Abstract
Objective
We sought to characterize associations between aminotransferase levels and cardiometabolic risk after accounting for visceral adipose tissue (VAT) and insulin resistance.
Methods and Results
Participants (n=2621) from the Framingham Heart Study (mean age 51, 49.8% women) were included. Sex-specific linear and logistic regressions were used to evaluate associations between aminotransferase levels and cardiometabolic risk factors. In multivariable models, increased ALT levels were associated with elevated blood pressure, fasting plasma glucose, and triglycerides and lower HDL levels (all p ≤ 0.007). Further, each 1 standard deviation (SD) increase in ALT corresponded to an increased odds of hypertension, diabetes, the metabolic syndrome, impaired fasting glucose, and insulin resistance estimated by HOMA-IR (OR 1.29–1.85, all p ≤ 0.002). Associations with ALT persisted after additional adjustment for VAT, insulin resistance, and BMI with the exception of HDL cholesterol in both sexes and blood pressure in women. Results were materially unchanged when moderate drinkers were excluded, when the sample was restricted to those with ALT<40 U/L, and when the sample was restricted to those without diabetes. Similar trends were observed for AST levels, but associations were more modest.
Conclusions
Aminotransferase levels are correlated with multiple cardiometabolic risk factors above and beyond VAT and insulin resistance.
Keywords: liver function tests, obesity, visceral fat, insulin resistance, cardiometabolic risk factors
Nonalcoholic fatty liver disease (NAFLD) is increasingly recognized as the hepatic manifestation of obesity and its metabolic and cardiovascular sequelae 1,2. Serum aminotransferase levels, particularly alanine aminotransferase (ALT), may be used to estimate NAFLD in obese and nonobese individuals 3,4, and serum ALT appears to be the liver enzyme most closely related to liver fat content 5. Elevated ALT levels have been associated with metabolic risk factors for cardiovascular disease, including type 2 diabetes mellitus and the metabolic syndrome 6–9. Such associations raise the possibility that NAFLD, obesity, and metabolic derangements may share a common pathogenesis. They also raise the possibility that serum aminotransferase levels may be useful indicators of cardiometabolic risk above and beyond adiposity and insulin resistance.
The goal of the present study was to explore the association between serum aminotransferase levels and metabolic risk factors for cardiovascular disease in a community-based sample unselected for NAFLD, obesity, or metabolic disease. We tested the hypothesis that aminotransferase levels are associated with cardiometabolic risk above and beyond generalized and central adiposity and insulin resistance. We also tested this hypothesis among those with both elevated and normal aminotransferase levels.
Methods
Study Sample
Subjects were drawn from the Framingham Heart Study, a prospective cohort study that began with 5,209 residents of Framingham, Massachusetts in 1948. In 1971, the offspring and spouses of the original cohort were enrolled in the Offspring Study, and in 2002 the children of the original cohort’s offspring were enrolled in the Third Generation Study. Between June 2002 and April 2005, 3,529 Offspring and Third Generation participants were enrolled in the Multi-Detector Computed Tomography (MDCT) substudy and underwent chest and abdominal computed tomographic (CT) scanning. The current study sample consisted of the 2,621 participants (1,305 women and 1,316 men) who had interpretable abdominal CT scans and a complete covariate profile. The institutional review boards of the Boston University Medical Center and Massachusetts General Hospital approved the study protocol. All subjects provided informed written consent.
Multidetector CT Scan Protocol and Measurement of VAT and SAT
Participants underwent eight-slice multidetector abdominal CT scanning in a supine position (LightSpeed Ultra, General Electric, Milwaukee, WI). Twenty-five contiguous 5-mm thick slices (120 kVp, 400 mA, gantry rotation time 500 ms, and table feed 3:1) were obtained, covering 125 mm above the level of S1. VAT and SAT volumes were quantified from CT scans using a semiautomatic segmentation technique at a dedicated offline workstation (Aquarius 3D Workstation; TeraRecon, San Mateo, CA). A reader manually traced the abdominal muscular wall separating the two layers. An image display window width of −195 to −45 Hounsfield units and a window center of −120 Hounsfield units were used to identify pixels containing fat. VAT was defined as adipose tissue inside the abdominal muscular wall and SAT as adipose tissue outside the abdominal muscular wall. Interclass correlations for inter-reader comparisons were 0.997 for SAT and 0.992 for VAT on a random sample of 100 scans; high intra-reader correlations were similarly obtained.
Baseline Measurements and Definitions
Serum ALT and AST levels were measured on fasting morning samples using the kinetic method (Beckman Liquid-Stat Reagent Kit) 10. Coefficients of variation were 4.1–4.4% and 3.8–4.5% for ALT and AST, respectively.
