Abstract
Essential oils extracted from the foliage of Mentha longifolia (L.) (Lamiales: Lamiaceae) and Pulicaria gnaphalodes Ventenat (Asterales: Asteraceae), and flowers of Achillea wilhelmsii C. Koch (Asterales: Asteraceae) were tested in the laboratory for volatile toxicity against two storedproduct insects, the flour beetle, Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) and the cowpea weevil, Callosobruchus maculatus F. (Coleoptera: Bruchidae). The chemical composition of the isolated oils was examined by gas chromatography-mass spectrometry. InM longifolia, the major compounds were piperitenon (43.9%), tripal (14.3%), oxathiane (9.3%), piperiton oxide (5.9%), and d-limonene (4.3%). In P. gnaphalodes, the major compounds were chrysanthenyl acetate (22.38%), 2L -4L-dihydroxy eicosane (18.5%), verbenol (16.59%), dehydroaromadendrene (12.54%), β-pinen (6.43%), and 1,8 cineol (5.6%). In A. wilhelmsii, the major compounds were 1,8 cineole (13.03%), caranol (8.26%), alpha pinene (6%), farnesyl acetate (6%), and p-cymene (6%). C maculatus was more susceptible to the tested plant products than T castaneum. The oils of the three plants displayed the same insecticidal activity against C. maculatus based on LC50 values (between 1.54µl/L air in P. gnaphalodes, and 2.65 µl/L air in A. wilhelmsii). While the oils of A. wilhelmsii and M. longifolia showed the same strong insecticidal activity against T. castaneum (LC50 = 10.02 and 13.05 µl/L air, respectively), the oil of P. gnaphalodes revealed poor activity against the insect (LC50 = 297.9 µl/L air). These results suggested that essential oils from the tested plants could be used as potential control agents for stored-product insects.
Keywords: fumigant toxicity, gas chromatography-mass spectrometry, mono terpenoids
Introduction
The global pest-harvest grain losses by insect damage and other bio-agents range from 10% to 40% (Papachristos and Stamopoulos 2002). Chemicals largely used as pesticides in crop protection could have undesirable effects such as ozone depletion, environmental pollution, toxicity to non-target organisms, pest resistance, pesticide residues, and direct toxicity to users (Isman 2006). With heightened concern for environmental problems and human health, the search for readily biodegradable and environmentally friendly insecticides is of interest among scientists (Shaaya et al. 1997; Isman 2000). Plants offer an alternative source of insectcontrol agents because they contain a range of bioactive chemicals, many of which are selective and have little or no harmful effect on non-target organisms and the environment (Shaaya et al. 1997; Rajendran and Sriranjini 2008).
The genus Mentha belongs to the family Lamiaceae (Labiatae), and consists of about 25-30 species, most of which are found in temperate regions of Eurasia, Australia and South Africa (Lange and Croteau 1999). Mentha longifolia (L.) (Lamiaceae), commonly known as wild mint, is a perennial herb that can grow 1–2 m high. Various biological activities have been reported for some species of Mentha, such as antibacterial (Oyedeji and Afolayan 2006; Hajlaoui et al. 2008), antifungal (Bouchra et al. 2003), and insecticidal properties (Franzios et al. 1997; Lamiri et al. 2001; Pavela 2005; Saljoqi et al. 2006). The oils of M. longifolia are known to contain numerous monoterpenoids with piperitone oxide, piperitone, piperitenone, pulegone, d-limonene, carvone, menthone, âcaryophyllene, 1,8-Cineole, and menthol as dominating compounds; however, there have been some variations in the constituents of this oil from different countries, and a chemogeographical variation has been observed in essential oil composition of this species (Oyedeji and Afolayan 2006).
