Abstract
Microbial entomopathogen—based bioinsecticides are recognized as alternatives to synthetic pesticides. Insects defend themselves against microbial pathogens by innate mechanisms, including increased phenoloxidase (PO) activity, but its relationship with microbial bioinsecticides efficacy is little known. This study evaluated the differences in PO activity at different developmental stages of the tobacco budworm Heliothis virescens Fabricius (Lepidoptera: Noctuidae), Indian meal moth Plodia interpunctella (Hübner) (Pyralidae), beet armyworm Spodoptera exigua (Hübner) (Noctuidae), and cabbage looper Trichoplusia ni (Hübner) (Noctuidae). Additionally, 2nd- and 4th-instars were exposed to the LC50 value of the commercial Bacillus thuringiensis (Bt) spray, Biobit®. The percentage of insecticidal activity (IA%) on 2nd-instar Biobit—exposed larvae was approximately the predicted 50 % mortality for all species except S. exigua. With all 4th instar Biobit—exposed larvae, mortality was not significantly different from that of unexposed larvae. Unexposed insects had a significantly higher PO activity in pre—pupae and pupae than early—instar larvae and adults, whereas PO activity was higher in adult females than in males. Correlation analysis between IA% and PO activity revealed significant r—values (p < 0.01) in 2nd instar H. virescens (r = 0.979) and P. interpunctella (r = 0.930). Second instar Biobit—exposed P. interpunctella had 10 times more PO activity than unexposed larvae. Similarly, the amount of total protein was lower in 4th instar Biobit—exposed H. virescens and higher in S. exigua. Therefore, the results indicated a relationship between Biobit susceptibility and PO activity in some cases. This information may be useful if the Biobit application period is timed for a developmental stage with low PO activity. However, more studies are needed to determine the correlation of each insect with a particular bioinsecticide.
Keywords : Heliothis virescens, innate humoral response, Plodia interpuctella, Spodoptera exigua, Trichoplusia ni
Introduction
Food—borne microbes can be affected by enzymes of the digestive tract. Ingested organisms also may be harmed by the harsh pH and redox conditions in the alimentary canal. If microorganisms are successful in evading the passive immune responses, they still may encounter innate immune defenses (Stanley and Shapiro 2007). Arthropods rely on diverse mechanisms of immune response, both passive and innate. Innate immunity includes the prophenoloxidase (proPO) system, which is confined inside the hemocytes, and is manifested in a series of cascading enzymatic reactions by the stimulation of peptidoglycan, β—glucans or lipopolysaccharides, and phenoloxidase (PO) enzyme activation. proPO is activated to PO by serine proteases and is responsible for initiating the biosynthesis of quinones to melanin (Ashida and Dohke 1980; Bidla et al. 2009). Melanin is a brown—black pigment that inhibits entomopathogenic bacterial and fungal enzymatic activity by encapsulation, as has been observed in Lepidoptera (Jiang et al. 1998), and may be related to the efficacy of certain bioinsecticides.
Lepidopteran pests are controlled mainly through synthetic chemical insecticides, but the risk of ecological disturbance and resistance development has prompted research to identify better choices. One alternative to chemicals is the application of bioinsecticides, considered effective for the control of some lepidopteran pests. Among microbial entomopathogens, Bacillus thuringiensis (Bt) commercial products, and transgenic plants expressing one or more Bt toxins, are used worldwide. However, insects can defend against microbial pathogens by innate mechanisms including PO activity as part of the humoral response (Cerenius et al. 2008). More importantly, the immune response to biopesticides in arthropods may be related to their lack of efficacy, although it is unknown if innate responses are relevant to formulations containing only toxin proteins. In the present study, differences were evaluated in the innate immune response to Bt as PO activity in different life—cycle phases of four species including the tobacco budworm Heliothis virescens Fabricius (Lepidoptera: Noctuidae), Indian meal moth Plodia interpunctella (Hübner) (Pyralidae), beet armyworm Spodoptera exigua (Hübner) (Noctuidae), and cabbage looper Trichoplusia ni (Hübner) (Noctuidae). Two of these species, P. interpunctata and T. ni were selected because they have demonstrated differences in their susceptibility to Bt in our laboratory (Rubio-Cota et al. 2010; Tamez-Guerra et al. 2006). In addition, the insecticidal activity was compared among 2nd and 4th instar larvae that were either unexposed or exposed to the Bt commercial product Biobit®, to evaluate whether an insect immune response can affect susceptibility to Bt.