Covariates and Risk Factor Assessment
Risk factors used in this study were measured at the seventh Framingham Offspring examination (1998–2001) and the first Third Generation examination (2002–2005). BMI was calculated as weight in kilograms divided by the square of height in meters. Waist circumference was measured at the umbilicus. Serum triglycerides, HDL cholesterol, and fasting plasma glucose were measured in fasting morning samples from participants. Diabetes was defined as fasting plasma glucose ≥ 126 mg/dl or treatment with a hypoglycemic agent or insulin; impaired fasting glucose was defined as a fasting plasma glucose level of 100–125 mg/dl in the absence of diabetes treatment. Hypertension was defined as systolic blood pressure ≥140 mmHg and diastolic blood pressure as ≥90 mmHg or antihypertensive treatment. Current smoking was defined as smoking ≥1 cigarette per day in the past year. Alcohol use was evaluated through a physician-administered questionnaire and patients were considered to have high alcohol intake if they reported more than 14 drinks per week (men) or 7 drinks per week (women). Women were classified as postmenopausal if periods had stopped for ≥1 year. Metabolic syndrome was defined by modified Adult Treatment Panel III criteria (impaired fasting glucose defined as fasting plasma glucose 100–125 mg/dl in the absence of treatment of diabetes, the high triglyceride component was defined as triglycerides ≥150 mg/dl or treatment with a lipid-lowering agent, and blood pressure criteria were modified to include treatment for hypertension). Insulin resistance was defined as having greater than the top quartile of the homeostasis model assessment of insulin resistance (HOMA-IR) (fasting glucose × fasting insulin/22.5) distribution among individuals without diabetes by sex 11 (for the analysis sample, > 3.06 for women and > 3.51 for men).
Statistical Analysis
ALT and AST were natural log-transformed to normalize their skewed distribution. Sex-specific, age-adjusted Pearson correlations between log ALT and log AST levels were calculated with adiposity traits and cardiometabolic risk factors.
Multivariable linear and logistic regression models were used to assess the significance of covariate-adjusted associations between aminotransferase levels and continuous and dichotomous cardiometabolic risk factors, respectively. The increment in covariate-adjusted risk factors per 1-SD increase in log ALT and log AST was estimated for continuous variables while the odds ratio of the risk factor per 1-SD increase in log aminotransferase level was estimated for dichotomous variables. Covariates included age, current smoking, high alcohol intake (>14 drinks/week for men or >7 drinks/week for women), physical activity, menopausal status (women only), and hormone replacement therapy (women only). For models of systolic and diastolic blood pressure, fasting plasma glucose, HDL cholesterol, and log triglycerides, an additional covariate of treatment for hypertension, diabetes, or lipid disorders, respectively, was included. Finally, additional models evaluated the impact of the associations after additional adjustment for VAT, HOMA-IR (where appropriate), and BMI (separately).
In secondary analyses, we restricted the sample to those with aminotransferase levels in the normal range (ALT<40 U/L or AST<40 U/L, respectively), to participants not reporting high alcohol intake (≤7 drinks per week [women] or ≤ 14 drinks per week [men]), and to individuals without diabetes.
Further, age-adjusted rates of CVD risk factors were calculated within tertiles of ALT stratified by tertiles of VAT and a test of trend (also age-adjusted) across ALT tertile levels within each VAT tertile was performed. Given the greater number and strength of associations between ALT and cardiometabolic risk factors than between AST and cardiometabolic risk factors, the analysis was only performed with ALT.
SAS version 9.2 was used to perform all computations. A 2-tailed p-value <0.05 was considered significant.
Results
Study Sample Characteristics
The characteristics of the study sample are summarized in Table 1. The mean age of study participants was 52 years for women and 50 years for men; 49.8% of participants were women. Mean ALT and AST levels were 20.4 U/L (SD 12.5) and 21.4 U/L (SD 8.5) in women and 31.2 U/L (SD 22.8) and 26.0 U/L (SD 15.9) in men, respectively.
Table 1.
Study Sample Characteristics
| Women (N=1305) | Men (N=1316) | |
|---|---|---|
| Age (years) | 52.2 (9.8) | 49.7 (10.7) |
| Postmenopausal (%) | 52.2 | NA |
| Hormone Replacement (%) | 19.6 | NA |
| Current Smoking (%) | 11.2 | 13.5 |
| High Alcohol Intake* (%) | 14.1 | 16.0 |
| Body Mass Index (kg/m2) | 27.2 (5.7) | 28.2 (4.4) |
| Waist Circumference (cm) | 93.7 (15.2) | 100.4 (11.5) |
| Visceral Adipose Tissue (cm3) | 1396.2 (834.5) | 2210.0 (1011.9) |
| Subcutaneous Adipose Tissue (cm3) | 3187.4 (1480.0) | 2576.3 (1168.0) |
| Systolic Blood Pressure (mm Hg) | 120.5 (17.5) | 123.2 (14.7) |
| Diastolic Blood Pressure (mm Hg) | 73.8 (9.1) | 77.9 (9.0) |
| Hypertension (%) | 27.5 | 30.7 |
| Hypertension Treatment (%) | 19.4 | 18.6 |
| Triglycerides (mg/dl)† | 97 (69–142) | 116 (78–174) |
| HDL Cholesterol (mg/dl) | 60.5 (16.1) | 45.6 (12.2) |
| Lipid Treatment (%) | 10.3 | 17.6 |
| Fasting Glucose (mg/dl) | 96.1 (17.4) | 101.6 (21.3) |
| Impaired Fasting Glucose ‡ (%) | 19.7 | 37.5 |
| HOMA-IR† | 2.4 (2.0–3.3) | 2.8 (2.3–3.8) |
| Insulin Resistance (%) | 29.2 | 28.3 |
| Diabetes (%) | 5.4 | 6.7 |
| Diabetes Treatment (%) | 3.0 | 3.3 |
| Metabolic Syndrome (%) | 29.0 | 37.0 |
| ALT (U/L)† | 18 (14–23) | 26 (20–36) |
| AST (U/L)† | 20 (17–24) | 23 (20–29) |
| Physical Activity Score | 20.0 (17.0–24.0) 36.8 (5.9) | 38.5 (8.2) |
Data are presented as the mean (standard deviation) for continuous traits, and percentage having that characteristic (n/N) for categorical data.