The Pulicaria genus belongs to the family Compositae (Asteraceae), tribe Inuleae, which contains more than 77 species that are widely distributed throughout Asia, Europe and Africa (Anderberg 1991). The chemical investigation of the genus showed the presence of terpenes such as monoterpenes and oxygenated monoterpenes (Weyerstahl et al. 1999), diterpenes (Muhammad et al. 1992), and sesquiterpenes (Weyerstahl et al. 1999; Dendougui et al. 2000). Various biological activities have been reported for some species of Pulicaria, such as antibacterial, antifungal (El-Kamali et al. 1998; Bahman et al. 2002; Liu et al. 2010), and insecticidal properties (Ross et al. 1997; Dubaie and El-Khulaidi 2005).
The herb Achillea, which belongs to the family Compositae (Asteraceae), is a genus with more than 100 species around the world. These plants are medicinal perennial rhizomous herbs, native to Europe and Western Asia, but also found in Australia, New Zealand, and North America (Chevallier 1996). Previous research has investigated the chemical composition of the essential oil of Achillea, such as its antibacterial (Barel et al. 1991; Magiatis et al. 2002) and insecticidal properties (Calmasur et al. 2006; Jovanovic et al. 2007; Magdy and Samir 2008). Previous work showed that the essential oil extracted from Achillea wilhelmsii C. Koch (Asteraceae) leaves exhibited volatile toxicity to Sitophilus granarius and Tribolium confusum (Calmasur et al. 2006).
In the present study, the chemical components of essential oils from M. longifolia L. (Lamiaceae) and P. gnaphalodes vent. (Asteraceae) aerial parts, and A. wilhelmsii C. Koch (Asteraceae) flowers, were determined, and the insecticidal activity of them was tested against the adult stages of the stored-products pests, Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) and Callosobruchus maculatus F. (Coleoptera: Bruchidae). No study has been reported concerning the activity of the three test oils as fumigants against these stored product insects.
Materials and Methods
Insect material
C. maculatus and T. castaneum were reared in plastic containers (20 cm length, 14 cm width, and 8 cm height, covered by a fine mesh cloth for ventilation) containing bean grain and wheat flour mixed with yeast (10:1, w/w), respectively. The culture was maintained in the dark, in a growth chamber set at 27±1°C and 65±5 relative humidity. All experiments were carried out under the same environmental conditions.
Plants and essential oils
Aerial parts (foliage) of M. longifolia and P. gnaphalodes, and flowers of A. wilhelmsii, were collected respectively in Masabi, Sarayan (33° 51′ N, 58° 31′ E; 1500 m a.s.l.), Birjand suburbs (32° 50′ N, 59° 13′ E; 1500 m asl) and Sade, Ghaenat (33° 1′ N, 59° 14 ′ E; 1900 m asl), located in South Khorasan province, Iran, from May to July, 2009. The plant material was dried naturally on laboratory benches at room temperature (23– 27 °C) until crisp. The dried material was stored at -24 °C, and then hydrodistilled to extract its essential oil. Essential oil was extracted from the plant samples using a Clevenger-type apparatus where the plant material is subjected to hydrodistillation. Conditions of extraction were 50 g of samples, 1:10 plant material/water volume ratio, and a four-hour distillation. The oil was dehydrated with anhydrous sodium sulphate (10 min), and immediately stored in airtight glassware in a refrigerator at 4 °C.
Gas chromatography-mass spectrometry
The essential oils were analyzed on a gas Chromatograph mass spectrometer (Shimadzu -17A-QP5050, Japan). The gas chromatography column was a super CP-SiI 5CB capillary column (50 m × 0.32 mm ID, 0.25 pm film thickness). The column oven temperature was set at 70 °C for 1 min, increased to 100 °C at a rate of 1.5 °C/min, increased to 180 °C at a rate of 4 °C/min, and held at 180 °C for 1 min. Next, it was increased to 200 °C at a rate of 10 °C/min, increased to 250 °C at a rate of 2.5 °C/min, and held at 250 °C for 5 min. Injector and detector temperatures were 280 °C and 300 °C respectively. The gas chromatography mass analysis was carried out with the same characteristics as used in gas chromatography. The ionization energy was 70 eV, with a scan time of 1 sec, and a mass range of 40–300 amu. Unknown essential oil was identified by comparing its gas chromatography retention time to that of known compounds, and its mass spectra to known compounds or published spectra.