Materials and Methods
Insects
The, S. exigua and H. virescens colonies were established from field collected insects in Northeast Mexico in 2000, whereas the T. ni colony was obtained from Dr. Howard T. Dulmage (USDA-ARS, Weslaco, TX), and reared since 1982 in León on artificial diet as described in Tamez-Guerra et al. (2006). The T. ni colony has been crossed with field—collected Mexican populations every 5–7 years to avoid homocygamy—related problems. The P. interpuctella colonies were from the Center for Grain and Animal Health Research (Manhattan, KS) and reared in León on a previously described cracked wheat artificial diet (McGaughey and Beeman 1988). Insects were incubated at 25 ± 2 °C, 55–60 ± 10% RH, and 16:8 L:D photoperiod.
Chemicals
All substrates and chemicals were from Sigma—Aldrich (www.sigmaaldrich.com) unless otherwise specified.
Biobit insecticidal activity
Initial tests were conducted to determine the fifty percent lethal concentration (LC50) for insects exposed to Biobit HP 32,000 IU/mg potency (Valent Biosciences Corporation, www.valentbiosciences.com), produced from a Bt var. kurstaki strain from DuPont (www.dupont.com) using an overlayer bioassay (Tamez-Guerra et al. 2006). Bioassays were performed in triplicate by exposing 30 neonates of each insect to six Biobit concentrations, prepared as serial doses (diluted 1:2) in distilled water. For H. virescens, the highest concentration tested was 0.16 IU/cm2; for T. ni, 0.19 IU/cm2; and for S. exigua, 1.9 IU/cm2. 35 mL of each dose or distilled water only used as a control were applied to 5 mL wheat germ artificial diet, 7.1 cm2 surface area. Doses were air dried for 30 min and then infested with two neonates per cup with S. exigua or T. ni, or one per cup with H. virescens. The insecticidal activity of Biobit against P. interpunctella was determined using a diet-incorporated bioassay with six doses (0, 0.6, 1.2, 2.4, 4.8, 9.6, and 19.2 µg/g of diet) (McGaughey and Johnson 1992). For this assay Biobit doses were prepared by incorporating 1.5 mL of each Biobit dose into 5 g of wheat-germ diet and allowed to air dry, and then infesting with 10 P. interpunctella neonates in triplicate. Treatments were incubated in 14:10 L:D photoperiod at 28 °C. To calculate LC50 values for Biobit, mortality data for each lepidopteran species were evaluated after five days and analyzed using POLO-Plus (LeOra 2007).
PO activity in unexposed and Biobit—exposed larvae
PO activity was measured from the hemolymph of different developmental stages of P. interpunctella, H. virescens, S. exigua, and T. ni using a technique first described by Ashida (1971) and Seed et al. (1978), and modified by Harizanova et al. (2004). The bioassay was conducted with 2nd or the 4th instar larvae of each lepidopteran species, using the LC50 values for Biobit-exposed neonates (Table 1), and using the overlayer bioassay for H. virescens, S. exigua, and T. ni, or the diet—incorporation bioassay for P. interpunctella as previously described. In these bioassays, 40 larvae of each insect species, either 2nd or 4th instar, were incubated for 24 hours on either the control or Biobit—treated diet; 20 larvae were used for insecticidal activity (IA) determination and the other 20 for phenoloxidase (PO) activity and protein analysis.
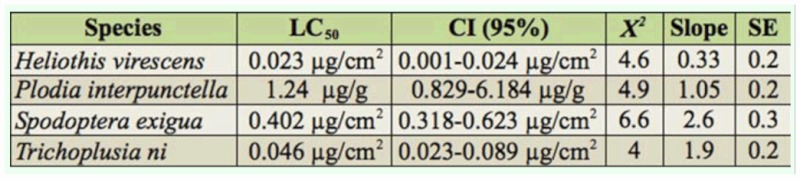
Table 1.
50% lethal concentrations (LC50) for Biobit in four lepidopteran pests.