Abbreviations: HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
>7 drinks/week in women, >14 drinks/week in men.
Median with 25th–75th percentiles.
Fasting plasma glucose 100 to 125 mg/dL and not on diabetes treatment.
Correlations Between Aminotransferase Levels and Cardiometabolic Risk Factors
Increasing log ALT levels were associated with most cardiometabolic risk factor levels in women and men after adjusting for age (Table 2). More specifically, higher levels of log ALT were associated with higher levels of SAT, VAT, waist circumference, BMI, log triglycerides, serum fasting glucose, insulin resistance, systolic and diastolic blood pressure and with lower HDL cholesterol. Correlations between log AST and risk factors showed similar trends as observed in ALT, but associations were attenuated and fewer significant correlations were observed. Higher log AST levels were correlated with higher diastolic blood pressure, and greater insulin resistance in men and women and additionally with increased BMI, waist circumference, systolic blood pressure, and VAT in men.
Table 2.
Sex-specific Pearson Correlation Coefficients between log ALT and log AST and Cardiometabolic Risk Factors, Age-Adjusted
| Log ALT | Log AST | |||
|---|---|---|---|---|
|
| ||||
| Women | Men | Women | Men | |
| Age | 0.04 | −0.29**** | 0.10*** | −0.12**** |
| Body Mass Index | 0.20**** | 0.26**** | 0.01 | 0.10*** |
| Waist Circumference | 0.22**** | 0.25**** | 0.03 | 0.07* |
| Visceral Adipose Tissue | 0.21**** | 0.31**** | 0.01 | 0.10*** |
| Subcutaneous Adipose Tissue | 0.17**** | 0.18**** | 0.01 | 0.05 |
| Systolic Blood Pressure | 0.11**** | 0.16**** | 0.05 | 0.08** |
| Diastolic Blood Pressure | 0.13**** | 0.21**** | 0.08** | 0.12**** |
| Log Triglycerides | 0.16**** | 0.20**** | 0.01 | 0.04 |
| HDL Cholesterol | −0.10*** | −0.07* | 0.07** | 0.08** |
| Fasting Glucose | 0.17**** | 0.14**** | 0.04 | 0.03 |
| Log HOMA-IR | 0.28**** | 0.27**** | 0.07** | 0.07* |
Abbreviations: HDL, high-density lipoprotein; HOMA-IR, homeostasis model of insulin resistance.
p<0.05;
p<0.01;
p<0.001;
p<0.0001
Multivariable-Adjusted Models
In multivariable adjusted models, log ALT levels were associated with systolic and diastolic blood pressure in both sexes. Among women, SBP was on average 1.19 mm Hg higher per 1-SD increase in log ALT (p=0.007) and among men, SBP was 2.07 mm Hg higher on average per 1-SD increase in log ALT (p<0.0001), after covariate adjustment. This association was attenuated slightly but persisted in men after additional adjustment for VAT, insulin resistance, and BMI (separately) and persisted in women after adjustment for insulin resistance, but significance was lost after adjustment for VAT and for BMI. Associations between DBP and log ALT levels remained significant in both sexes after adjustments for VAT, IR, and BMI (Table 3).
Table 3.
Sex-Specific Multivariable-Adjusted* Regressions for ALT with Cardiometabolic Risk Factors
| Women | Men | ||||
|---|---|---|---|---|---|
|
| |||||
| MV adjusted beta coefficient or OR (95% CI) | p-value | MV adjusted beta coefficient or OR (95% CI) | p-value | p-value for sex interaction | |
| Continuous Variables | |||||
| Systolic Blood Pressure | 0.21 | ||||
| Base Model* | 1.19 (0.33, 2.06) | 0.007 | 2.07 (1.29, 2.84) | <0.0001 | |
| +VAT | 0.48 (−0.37, 1.33) | 0.27 | 1.42 (0.62, 2.22) | 0.0005 | |
| +HOMA-IR | 0.91 (0.03, 1.78) | 0.04 | 1.89 (1.10, 2.67) | <0.0001 | |
| +BMI | 0.55 (−0.30, 1.40) | 0.20 | 1.44 (0.65, 2.23) | 0.0004 | |
| Diastolic Blood Pressure | 0.046 | ||||