Fumigant toxicity
To determine the fumigant toxicity of the oil, glass vials (volume 70 mL) were prepared, each containing 10 adults (1–7 days old of undefined sex) of each species. Filter papers (2 cm diameter) were prepared by adding 5, 15, or 30 µl of oil to individual papers (without using any solvent). Then, each filter paper was attached to the under-surface of a screw cap and the cap was screwed tightly on the vial in order to generate concentrations of 71.43, 214.29, and 428.57 µl/L air, respectively. Each concentration and control was replicated five times. Mortality was determined 3, 6, 9, 12, and 24 hours after exposure. When no signs of leg or antennal movement were observed, insects were considered dead.
Another experiment was designed to assess insect mortality using 50% lethal doses (LC50). The concentrations of the essential oils were chosen based on range-finding tests (to cause mortality between 5 and 90%). For each bioassay, five different concentrations, each with five replicates and ten individuals per replicate, were used. The volumes of the glass vials were 300 mL and 500 mL for T. castaneum and C. maculatus, respectively. The dead and living insects in each bottle were counted 24 hours after initial exposure to the essential oil. The mortality was determined as described in the previous experiment. The treatment bottles were monitored for 48 hours after recording the data, and no affected insect recovered. Data obtained from each dose response bioassay were subjected to probit analysis. LC50 values were determined by log-probit regression using SPSS 16.0 for Windows.
Results and Discussion
Chemical composition of essential oils
The chemical composition of the essential oils of the studied plants, M. longifolia and P. gnaphalodes aerial parts, and A. wilhelmsii flowers, are presented in Table 1.
Table 1.
Chemical composition of essential oils of Mentha longifolia and Pulicaria gnaphalodes vegetative parts and Achillea wilhelmsii flowers.
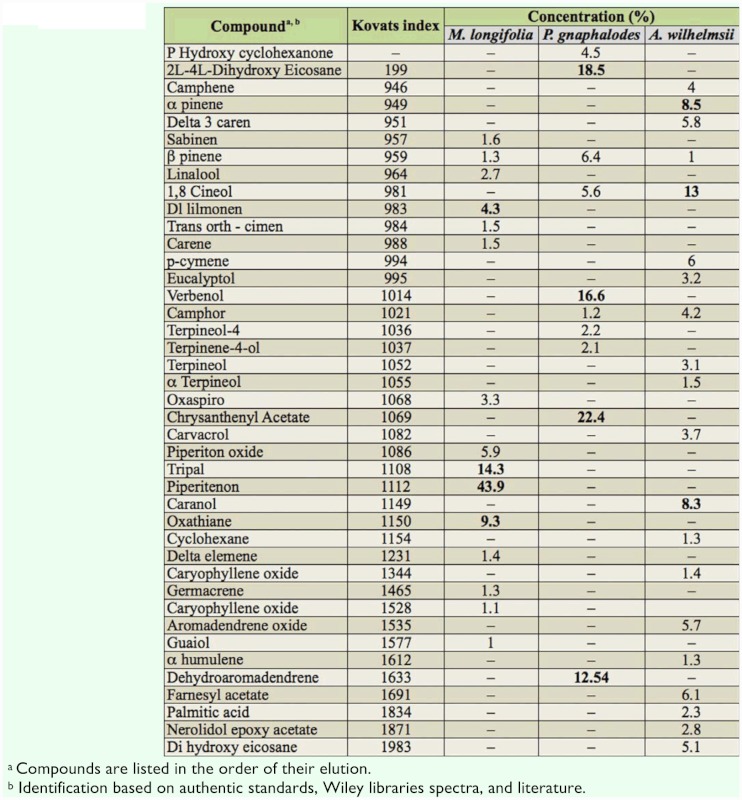
In M. longifolia, the major compounds were piperitenone (43.9%), tripal (14.3%), oxathiane (9.3%), piperitone oxide (5.9%), and d-limonen (4.3%). The major compounds of the Iranian M. longifolia oil were piperitone (43.9%), limonene (13.5%), and transpiperitol (12.9%) (Rasooli and Rezaei 2002). However, identification of piperitone as the major compound in the M. longifolia oil is in sharp contrast to other reports where the oil had carvone (Monfared et al. 2002) or ciscarveol (Zeinali et al. 2005) as the major component.