For PO activity and protein analyses, hemolymph was collected by gently removing an anterior proleg, using a 14 cm sterile entomological dissection scissor. Hemolymph was collected directly into a chilled 1.5 mL microcentrifuge tube on ice (Shelby and Popham 2006) and was diluted 1:24 with ice—cold PBS. Hemolymph was frozen for 48 hours to lyse the hemocytes and release the inner—cell plasma. Samples were thawed and centrifuged at 5000 rpm for 1 min to separate the plasma containing PO. 50 mL aliquots of plasma sample were placed in a microplate well, and 150 µL of 10 mM DL-DOPA were added to each well as substrate. PO activity was measured and calculated as previously described. Two hundred microliters of substrate 10 mM DL-dihydroxyphenylalanine (DL-DOPA) were added to each well. Microplates were incubated in the dark at room temperature, and absorbance was read at 490 nm every 5 min for 30 min, using a microplate reader (Multimode detector DTX 880, Beckman Coulter Inc., Austria). As a negative control, phosphate buffered saline (PBS, 1.48 g of Na2HPO4, 0.43 g of NaH2PO4, 7.2 g NaCl, 1000 mL distilled water, pH 7.2) with substrate only was monitored over the same time periods and was subtracted as background. PO-specific activity was defined as the change in optical density over time. Tests were in triplicate with insects from different rearing lots. Data were analyzed using ANOVA posthoc Tukey α = 0.05 SPSS version 17.0 (SPSS 2008). This bioassay was performed in triplicate.
Protein determination was performed in triplicate using the diluted hemolymph from the PO activity assay (Bradford 1976). A standard curve was prepared with standard concentrations of 12 serial BSA dilutions, from 0.0 to 2.0 mg/mL, using PBS as diluent. For treatment analysis, 5.0 µL of diluted hemolymph from each sample and 200 µL of Bradford reagent were mixed and transferred to a 96—well plate. Absorbance was read in a spectrophotometer (Beckman Coulter Inc.) at 595 nm 2–10 min after mixing. The reaction setting time was selected to allow adequate reaction development, but no longer than 10 min to prevent oxidation. Total protein was calculated by comparison to the standard curve value.
Correlation analysis
Correlation analyses comparing insecticidal activity versus PO activity, total protein versus PO activity, or insecticidal activity versus total protein of each 2nd and 4th instar larvae from unexposed or Biobit—exposed insects was performed by using Pearson's analysis (SPSS 2008) using a cutoff for significance p < 0.05.
Results
Susceptibility to Biobit
Susceptibility to Biobit—exposed 2nd or 4th instar larvae showed that the most susceptible were H. virescens neonates, whereas the bioinsecticide was least toxic when tested against P. interpunctella neonates (Table 1). ANOVA comparison among all 24—hour Biobit—exposed 2nd or 4th instar larvae demonstrated a significantly higher IA in 2nd than 4th instar larvae (p ≤ 0.05, Table 2). The IA of Biobit in 2nd instar H. virescens, P. interpunctella, and T. ni was close to the expected 50% mortality (50, 44, and 46%, respectively). However, 2nd instar S. exigua larvae exposed to Biobit resulted in an IA of only 20.0%. IA among of all 4th instar lepidopterans demonstrated a lack of susceptibility to Biobit, as survival was similar to that of unexposed larvae.
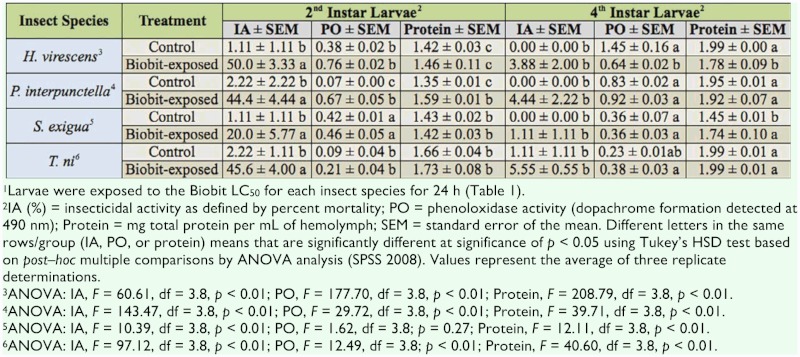
Table 2.
Insecticidal activity, phenoloxidase activity, and total protein (mg/mL) of 2nd or 4th instar Heliothis virescens, Plodia interpunctella, Spodoptera exigua, and Trichoplusia ni larvae, either control or exposed to Biobit for 24 hours. 1
Phenoloxidase activity in Biobit—treated Lepidoptera
PO activity in the hemolymph of Biobit—exposed larvae was compared to that of control (unexposed) larvae (Table 2). In general, PO activity was higher in H. virescens and lowest in T. ni larvae. Increased PO activity was found in 2nd instar Biobit—exposed larvae, but the increase was significant only in P. interpunctella larvae. PO activity was highest in 4th instar control H. virescens larvae, and significantly lower PO activity was found when larvae were exposed to Biobit.