| Base Model* | 0.94 (0.45, 1.43) | 0.0002 | 1.86 (1.37, 2.36) | <0.0001 | |
| +VAT | 0.58 (0.09, 1.07) | 0.02 | 1.37 (0.86, 1.88) | <0.0001 | |
| +HOMA-IR | 0.86 (0.36, 1.37) | 0.0008 | 1.76 (1.25, 2.26) | <0.0001 | |
| +BMI | 0.61 (0.12, 1.10) | 0.02 | 1.44 (0.94, 1.94) | <0.0001 | |
| Fasting Plasma Glucose | 0.48 | ||||
| Base Model* | 1.79 (1.02, 2.55) | <0.0001 | 2.75 (1.71, 3.79) | <0.0001 | |
| +VAT | 0.82 (0.09, 1.55) | 0.03 | 2.00 (0.92, 3.08) | 0.0003 | |
| +BMI | 0.99 (0.25, 1.73) | 0.01 | 1.82 (0.76, 2.88) | 0.0008 | |
| Log Triglycerides | 0.06 | ||||
| Base Model* | 0.08 (0.06, 0.11) | <0.0001 | 0.12 (0.09, 0.15) | <0.0001 | |
| +VAT | 0.04 (0.02, 0.07) | 0.001 | 0.07 (0.04, 0.10) | <0.0001 | |
| +HOMA-IR | 0.05 (0.03, 0.08) | 0.0001 | 0.09 (0.06, 0.12) | <0.0001 | |
| +BMI | 0.05 (0.03, 0.08) | <0.0001 | 0.09 (0.06, 0.12) | <0.0001 | |
| HDL Cholesterol | 0.10 | ||||
| Base Model* | −1.40 (−2.26, −0.55) | 0.001 | −1.05 (−1.71, −0.38) | 0.002 | |
| +VAT | −0.29 (−1.11, 0.53) | 0.49 | 0.16 (−0.50, 0.82) | 0.63 | |
| +HOMA-IR | −0.56 (−1.41, 0.28) | 0.19 | −0.45 (−1.11, 0.20) | 0.18 | |
| +BMI | −0.50 (−1.33, 0.33) | 0.23 | −0.23 (−0.90, 0.44) | 0.50 | |
| HOMA-IR | 0.76 | ||||
| Base Model* | 0.41 (0.31, 0.51) | <0.0001 | 0.48 (0.35, 0.61) | <0.0001 | |
| +VAT | 0.23 (0.15, 0.32) | <0.0001 | 0.22 (0.09, 0.34) | 0.0007 | |
| +BMI | 0.26 (0.17, 0.35) | <0.0001 | 0.21 (0.09, 0.33) | 0.0006 | |
| Dichotomous Variables | |||||
| Hypertension | 0.75 | ||||
| Base Model* | 1.37 (1.20, 1.57) | <0.0001 | 1.34 (1.17, 1.54) | <0.0001 | |
| +VAT | 1.22 (1.06, 1.40) | 0.005 | 1.16 (1.00, 1.33) | 0.04 | |
| +HOMA-IR | 1.21 (1.05, 1.39) | 0.01 | 1.26 (1.09, 1.44) | 0.002 | |
| +BMI | 1.25 (1.09, 1.43) | 0.002 | 1.20 (1.04, 1.38) | 0.01 | |
| IFG | 0.52 | ||||
| Base Model* | 1.30 (1.13, 1.49) | 0.0002 | 1.29 (1.14, 1.45) | <0.0001 | |
| +VAT | 1.14 (0.99, 1.32) | 0.07 | 1.14 (1.01, 1.30) | 0.04 | |
| +BMI | 1.17 (1.02, 1.36) | 0.03 | 1.19 (1.05, 1.35) | 0.006 | |
| Diabetes | 0.40 | ||||
| Base Model* | 1.62 (1.30, 2.03) | <0.0001 | 1.42 (1.14, 1.77) | 0.002 | |
| +VAT | 1.48 (1.17, 1.87) | 0.001 | 1.27 (1.01, 1.60) | 0.04 | |
| +BMI | 1.50 (1.18, 1.92) | 0.001 | 1.23 (0.97, 1.54) | 0.08 | |
| Metabolic Syndrome | 0.74 | ||||
| Base Model* | 1.61 (1.41, 1.84) | <0.0001 | 1.72 (1.50, 1.96) | <0.0001 | |
| +VAT | 1.33 (1.15, 1.55) | 0.0002 | 1.29 (1.11, 1.49) | 0.0006 | |
| +HOMA-IR | 1.26 (1.09, 1.47) | 0.002 | 1.43 (1.24, 1.65) | <0.0001 | |
| +BMI | 1.42 (1.23, 1.64) | <0.0001 | 1.41 (1.22, 1.64) | <0.0001 | |
| Insulin Resistance | 0.65 | ||||
| Base Model* | 1.68 (1.47, 1.91) | <0.0001 | 1.91 (1.66, 2.20) | <0.0001 | |
| +VAT | 1.42 (1.23, 1.65) | <0.0001 | 1.51 (1.30, 1.75) | <0.0001 | |
| +BMI | 1.49 (1.29, 1.72) | <0.0001 | 1.61 (1.38, 1.89) | <0.0001 | |
Data presented include effect size (SE) expressed per 1 SD increase in log ALT for continuous data, and the odds of the condition per 1 SD increase in log ALT with 95% confidence intervals for dichotomous data.
Adjusted for age, current smoking, high alcohol intake, physical activity, and menopausal status (women only), hormone replacement therapy (women only); for blood pressure, FPG, HDL cholesterol, and log triglycerides, an additional covariate of treatment for HTN, diabetes, or lipid disorders, respectively, was included.
Abbreviations: VAT, visceral adipose tissue; HOMA-IR, homeostasis model of insulin resistance; BMI, body mass index; HDL, high-density lipoprotein; IFG, impaired fasting glucose.
Fasting plasma glucose was associated with ALT level in both men and women; these associations persisted after additional adjustments for VAT, IR, and BMI.
For each 1-SD increase in log ALT, HDL was 1.4 mg/dL lower in women (p=0.001) and 1.1 mg/dL lower in men (p=0.002); however, these associations did not persist after additional adjustment for VAT, IR, or BMI. For each 1-SD increase in log ALT, log triglycerides were 0.08 mg/dL higher in women (p<0.0001) and 0.12 mg/dL higher in men (p<0.0001). These associations remained significant with slight attenuation in both sexes after adjustment for VAT, IR, and BMI.
Associations between log ALT and dichotomous traits were fairly strong (all OR > 1.29, all p ≤ 0.0002) and generally persisted after adjustment for VAT, BMI, and HOMA-IR. For example, for every 1-SD increase in log ALT, the odds ratio of metabolic syndrome was 1.61 times higher in women and 1.72 times higher in men. These relations remained significant after adjustment for VAT, IR, and BMI.
Far fewer significant associations were observed between log AST and cardiometabolic risk factors than between log ALT and risk factors (Table 4). Of the risk factors tested, only DBP and HDL were significantly associated with AST level in both men and women before and after additional adjustment for VAT, IR, and BMI.
Table 4.
Sex-Specific Multivariable-Adjusted* Regressions for AST with Cardiometabolic Risk Factors
| Women | Men | ||||
|---|---|---|---|---|---|
|
| |||||
| MV adjusted beta coefficient or OR (95% CI) | p-value | MV adjusted beta coefficient or OR (95% CI) | p-value | p-value for sex interaction | |
| Continuous Variables | |||||
| Systolic Blood Pressure | 0.57 | ||||
| Base Model* | 0.39 (−0.47, 1.26) | 0.37 | 0.88 (0.13, 1.63) | 0.02 | |
| +VAT | 0.45 (−0.39, 1.29) | 0.29 | 0.65 (−0.09, 1.40) | 0.09 | |
| +HOMA-IR | 0.34 (−0.52, 1.20) | 0.44 | 0.81 (0.06, 1.57) | 0.03 | |
| +BMI | 0.44 (−0.40, 1.28) | 0.30 | 0.63 (−0.11, 1.37) | 0.10 | |
| Diastolic Blood Pressure | 0.40 | ||||
| Base Model* | 0.54 (0.05, 1.04) | 0.03 | 0.99 (0.50, 1.47) | <0.0001 | |
| +VAT | 0.57 (0.09, 1.05) | 0.02 | 0.81 (0.34, 1.29) | 0.0008 | |
| +HOMA-IR | 0.52 (0.03, 1.02) | 0.04 | 0.94 (0.46, 1.43) | 0.0001 | |
| +BMI | 0.57 (0.08, 1.05) | 0.02 | 0.81 (0.34, 1.29) | 0.0008 | |
| Fasting Plasma Glucose | 0.23 | ||||
| Base Model* | 0.09 (−0.68, 0.86) | 0.82 | 1.20 (0.19, 2.21) | 0.02 | |
| +VAT | 0.08 (−0.64, 0.80) | 0.82 | 0.91 (−0.10, 1.91) | 0.08 | |
| +BMI | 0.08 (−0.65, 0.81) | 0.83 | 0.79 (−0.20, 1.79) | 0.12 | |
| Log Triglycerides | 0.50 | ||||
| Base Model* | 0.01 (−0.02, 0.03) | 0.62 | 0.02 (−0.01, 0.05) | 0.19 | |
| +VAT | 0.01 (−0.02, 0.03) | 0.58 | 0.00 (−0.03, 0.03) | 0.77 | |
| +HOMA-IR | 0.00 (−0.03, 0.03) | 0.98 | 0.01 (−0.02, 0.04) | 0.42 | |
| +BMI | 0.01 (−0.02, 0.03) | 0.62 | 0.01 (−0.02, 0.04) | 0.61 | |
| HDL Cholesterol | 0.46 | ||||
| Base Model* | 1.25 (0.39, 2.11) | 0.004 | 0.68 (0.04, 1.32) | 0.04 | |
| +VAT | 1.24 (0.44, 2.05) | 0.002 | 1.04 (0.43, 1.65) | 0.0008 | |
| +HOMA-IR | 1.43 (0.60, 2.26) | 0.0007 | 0.84 (0.22, 1.46) | 0.008 | |
| +BMI | 1.26 (0.44, 2.08) | 0.003 | 0.98 (0.35, 1.60) | 0.002 | |
| HOMA-IR | 0.90 | ||||
| Base Model* | 0.10 (0.00, 0.20) | 0.049 | 0.13 (0.01, 0.26) | 0.04 | |
| +VAT | 0.09 (0.01, 0.18) | 0.04 | 0.05 (−0.07, 0.17) | 0.41 | |
| +BMI | 0.09 (0.01, 0.18) | 0.04 | 0.03 (−0.08, 0.15) | 0.56 | |
| Dichotomous Variables | |||||
| Hypertension | 0.39 | ||||
| Base Model* | 1.23 (1.08, 1.41) | 0.002 | 1.13 (1.00, 1.28) | 0.05 | |
| +VAT | 1.23 (1.07, 1.41) | 0.003 | 1.09 (0.96, 1.23) | 0.21 | |
| +HOMA-IR | 1.19 (1.04, 1.37) | 0.01 | 1.11 (0.98, 1.26) | 0.11 | |
| +BMI | 1.25 (1.09, 1.43) | 0.002 | 1.09 (0.96, 1.24) | 0.19 | |
| IFG | 0.77 | ||||
| Base Model* | 1.01 (0.88, 1.17) | 0.85 | 1.08 (0.97, 1.22) | 0.16 | |
| +VAT | 1.00 (0.87, 1.15) | 0.98 | 1.04 (0.93, 1.17) | 0.46 | |
| +BMI | 1.01 (0.87, 1.16) | 0.91 | 1.05 (0.94, 1.18) | 0.39 | |
| Diabetes | 0.40 | ||||
| Base Model* | 1.14 (0.89, 1.46) | 0.31 | 1.00 (0.80, 1.25) | 0.98 | |
| +VAT | 1.16 (0.92, 1.47) | 0.21 | 0.97 (0.78, 1.21) | 0.79 | |
| +BMI | 1.16 (0.91, 1.48) | 0.23 | 0.96 (0.78, 1.18) | 0.70 | |
| Metabolic Syndrome | 0.78 | ||||
| Base Model* | 1.11 (0.98, 1.26) | 0.11 | 1.16 (1.03, 1.30) | 0.01 | |
| +VAT | 1.09 (0.94, 1.27) | 0.24 | 1.07 (0.93, 1.22) | 0.34 | |
| +HOMA-IR | 1.03 (0.89, 1.19) | 0.72 | 1.12 (0.98, 1.28) | 0.11 | |
| +BMI | 1.13 (0.98, 1.30) | 0.10 | 1.08 (0.94, 1.24) | 0.28 | |
| Insulin resistance | 0.49 | ||||
| Base Model* | 1.12 (0.99, 1.27) | 0.07 | 1.22 (1.08, 1.38) | 0.001 | |
| +VAT | 1.11 (0.97, 1.29) | 0.14 | 1.15 (1.00, 1.32) | 0.04 | |
| +BMI | 1.14 (0.97, 1.31) | 0.07 | 1.15 (1.00, 1.33) | 0.051 | |
Data presented include effect size (SE) expressed per 1 SD increase in log AST for continuous data, and the odds of the condition per 1 SD increase in log AST with 95% confidence intervals for dichotomous data.
Adjusted for age, current smoking, high alcohol intake, physical activity, and menopausal status (women only), hormone replacement therapy (women only); for blood pressure, FPG, HDL cholesterol, and log triglycerides, an additional covariate of treatment for HTN, diabetes, or lipid disorders, respectively, was included.
Abbreviations: VAT, visceral adipose tissue; HOMA-IR, homeostasis model of insulin resistance; BMI, body mass index; HDL, high-density lipoprotein; IFG, impaired fasting glucose.
Associations between ALT and AST and cardiometabolic risk factors were generally similar between men and women, and sex interactions were generally negative (Tables 3 and 4).
Secondary Analyses
Secondary analyses excluded participants with high alcohol intake (>7 drinks per week [women], >14 drinks per week [men], n = 394), participants with diabetes (n = 159), and participants with ALT levels above the normal range (≥40 U/L, n = 322), respectively. When moderate drinkers were excluded from the analysis, results were essentially unchanged (data not shown). Among individuals without diabetes, there was attenuation of the effect size of the association between log ALT and fasting glucose level (beta coefficient 1.79 to 1.22 [women], beta coefficient 2.75 to 1.38 [men], all p-values <0.0001) and between log ALT and HOMA-IR (beta coefficient 0.41 to 0.33 [women], beta coefficient 0.48 to 0.38 [men], all p-values <0.0001); other associations were materially unchanged and persisted after additional adjustment for VAT, IR, and BMI (data not shown). When multivariable and logistic regressions were simultaneously adjusted for BMI, VAT, and HOMA-IR (with the exception of glycemic traits, which were not adjusted for HOMA-IR, and HOMA-IR, which was not adjusted for glucose), results were not appreciably different (data not shown).
When the sample was limited to those with ALT levels in the normal range (<40 U/L), significant associations persisted without attenuation of effect size (supplementary table). Of note, the odds of impaired fasting glucose was similar for each 1-SD increase in log ALT for both men and women in the normal range ALT sample (OR 1.44, p=0.0001 [women], OR 1.62, p<0.0001[men]) relative to the study population as a whole (OR 1.30, p=0.0002 [women], OR 1.29, p<0.0001 [men]). These associations persisted after additional adjustment for VAT and BMI. Among women with ALT in the normal range, the odds of the metabolic syndrome per 1-SD increase in log ALT was increased relative to the entire sample (OR 1.78, p<0.0001 versus 1.61, p<0.0001). This relationship remained after adjustment for VAT, insulin resistance, and BMI. Among men with ALT in the normal range, the effect sizes of each 1-SD increase in log ALT on SBP and DBP were increased relative to the entire sample.
Combination of log ALT and VAT
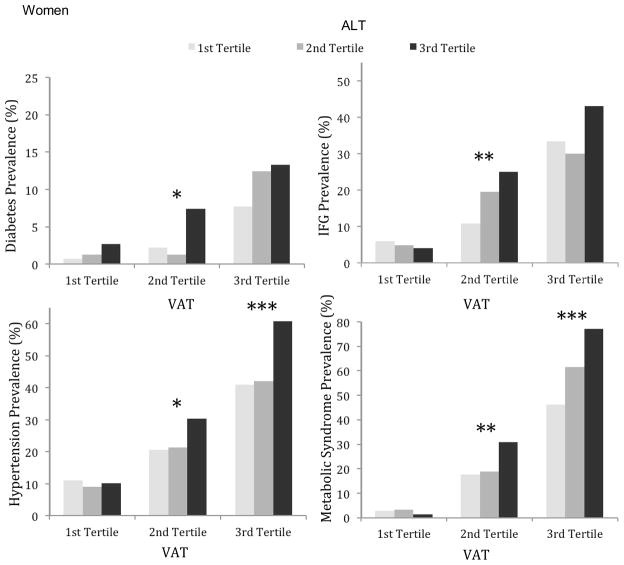
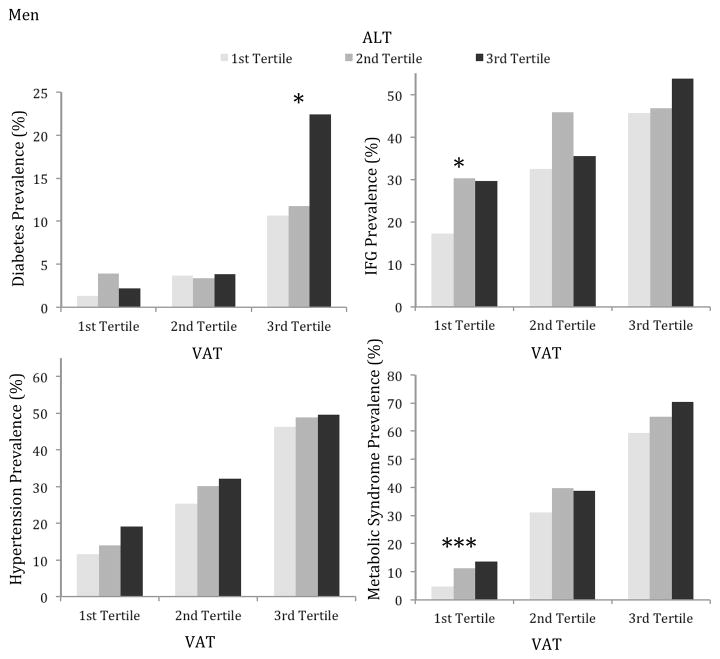
Age-adjusted rates of cardiometabolic risk factors within tertiles of ALT stratified by tertiles of VAT are shown in the figure. In women and men, the age-adjusted prevalence of diabetes, impaired fasting glucose (IFG), hypertension, and metabolic syndrome (MetS) increased across increasing tertiles of VAT. Of note, rates of these risk factors also increased across increasing ALT tertiles in the higher VAT tertiles for both sexes.
Figure.
Age-adjusted prevalence of diabetes mellitus, impaired fasting glucose (IFG), hypertension, and metabolic syndrome by tertiles of ALT within tertiles of VAT. Results are shown for women (top) and men (bottom). Significant p-values for trend across ALT tertiles within each VAT tertile are designated by *p<0.05, **p<0.01, ***p<0.001.
Discussion
Principal Findings
Our results demonstrate a robust association between serum alanine aminotransferase levels and multiple cardiometabolic risk factors in a community-based sample. The most important finding in our study is the strong association between serum ALT level and cardiometabolic risk after adjustment for visceral adipose tissue and insulin resistance. These associations remained significant when participants reporting moderate alcohol intake (>7 drinks per week [women], >14 drinks per week [men]) and those with diabetes were excluded from the analysis. In addition, among those with serum ALT in the normal range (<40 U/L), the associations of log ALT and cardiometabolic risk factors remained significant after multivariable adjustment. These findings suggest that serum ALT levels are associated with cardiometabolic risk independent of visceral adiposity and insulin resistance and that even modest elevations in ALT that fall in the “normal range” may be a clinically significant precursor to cardiometabolic disease.
In the Context of Current Literature
Multiple studies have linked NAFLD with metabolic risk factors for cardiovascular disease, including type 2 diabetes mellitus and the metabolic syndrome, in a variety of populations 6–9,12,13. The majority of individuals with NAFLD are overweight or obese or have type 2 diabetes, but NAFLD is associated with insulin resistance in normal weight individuals without diabetes 14. This suggests that NAFLD may be an early predictor of metabolic disease that is distinct from other adiposity traits. Many studies have adjusted for easily measurable adiposity traits such as waist circumference and BMI. In a small study of male subjects, Targher et al. 15 found that nonalcoholic steatohepatitis (NASH) was predictive of an atherogenic biomarker profile independent of visceral adiposity, as measured by ultrasound. This finding suggests that fat in the liver may confer CVD risk beyond that associated with more generalized obesity. Previous work from the Framingham Heart Study found that fatty liver as measured by CT is associated with lipid and glucose traits independent of VAT 16. Our results extend these findings to show that serum ALT levels, as surrogate biomarkers of NAFLD, are also associated with cardiometabolic risk factors independent of VAT. In addition, we show that ALT levels are associated with metabolic risk independent of BMI and insulin resistance.
Many studies using aminotransferase levels to examine the link between NAFLD and cardiometabolic risk have considered 40 U/L as the upper limit of normal for both ALT and AST. These values were derived from the mean + 2 SD in a “healthy referent sample” from the 1950s 17. More recently, some have issued calls to adjust normal ALT values on the basis of sex and BMI 18,19. Prati et al 20 found that lowering the upper limit of normal ALT levels to 30 U/L in men and 19 U/L in women would increase the sensitivity of liver injury detection from 55% to 76%, mostly due to increased detection of fatty liver. The presence of histological damage from NAFLD in the context of normal ALT levels has also been observed 21,22. Our finding that serum ALT levels <40 U/L are strongly associated with cardiometabolic risk suggests that ALT levels represent a continuum of risk rather than a cutoff below which can be considered healthy and that even subtle ALT elevations may be clinically relevant.
Potential Mechanisms
There are several possible mechanisms linking NAFLD, NASH, and cardiometabolic risk. A “2-hit” hypothesis of lipid accumulation in hepatocytes in the presence of hepatic insulin resistance and has been proposed to explain the development of NAFLD and NASH 23. Intrahepatic fatty acid accumulation results mostly from the lipolysis of abdominal adipose tissue, but also from dietary chylomicrons and de novo lipogenesis in the liver 24,25. The relative contributions of fat in the liver versus other fat depots to cardiometabolic risk is not entirely clear. Previous work from the Framingham Heart Study found that both fatty liver and VAT are associated with cardiometabolic risk 16,26. Fabbrini et al. found that fatty liver, but not VAT, is associated with metabolic abnormalities 27, while work from the Jackson Heart Study found that fatty liver and VAT were independently associated with cardiometabilic risk, but that associations with triglycerides and HDL cholesterol were stronger for VAT than fatty liver28.
Secretion by adipose tissue of adipokines, such as adiponectin, leptin, and resistin, may play a role in the development of NAFLD and other cardiometabolic abnormalities 29–31. For example, patients with NAFLD have reduced levels of adiponectin 32, a hormone with anti-inflammatory and anti-fibrotic properties, lower levels of which have been associated with cardiometabolic risk 33,34. In addition, the liver is the site of production for biomarkers of inflammation and endothelial dysfunction, including C-reactive protein (CRP), fibrinogen, and plasminogen activator inhibitor-1 (PAI-1) all of which are elevated in fatty liver disease and which are considered biomarkers of CVD risk 15,31.
Our findings showed a greater number and strength of associations between ALT and cardiometabolic risk factors than between AST and cardiometabolic risk factors. This is likely due to the stronger association of ALT with NAFLD 35 and obesity 36. It may also reflect the greater specificity of ALT for liver injury since ALT is restricted to hepatocytes while AST is also released from the heart, skeletal muscle, kidney, brain, pancreas, and red blood cells 37.
Strengths and Limitations
Compared with prior work on aminotransferase levels and cardiometabolic risk, the strengths of the present study include a large population-based sample not selected for NAFLD, obesity, or metabolic disease with rich covariate data, including VAT. The richness of the covariate data allows us to adjust for several important confounders in exploring the association between aminotransferase levels and cardiometabolic risk and to further adjust for visceral adiposity and insulin resistance. We were able to conduct these analyses in a sample that included participants with normal and elevated aminotransferase levels.
Some limitations deserve mention. First, this study is cross-sectional and observational, so we are unable to assess temporality and causality. Second, we relied on a single measurement of aminotransferase levels for each participant, so we were unable to account for inter-individual variation in these levels. Such variability, however, is likely to bias our results towards the null hypothesis, and is unlikely to account for our significant findings. We were also unable to exclude individuals with non-NAFLD liver disease such as viral hepatitis or drug-induced liver injury as the cause of aminotransferase elevations, although the absolute numbers of such conditions are likely to be low in our community-based population study. We were unable to separate out the retroperitoneal VAT compartment in our analyses. Waist circumference measured at the umbilicus can be problematic in those with a pendulant abdomen. Participants taking metformin were classified as having diabetes, although they might have been taking the medication for other reasons. We made use of surrogate markers for both insulin resistance using the HOMA-IR and NAFLD using serum aminotransferase levels, which are insensitive for detecting NAFLD, NASH, advanced fibrosis, and cirrhosis. Finally, our sample is comprised of Caucasians, which limits the generalizability of our results to other populations or ethnic groups.
Conclusions
Serum ALT levels, including those in the normal range, are associated with cardiometabolic risk above and beyond visceral fat and insulin resistance.
Supplementary Material
Supplementary Table. Sex-Specific Multivariable-Adjusted* Regressions for ALT<40 U/L with Cardiometabolic Risk Factors
Acknowledgments
Sources of Funding: From the Framingham Heart Study of the National Heart, Lung, and Blood Institute of the National Institutes of Health and Boston University School of Medicine. This work was supported by the National Heart, Lung, and Blood Institute’s Framingham Heart Study (contract N01-HC-25195).
Footnotes
Disclosures: Alison Pedley is an employee of Merck.
References
- 1.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 2.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N, Rizzetto M. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 3.Nanji AA, French SW, Freeman JB. