage.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Wu T, Long X, Wang L, Hallett M, Zang Y, Li K, Chan P. Functional connectivity of cortical motor areas in the resting state in Parkinson's disease. Hum Brain Mapp. 2011;32:1443–1457. doi: 10.1002/hbm.21118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JX, Feng CM, Fox PT, Gao JH, Tan LH. Is left inferior frontal gyrus a general mechanism for selection? Neuroimage. 2004;23:596–603. doi: 10.1016/j.neuroimage.2004.06.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.