The analysis of P. gnaphalodes essential oil revealed that chrysanthenyl acetate (22.38%), 2L -4L-dihydroxy eicosane (18.5%), verbenol (16.59%), dehydroaromadendrene (12.54%), β-pinen (6.43%), and 1,8 cineol (5.6%) were the main products. Weyerstahl et al. (1999) reported the oil prepared from aerial parts of P. gnaphalodes collected in the Elbrus mountains, Tehran province, Iran contained about 65% monoterpenes, with α-pinene (34%) and 1,8-cineole (12%) as main compounds, and β-pinen (0.6%), alloaromadendreneand (0.4%) and trans-verbenol (0.2%) as minor compounds. Also, cischrysanthenol (oxidized monoterpenes) (2.3%) and its esters cis-chrysanthenyl formate (2.9%), cis-chrysanthenyl acetate (0.2%), chrysanthenone (monoterpene) (2%), and its related product isochrysanthenone (0.7%) were identified in the oil of this plant. Chrysanthenyl acetate was reported as the main component in the oil of Artemisia vulgaris L. collected from some localities in North Lithuania (Judžzentienè and Buzelytè 2006), Tanacetum balsamita subsp. balsamita (Asteraceae) from Turkey (Bagci et al. 2008b), and Tanacetum parathenium (L.) from England and the Netherlands (Hendriks et al. 1996; Christensen et al. 1999). However, there are no data in the literature on the prevalence of chrysanthenyl acetate, 2L-4Ldihydroxy eicosane, verbenol, and dehydroaromadendrene in P. gnaphalodes essential oil.
Gas chromatography-mass spectrometry analysis indicated that there are 20 major compounds in the oil of A. wilhelmsii flowers, comprising 88% of the total weight. 1,8-cineole was the most abundant compound (13.03%), followed by caranol (8.26%), α pinene (6%), farnesyl acetate (6%), p-cymene (6%), together with lesser amounts of the other important insecticidal compounds, including camphor (4.2%), carvacrol (3.7%), and terpineol (3.1%). The results of our analysis are in agreement with previous reports that have also reported carvacrol, 1,8-cineol, camphor, and α-pinene as the major components of the oil of A. wilhelmsii from Iran (Afsharypour et al. 1996; Javidnia et al. 2004; Ghani et al. 2008) and Turkey (Bagci et al. 2008a).
Fumigant toxicity
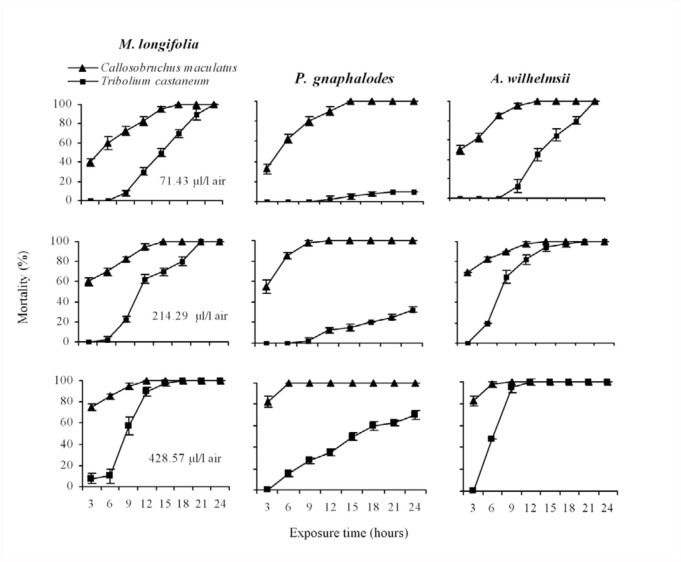
In all cases, considerable differences in insect mortality due to essential oil vapor were observed using different concentrations and exposure times. The mortality increased with rising concentrations and exposure time (Figure 1). Results indicated that the oils of all three plants were significantly more toxic against C. maculatus than T. castaneum, as inferred by the confidence intervals of LC50 (Figure 1). Furthermore, a difference in the response of the insect species to the essential oils has previously been reported for storedproduct insects (Lee et al. 2003; Negahban et al. 2007).