Total protein determination
Differences in total protein among different insect species or treatments were significant in some cases (Table 2). In general, protein values were higher in 4th than in 2nd instar larvae. In comparing treatment effects, the total protein was significantly more in 4th instar H. virescens control than in Biobit—treated larvae. However, the total protein in Biobit—exposed 2nd instar P. interpunctella and 4th instar S. exigua larvae was significantly more than found in that of the respective control larvae.
Correlation analysis
Three correlation analyses were performed: IA versus PO activity, IA versus total protein, and PO activity versus total protein (Table 3). IA versus PO activity correlation analysis resulted in a negative value only with 4th instar H. virescens larvae (r = -0.677), but the correlation was not significant (p = 0.140). Significant positive correlation (p < 0.01) was observed in IA versus PO activity among 2nd instar Biobit—exposed H. virescens (p < 0.01) and P. interpunctella (p < 0.01) larvae, as well as in 4th instar Biobit—exposed T. ni larvae (p < 0.01). However, there was no significant correlation between IA and PO in Biobit—exposed S. exigua larvae.
Table 3.
Correlation analysis of insecticidal activity, phenoloxidase activity, and protein (me/mL)1.
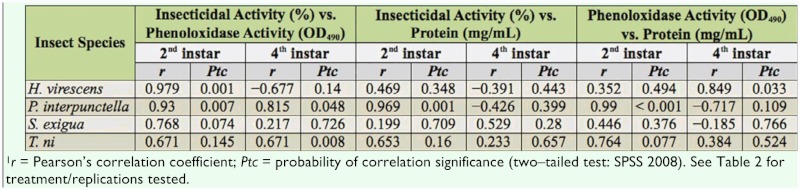
The correlation analysis of IA versus total protein was significant only in 2nd instar Biobit—treated P. interpunctella (p < 0.01). There was no significant correlation of IA and total protein in H. virescens, S. exigua, and T. ni treated larvae.
Correlation analysis of PO activity versus total protein was significant in 4th instar Biobit—exposed H. virescens larvae (p < 0.05). Similarly, PO and total protein were correlated in 2nd instar Biobit—exposed P. interpunctella larvae (p < 0.01). No significant correlation of PO activity and total protein was observed in T. ni or S. exigua.
Discussion
In the present study, we evaluated the relationship among Bt susceptibility and PO activity levels (as representative of the innate immune response) among laboratory colonies of four selected Lepidoptera. Samples included hemolymph from unexposed or Biobit-exposed 2nd- and 4th-instar larvae.
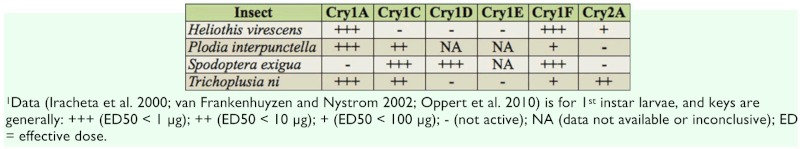
Of the species tested, Heliothis virescens was the most susceptible lepidopteran to Biobit, whereas the least susceptible was P. interpunctella. The percentage mortality of 2nd instar larvae exposed to the LC50 value of Biobit was similar to the predicted 50% in all of the insects tested, except S. exigua in which mortality was only 20%. Although the product recommendation dose is similar for H. virescens and S. exigua (http://www.proagro.com.mx/prods/valent/valent02.htm), we observed that H. virescens susceptibility was 17-fold higher than that of S. exigua in the laboratory. According to previous bioassays, H. virescens and T. ni were more susceptible to Cry 1A and 2A toxins, P. interpunctella was susceptible only to 1A, and S. exigua was not susceptible to any of the these toxins (Table 4), whereas P. interpunctella was susceptible to Cry1A toxins (Cry toxins in Biobit are 1Aa, 1Ab, 1Ac, 2Aa, and 2Ab). The different bioassay method, in which the toxin was incorporated into the diet instead of concentrated on the surface, may have contributed at least in part to an increased LC50.
Table 4.
Relative sensitivity of Heliothis virescens, Plodia interbunctella, Sbodobtera exigua, and Trichoblusia ni to some Bacillus thuringiensis Cry toxins1.
In our study, Biobit was not effective on 4th instar larvae. Similarly, Kwon and Kim (2008) reported that 5th instar S. exigua larvae exposed either to Bt svar. kurstaki (Btk, Thuricide®) or to Bt svar. aizawai (Bta GB413, GreenBioTech Chungju, Korea) showed no differences in mortality compared with that of unexposed controls. However, if they applied the immune suppressor benzylideneacetone, mortality increased to 60 and 80% with Bta— and Btk, respectively. Previous reports have indicated that earlier stages in Lepidoptera were more susceptible to Bt toxins and viruses (Morris 1969; Huang et al. 1999; Fuxa et al. 1999). In our study, this was also true in all species except S. exigua (Figure 1). Increased specific activity of gut proteases was proposed to be associated with the larval age and the loss of Bt toxin sensitivity in 5th instar S. littoralis larvae (Keller et al. 1996). The relationship of susceptibility to Bt and larval age has been studied in P. interpunctella (Nwanze et al. 1975) and T. ni (Engelhard and Volkman 1995). Nwanze et al. (1975) found that by using a dose of 25 mg/kg of whole wheat diet using Dipel® in a diet-incorporation bioassay resulted in 100% mortality of 1st instar P. interpunctella larvae, whereas 200 mg/kg was needed to obtain the same mortality level in larvae 18–21 days old. T. ni exposed to Autographa californica multiple nucleocapsid nucleopolyhedrovirus (AcMNPV) were more resistant as 4th instar larvae than earlier instars, probably due to their ability to completely clear the AcMNPV infection from the midgut epithelium (Engelhard and Volkman, 1995). Similar results were observed with 4th instar Lymantria dispar larvae and the Lymantria dispar multiple nucleocapsid nucleopolyhedrovirus (LdMNPV) compared to control (McNeil et al., 2010).
Popham et al. (2004) demonstrated that PO in the plasma of H. virescens might provide a constitutive, humoral innate antiviral immune response to Helicoverpa zea single capsid nucleopolyhedrovirus (HzSNPY) infection. In our study, it was observed that the most Biobit—susceptible H. virescens larvae had lower levels of PO present in the hemolymph as one of the key enzymes of insect immune response, but it is also found in hemocytes of the cuticular matrix where it is involved in the molting process (Ashida and Brey 1995; Hillyer and Christensen 2005). In this regard, PO is involved in sclerotization of the cuticle and melanization associated with nodulation, encapsulation, and wound healing, and may provide cytotoxic quinonoid compounds to kill opportunistically invading microorganisms (Nappi and Christensen 2005). PO activation has been positively correlated to wounding or infection as part of the immune response (Kanost and Gorman 2008). PO activity was significantly lower in 2nd instar P. interpunctella larvae compared with that of H. virescens, S. exigua, and T. ni. However, exposure to Biobit resulted in significantly increased PO activity only in 2nd instar P. interpunctella, and may correlate to the relatively higher LC50 for this lepidopteran. Furthermore, the most Biobit—sensitive insect in our test, H. virescens, had significantly lower PO activity when 4th instar larvae were exposed to Biobit. It was previously reported that PO levels increased in succeeding larval instars of S. littoralis and P. interpunctella (Ishaaya et al. 1974; Hartzer et al. 2005; Valadez-Lira et al. 2010). With P. interpunctella and S. exigua, an increase in immune response was related to decreased susceptibility to entomopathogens (Gassmann et al. 2009) and chemical insecticides (Liu et al. 2009). However, increased tolerance to Bt svar. kurstaki resulted in a reduced immune response and lower PO activity in susceptible—resistant T. ni (Ericsson et al. 2009) and increased in Biobit—exposed 2nd instar P. interpunctella and 4th instar S. exigua larvae, whereas total protein was lower in Biobit—exposed 4th instar H. virescens larvae.
In general, the total protein calculated value was higher among 4th instar compared with 2nd instar larvae, and was increased in Biobit—exposed 2nd instar P. interpunctella and 4th instar S. exigua larvae, whereas total protein was lower in Biobit—exposed 4th instar H. virescens larvae.
Certainly, the major factors associated with sensitivity to Bt toxins have been previously characterized as toxin receptors and protoxin activation/solubilization (reviewed in Ferré and Van-Rie 2002). We found that IA and PO activity were positively correlated in Biobit—treated 2nd instar H. virescens and P. interpunctella and 4th instar T. ni larvae, suggesting that PO activity may contribute to the efficacy of Bt toxins. If PO is a factor in toxicity, elucidation of the PO levels in different developmental stages of lepidopteran pests may be used to enhance bioinsecticide performance in pest management strategies, particularly if application time is programmed accordingly.
Overall, our results may help to understand why bioinsecticides are more effective when applied to earlier instars in some insects, and may be useful as a tool to improve bioinsecticide efficacy and lead to further understanding of the mechanisms of innate immunity. Using PO activity as a physiological parameter may also help to determine immune response activation against entomopathogenic microbial infections (Narayanan 2004). Our results suggest that PO protects insects from microbial infection more effectively during later instars, but more studies are needed to determine the relationship between PO activity and susceptibility of an insect to a particular entomopathogen.
Abbreviations
- IA
insecticidal activity
- PO
phenoloxidase
Acknowledgements
This study was supported by the research and scientific exchange division, United States Department of Agriculture, Agricultural Research Service 210-22310-003-03S to CRP, and FGP 542/09, Fundación Guanajuato Produce A. C. 542/09, and CONACyT 155771 to PTG. The mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the United States Department of Agriculture. USDA is an equal opportunity provider and employer.
References
- Adamo SA, Jensen M, Younger M. Changes in lifetime immunocompetence in male and female Gryllus texensis (formerly G. integer): trade—offs between immunity and reproduction. Animal Behavior. 2001;62:417–425. [Google Scholar]
- Ashida M. Purification and characterization of pre—phenoloxidase from hemolymph of the silkworm Bombyx mori. Archives of Biochemistry and Biophysics. 1971;144:749–762. doi: 10.1016/0003-9861(71)90383-3. [DOI] [PubMed] [Google Scholar]
- Ashida M, Dohke K. Activation of pro—phenol oxidase by the activating enzyme of the silkworm, Bombyx mori. Insect Biochemistry. 1980;10:37–47. [Google Scholar]
- Ashida M, Brey PT. Role of the integument in insect defense: pro—phenol oxidase cascade in the cuticular matrix. Proceedings of the National Academy of Sciences USA. 1995;92:10698–10702. doi: 10.1073/pnas.92.23.10698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidla G, Hauling T, Dushay MS, Theopold U. Activation of insect phenoloxidase after injury: endogenous versus foreign elicitors. Journal of Innate Immunology. 2009;1:301–308. doi: 10.1159/000168009. [DOI] [PubMed] [Google Scholar]
- Bradford MM. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein—dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cerenius L, Lee BL, Soderhall K. The proPO—system: pros and cons for its role in invertebrate immunity. Trends in Immunology. 2008;29:263–271. doi: 10.1016/j.it.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Engelhard EK, Volkman LE. Developmental resistance in fourth instar Trichoplusia ni orally inoculated with Autographa californica M nuclear polyhedrosis virus. Virology. 1995;209:384–389. doi: 10.1006/viro.1995.1270. [DOI] [PubMed] [Google Scholar]
- Ericsson JD, Janmaat AF, Lowenberger C, Myers JH. Is decreased generalized immunity a cost of Bt resistance in cabbage loopers Trichoplusia ni? Journal of Invertebrate Pathology. 2009;100:61–67. doi: 10.1016/j.jip.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Ferré J, Van-Rie J. Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annual Review of Entomology. 2002;47:501–533. doi: 10.1146/annurev.ento.47.091201.145234. [DOI] [PubMed] [Google Scholar]
- Fuxa JR, Sun J-Z, Weidner EH, LaMotte LR. Stressors and rearing diseases of Trichoplusia ni: evidence of vertical transmission of NPV and CPV. Journal of Invertebrate Pathology. 1999;74:149–155. doi: 10.1006/jipa.1999.4869. [DOI] [PubMed] [Google Scholar]
- Gassmann AJ, Fabrick JA, Sisterson MS, Hannon ER, Stock SP, Carrière Y, Tabashnik BE. Effects of pink bollworm resistance to Bacillus thuringiensis on phenoloxidase activity and susceptibility to entomopathogenic nematodes. Journal of Economic Entomology. 2009;102:1224–1232. doi: 10.1603/029.102.0348. [DOI] [PubMed] [Google Scholar]
- Hartzer KL, Zhu KY, Baker JE. Phenoloxidase in larvae of Plodia interpunctella (Lepidoptera: Pyralidae): molecular cloning of the proenzyme cDNA and enzyme activity in larvae paralyzed and parasitized by Habrobracon hebetor (Hymenoptera: Braconidae). Archives of Insect Biochemistry. 2005;59:67–79. doi: 10.1002/arch.20056. [DOI] [PubMed] [Google Scholar]
- Harizanova N, Tchorbadjieva M, Ivanova P, Dimov S, Ralchev K. Developmental and organ—specific expression of transferrin in Drosophila melanogaster. Biotechnology and Biotechnological Equipment. 2004;18:118–121. [Google Scholar]
- Hillyer JF, Christensen BM. Mosquito phenoloxidase and defensin colocalize in melanization innate immune responses. Journal of Histochemistry and Cytochemistry. 2005;53:689–698. doi: 10.1369/jhc.4A6564.2005. [DOI] [PubMed] [Google Scholar]
- Huang F, Buschman LL, Higgins RA. Susceptibility of different instars of European corn borer (Lepidoptera: Crambidae) to diet containing Bacillus thuringiensis. Journal of Economic Entomology. 1999;92:547–550. [Google Scholar]
- Iracheta MM, Pereyra-Alferez B, Galan-Wong L, Ferre J. Screening for Bacillus thuringiensis crystal proteins active against the cabbage looper, Trichoplusia ni. Journal of Invertebrate Pathology. 2000;76:70–75. doi: 10.1006/jipa.2000.4946. [DOI] [PubMed] [Google Scholar]
- Ishaaya I, Navon A, Gurevitz E. Comparative toxicity of chlorfluazuron (IKI-7899) and Cypermethrin to Spodoptera littoralis, Lobesia botrana and Drosophila melanogaster. Crop Protection. 1974;5:385–388. [Google Scholar]
- Jiang H, Yang W, Kanost MR. Prophenol oxidase activating proteinase from an insect, Manduca sexta: A bacteria—inducible protein similar to Drosophila melanogaster. Proceedings of the National Academy of Sciences USA. 1998;95:12220–12225. doi: 10.1073/pnas.95.21.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanost MR, Gorman MJ. Phenol oxidases in insect immunity. In: Beckage N, editor. Insect Immunology. Elsiever: 2008. pp. 69–96. [Google Scholar]
- Keller M, Sneh B, Strizhov N, Prudovsky E, Regev A, Koncz C, Schell J, Zilberstein A. Digestion of δ—endotoxin by gut proteases may explain reduced sensitivity of advanced instar larvae of Spodoptera littoralis to CryIC. Insect Molecular Biology and Biochemistry. 1996;26:365–373. doi: 10.1016/0965-1748(95)00102-6. [DOI] [PubMed] [Google Scholar]
- Kwon B, Kim Y. Benzylideneacetone, an immunosuppressant, enhances virulence of Bacillus thuringiensis against beet armyworm (Lepidoptera: Noctuidae). Journal of Economic Entomology. 2008;101:36–41. doi: 10.1603/0022-0493(2008)101[36:baievo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- LeOra. Polo Plus, Polo Encore, PoloDose and Polo Mix. LeOra Sofware Company; 2007. [Google Scholar]
- Li H, Tang H, Sivakumar S, Philip J, Harrison RL, Gatehouse JA, Bonning BC. Insecticidal activity of a basement membrane—degrading protease against Heliothis virescens (Fabricius) and Acyrthosiphon pisum (Harris). Journal of Insect Physiology. 2008;54:777–789. doi: 10.1016/j.jinsphys.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Liu S, Mu H, Xiao T, Xue C, Liu Z, Luo W. Does phenoloxidase contributed to the resistance? Selection with Butane—Fipronil enhanced its activities from diamondback moths. Open Biochemistry Journal. 2009;3:9–13. doi: 10.2174/1874091X00903010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaughey WH, Beeman RW. Resistance to Bacillus thuringiensis in colonies of Indianmeal moth and almond moth (Lepidoptera: Pyralidae). Journal of Economic Entomology. 1988;81:28–33. [Google Scholar]
- McGaughey WH, Johnson DE. Indianmeal Moth (Lepidoptera: Pyralidae) resistance to different strains and mixtures of Bacillus thuringiensis. Journal of Economic Entomology. 1992;85:1594–1600. [Google Scholar]
- McNeil J, Cox-Foster D, Slavicek J, Hoover K. Contributions of immune responses to developmental resistance in Lymantria dispar challenged with baculovirus. Journal of Insect Physiology. 2010;56:1167–1177. doi: 10.1016/j.jinsphys.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Morris ON. Susceptibility of several forest insects of British Columbia to commercially produced Bacillus thuringiensis: II. Laboratory and field pathogenicity tests. Journal of Invertebrate Pathology. 1969;13:285–295. doi: 10.1016/0022-2011(69)90221-3. [DOI] [PubMed] [Google Scholar]
- Nappi AJ, Christensen BM. Melanogenesis and associated cytotoxic reactions: Applications to insect innate immunity. Insect Biochemistry and Molecular Biology. 2005;35:443–459. doi: 10.1016/j.ibmb.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Narayanan K. Insect defense: its impact on microbial control of insect pests. Current Science India. 2004;86:800–814. [Google Scholar]
- Nwanze KF, Partida GJ, McGaughey WH. Susceptibility of Cadra cautella and Plodia interpunctella to Bacillus thuringiensis on wheat. Journal of Economic Entomology. 1975;68:751–752. [Google Scholar]
- Oppert B, Ellis RT, Babcock J. Effects of Cry1F and Cry34Ab1/35Ab1 on storage pests. Journal of Stored Product Research. 2010;46:143–148. [Google Scholar]
- Popham HJR, Shelby KS, Brandt SL, Coudron TA. Potent virucidal activity in larval Heliothis virescens plasma against Helicoverpa zea single capsid nucleopolyhedrovirus. Journal of General Virology. 2004;85:2255–2261. doi: 10.1099/vir.0.79965-0. [DOI] [PubMed] [Google Scholar]
- Rubio-Cota E., Oppert B, Iracheta-Cárdenas M, Valadez-Lira A, Tamez-Guerra P. Susceptibility of Plodia interpunctella to Bacillus thuringiensis and its relation with microbial flora and hemolin expression. 58th Annual Meeting of the ESA-Southwestern Branch. Cancun; Quintana Roo, México: 2010. [Google Scholar]
- Russell V, Dunn PE. Antibacterial proteins in the midgut of Manduca sexta during metamorphosis. Mediators of Insect Immunity. Journal of Insect Physiology. 1996;42:65–71. doi: 10.1002/arch.940170202. [DOI] [PubMed] [Google Scholar]
- Seed JL, Boff M, Bennett JL. Phenol oxidase activity: induction in female schistosomes by in vitro incubation. The Journal of Parasitology. 1978;64:283–289. [PubMed] [Google Scholar]
- Shelby KS, Popham HJR. Plasma phenoloxidase of the larval tobacco budworm, Heliothis virescens, is virucidal. Journal of Insect Science. 2006;6:13. doi: 10.1673/2006_06_13.1. Available online, http://www.insectscience.org/6.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPSS. SPSS Inc. IBM; 2008. http://www.ibm.com/software/analytics/spss. [Google Scholar]
- Stanley D, Shapiro M. Eicosanoid biosynthesis inhibitors increase the susceptibility of Lymantria dispar to nucleopolyhedrovirus LdMNPV. Journal of Invertebrate Pathology. 2007;95:119–124. doi: 10.1016/j.jip.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Tamez-Guerra P, Damas G, Iracheta MM, Gomez-Flores RA, Oppert B, RodríiguezPadilla C. Differences in susceptibility and physiological fitness of Trichoplusia ni (Hübner) strains to Bacillus thuringiensis exposure. Journal of Economic Entomology. 2006;99:937–945. doi: 10.1603/0022-0493-99.3.937. [DOI] [PubMed] [Google Scholar]
- Teakle RE, Jensen JM, Giles JE. Age—related susceptibility of Heliothis punctiger to a commercial formulation of nuclear polyhedrosis virus. Journal of Invertebrate Pathology. 1986;47:82–92. [Google Scholar]
- Valadez-Lira JA, Damas G, Jalomo-Ortiz P, Terrazas-Castelan E, Nuñez-Mejía G, Oppert B, Gomez-Flores R, Rodriguez-Padilla C, Alcocer-Gonzalez JM, Tamez-Guerra P. 58th Annual Meeting of the ESA-Southwestern Branch. In: van Frankenhuyzen K, Nystrom C., editors. The Bacillus thuringiensis toxin specificity database. Cancun, Quintana Roo; México: 2010. 2002. Available online, www.glfc.cfs.nrcan.gc.ca/Bacillus/btsearch.cfm. [Google Scholar]
- van Frankenhuyzen K, Nystrom C. The Bacillus thuringiensis toxin specificity database. 2002. Available online, www.glfc.cfs.nrcan.gc.ca/Bacillus/btsearch.cfm.
- Welzel-Gramkow A, Perecmanis S, Barbosa-Sousa RL, Ferreira-Noronha E, Felix CR, Nagata T, Morais-Ribeiro B. Insecticidal activity of two proteases against Spodoptera fiugiperda larvae infected with recombinant baculoviruses. Virology Journal. 2010;7:143. doi: 10.1186/1743-422X-7-143. [DOI] [PMC free article] [PubMed] [Google Scholar]