Figure 1.
Mean (5 replications, 10 individuals each) cumulative percentage mortality of Tribolium. castaneum and Callosobruchus maculatus exposed to various concentrations of essential oils from Mentha longifolia and Pulicaria gnaphalodes aerial parts and A. wilhelmsii flowers at various periods of time. High quality figures are available online.
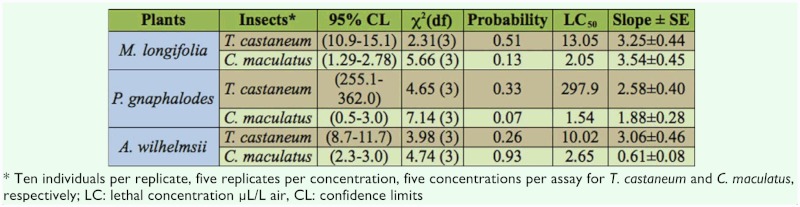
Based on LC50 (Table 1) and fumigant toxicity experiments (Figure 1), the oils of the three plants displayed the same strong insecticidal activity against C. maculatus (between 1.54 µl/L air in P. gnaphalodes, and 2.65 µl/L air in A. wilhelmsii). While the oils of A. wilhelmsii and M. longifolia showed the same strong insecticidal activity against T. castaneum (LC50=10.02 and 13.05 µl/L air, respectively), the oil of P. gnaphalodes showed poor activity against the insect (LC50=297.9 µl/Lair) (Table 2).
Table 2.
Efficiency of essential oil extracted from three plants against Tribolium castaneum and Callosobruchus maculatus adults.
No study has been previously reported on the insecticidal activities of the oils of three tested plants against C. maculatus and T. castaneum; however, it has been reported that the oils and extracts of the tested plants had insecticidal activity against other insects. For example, the ethanol extracts from the leaves of M. longifolia revealed insecticidal activity with a maximum of 70% mortality six days after grain pollution (5 grams of plant powder extracted with 500 mL ethanol, diluted to 10%, and used at an amount of 4 mL per 20 grams of grains in plastic vials with 100 mL capacity) (Saljoqi et al. 2006). Contact and fumigant insecticidal actions of Achillea wilhelmsii (Calmasur et al. 2006) and other Achillea species (Jovanovic et al. 2007; Magdy and Samir 2008) have been demonstrated against stored product pests. One hundred percent mortality was achieved with 2 µl/L air doses of the essential oils extracted from the leaves of A. wilhelmsii against Sitophilus granarius after an exposure time of 48 hours. However, mortality of Tribolium confusum at the same condition was about 90% (Calmasur et al. 2006).
Monoterpenes have been well documented as active fumigants, repellents, and insecticides toward stored-product insects (Papachristos et al. 2004). The insecticidal activity of the essential oils investigated in our study may be attributed to their having major monoterpenes components, because some major compounds of the test oils, such as carvacrol, camphor, 1,8-cineole, α-pinene, p-cymene, piperitenone oxide, and terpineol possessed insecticidal effects against the test insects (Traboulsi et al. 2002; Papachristos et al. 2004; Miresmailli et al. 2006; Cetin et al. 2007; Kordali et al. 2008; Magdy and Samir 2008).
The essential oils from these plants could become a viable alternative to conventional chemical control strategies. However, further studies need to be conducted in order to evaluate the safety of these oils before practical use in stored-product insect control.
Acknowledgements
We wish to thank Dr. V.A. Mozafarian, member of the scientific board at Research Institute of Forests and Rangelands, for plant species identification, and M.A. Allah Resani, expert of basic science faculty of Birjand University, for gas chromatography-mass spectrometry analysis. This research was supported by the University of Zabol, which is greatly appreciated.
References
- Afsharypour S, Asgary S, Lockwood B. Constituents of the essential oil of Achillea wilhelmsii from Iran. Planta Medica. 1996;62:77–78. doi: 10.1055/s-2006-957810. [DOI] [PubMed] [Google Scholar]
- Anderberg AA. Taxonomy and phylogeny of the tribe Inuleae (Asteraceae). Plant Systematic and Evolution. 1991;176:75–123. [Google Scholar]
- Bagci E, Koçak A, Yüce E. The Composition of the essential oils of two Achillea L. (Achillea wilhelmsii C. Koch. ve Achillea schischkinii Sosn.) species. Science and Engineering Journal of Firat University. 2008a;20(2):251–255. [Google Scholar]
- Bagci E, Kursat M, Kocak A, Gur S. Composition and Antimicrobial Activity of the Essential Oils of Tanacetum balsamita L. subsp. balsamita and T. chiliophyllum (Fisch. et Mey.) Schultz Bip. var. chiliophyllum (Asteraceae) from Turkey. Journal of Essential Oil Bearing Plants. 2008b;11(5):476–484. [Google Scholar]
- Barel S, Segal R, Yashphe J. The antimicrobial activity of the essential oil from Achillea fragrantissima. Journal of Ethnopharmacology. 1991;33:187–191. doi: 10.1016/0378-8741(91)90177-f. [DOI] [PubMed] [Google Scholar]
- Bouchra C, Achouri M, Idrissi Hassani LM, Hmamouchi M. Chemical composition and antifungal activity of essential oils of seven Moroccan Labiatae against Botrytis cinerea Pers. Journal of Ethnopharmacology. 2003;89:165–169. doi: 10.1016/s0378-8741(03)00275-7. [DOI] [PubMed] [Google Scholar]
- Calmasur O, Kordali S, Kaya O, Aslan I. Toxicity of essential oil vapours obtained from Achillea spp. to Sitophilus granarius (L.) and Tribolium confusum. Journal of Plant Diseases and Protection. 2006;113(1):37–41. [Google Scholar]
- Cetin H, Erler F, Yanikoglu A. Comparative evaluation of Origanum onites essential oil and its four major components as larvicides against the pine processionary moth, Thaumetopoea wilkinsoni Tams. Pest Management Science. 2007;63(8):830–833. doi: 10.1002/ps.1401. [DOI] [PubMed] [Google Scholar]
- Chevallier A. The Encyclopedia of Medicinal Plants. Dorling Kindersley Publishing; 1996. [Google Scholar]
- Christensen LP, Jakobsen HB, Paulsen E, Hodal L, Andersen KE. Airborne compositae dermatitis: Monoterpenes and no parthenolide are released from flowering Tanacetum parthenium (feverfew) plants. Archives of Dermatological Research. 1999;291:425–431. doi: 10.1007/s004030050433. [DOI] [PubMed] [Google Scholar]
- Dendougui H, Benayache S, Benayache F, Connoly JD. Sesquiterpene lactones from Pulicaria crispa. Fitoterapia. 2000;71:373–378. doi: 10.1016/s0367-326x(00)00133-7. [DOI] [PubMed] [Google Scholar]
- Dubaie AS, El-Khulaidi AA. Medicinal and aromatic plants in Yemen, deploymentcomponents of effective-uses. Ebadi Center for Studies and Publishing; 2005. [Google Scholar]
- El-Kamali HH, Ahmed AH, Mohammed AS, Yahia AAM, El-Tayeb IH, Ali AA. Antibacterial properties of essential oils from Nigella sativa seeds, Cymbopogon citratus leaves, and Pulicaria undulata aerial parts. Fitoterapia. 1998;69(1):77–78. [Google Scholar]
- Franzios G, Mirotsou M, Hatziapostolou E, Kral J, Scouras ZG, Mavragani-Tsipidou P. Insecticidal and genotoxic activities of mint essential oils. Journal of Agricultural and Food Chemistry. 1997;45:2690–2694. [Google Scholar]
- Ghani A, Azizi M, Hassanzadeh-Khayyat M, Pahlavanpour AA. Essential Oil Composition of Achillea eriophora, A. nobilis, A. biebersteinii and A. wilhelmsii from Iran. Journal of Essential Oil Bearing Plants. 2008;11(5):460–467. [Google Scholar]
- Hajlaoui H, Snoussi M, Ben Jannet H, Mighri Z, Bakhrouf A. Comparison of chemical composition and antimicrobial activities of Mentha longifolia L. ssp. longifolia essential oil from two Tunisian localities (Gabes and Sidi Bouzid). Annals of Microbiology. 2008;58(3):103–110. [Google Scholar]
- Hendriks H, Bos R, Woerdenbag J. The essential oil of Tanacetum parthenium (L.) Schultz-Bip. Flavour and Fragrance Journal. 1996;11:367–371. [Google Scholar]
- Isman MB. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annual Review of Entomology. 2006;51:45–66. doi: 10.1146/annurev.ento.51.110104.151146. [DOI] [PubMed] [Google Scholar]
- Isman MB. Plant essential oils for pest and disease management. Crop Protection. 2000;19:603–608. [Google Scholar]
- Javidnia K, Miri R, Sadeghpour H. Composition of the volatile oil of Achillea wilhelmsii C. Koch from Iran. Daru. 2004;12(2):63–66. [Google Scholar]
- Jovanovic Z, Kosti M, Popovic Z. Grain-protective properties of herbal extract against the bean weevil Acanthoscelides obtectus Say. Industrial Crops and Products. 2007;26:100–104. [Google Scholar]
- Judžentienè A, Buzelytè J. Chemical composition of essential oils of Artemisia vulgaris L. (mugwort) from North Lithuania. CHEMIJA. 2006;17(1):12–15. [Google Scholar]
- Kordali S, Cakir A, Ozer H, Cakmakci R, Kesdek M, Mete E. Antifungal, phytotoxic and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Bioresource Technology. 2008;99(18):8788–8795. doi: 10.1016/j.biortech.2008.04.048. [DOI] [PubMed] [Google Scholar]
- Lamiri A, Lhaloui S, Benjilali B, Berrada M. Insecticidal effects of essential oils against Hessian fly, Mayetiola destructor (Say). Field Crops Research. 2001;71:9–15. [Google Scholar]
- Lange BM, Croteau R. Genetic engineering of essential oil production in mint. Current Opinion in Plant Biotechnology. 1999;2:139–144. doi: 10.1016/s1369-5266(99)80028-4. [DOI] [PubMed] [Google Scholar]
- Lee S, Peterson CJ, Coats JR. Fumigation toxicity of monoterpenoids to several stored product insects. Journal of StoredProduct Research. 2003;39:77–85. [Google Scholar]
- Liu LL, Yang JL, Shi YP. Phytochemicals and Biological Activities of Pulicaria Species. Chemistry and Biodiversity. 2010;7(2):327–349. doi: 10.1002/cbdv.200900014. [DOI] [PubMed] [Google Scholar]
- Magdy IEM, Samir AMA. Chemical composition and insecticidal potential of essential oils from Egyptian plants against Sitophilus oryzae (L.) (Coleoptera: Curculionidae) and Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Applied Entomology and Zoology. 2008;43(4):599–607. [Google Scholar]
- Magiatis P, Skaltsounis AL, Chinov I, Haroutounian SA. Chemical composition and in vitro antimicrobial activity of the essential oils of three greek Achillea species. Zeitschrift für Naturforschung. 2002;57:287–290. doi: 10.1515/znc-2002-3-415. [DOI] [PubMed] [Google Scholar]
- Miresmailli S, Bradbury R, Isman MB. Comparative toxicity of Rosmarinus officinalis L. essential oil and blends of its major constituents against Tetranychus urticae Koch (Acari: Tetranychidae) on two different host plants. Pest Management Science. 2006;62(4):366–371. doi: 10.1002/ps.1157. [DOI] [PubMed] [Google Scholar]
- Monfared A, Rustaiyan A, Nabid M. Composition of a carvone chemotype of Mentha longifolia (L.) Huds. from Iran. Journal of Essential Oil Research. 2002;14:51–52. [Google Scholar]
- Muhammad I, El-Feraly FS, Mossa JS, Ramadan AF. Terpenoids from Pulicaria glutinosa. Phytochemistry. 1992;31(12):4245–4248. [Google Scholar]
- Negahban M, Moharramipour S, Sefidkon F. Fumigant toxicity of essential oil from Artemisia sieberi Besser against three stored product insects. Journal of Stored Product Research. 2007;43:123–128. [Google Scholar]
- Nickavar B, Amin GR, Ghavamian P. Antimicrobial activity of Pulicaria dysenterica. Iranian Journal of Pharmaceutical Research. 2002;1:31–32. [Google Scholar]
- Oyedeji OA, Afolayan AJ. Chemical composition and antibacterial activity of the essential oil isolated from South African Mentha longifolia (L.) subsp. capensis (Thunb.) Briq. Journal of Essential Oil Research. 2006;18:57–59. [Google Scholar]
- Papachristos DP, Karamanoli KI, Stamopoulos DC, Menkissoglu-Spiroudi U. The relationship between the chemical composition of three essential oils and their insecticidal activity against Acanthoscelides obtectus (Say). Pest Management Science. 2004;60(5):514–520. doi: 10.1002/ps.798. [DOI] [PubMed] [Google Scholar]
- Papachristos DP, Stamopoulos DC. Repellent, toxic and reproduction inhibitory effects of essential oil vapors on Acanthoscelides obtectus (Say) (Coleoptera: Bruchidae). Journal of Stored Product Research. 2002;38(2):117–128. [Google Scholar]
- Pavela R. Insecticidal activity of some essential oils against larvae of Spodoptera littoralis. Fitoterapia. 2005;76:691–696. doi: 10.1016/j.fitote.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Rajendran S, Sriranjini V. Plant products as fumigant for stored-product insect control. Journal of Stored Product Research. 2008;44:126–135. [Google Scholar]
- Rasooli I, Rezaei MB. Bioactivity and chemical properties of essential oils from Zataria multiflora Bois s and Mentha longifolia (L.) Huds. Journal of Essential Oil Research. 2002;14:141–146. [Google Scholar]
- Ross SA, El-Sayed KA, El-SoMy MA, Hamann MT, Abdel-Halim OB, Ahmed AF, Ahmed MM. Phytochemical Analysis of Geigeria alata and Francoeuria crispa essential oils. Planta Medica. 1997;63:479–482. doi: 10.1055/s-2006-957743. [DOI] [PubMed] [Google Scholar]
- SaIjoqi AUR, Afridi MK, Khan SA, Rehman S. Effects of six plant extracts on rice weevil Sitophilus oryzae L. in the stored wheat grains. Journal of Agricultural and Biological Science. 2006;1(4):1–5. [Google Scholar]
- Shaaya E, Kostyukovsky M, Eilberg J, Sukprakarn C. Plant oils as fumigants and contact insecticides for the control of stored-product insect. Journal of Stored Product Research. 1997;33:7–15. [Google Scholar]
- Traboulsi AF, Taoubi K, El-Haj S, Bessiere JM, Rammal S. Insecticidal properties of essential plant oils against the mosquito Culexpipiens molestus (Diptera: Culicidae). Pest Management Science. 2002;58(5):491–495. doi: 10.1002/ps.486. [DOI] [PubMed] [Google Scholar]
- Weyerstahl P, Marschall H, Wahlburg HC, Christiansen C, Rustaiyan A, Mirdjalili F. Constituents of the essential oil of Pulicaria gnaphalodes (Vent.) Boiss. from Iran. Flavour and Fragrance Journal. 1999;14(2):121–130. [Google Scholar]
- Zeinali H, Arzani A, Razmjoo K, Rezaee M. Evaluation of oil compositions of Iranian mints (Mentha ssp.). Journal of Essential Oil Research. 2005;17:156–159. [Google Scholar]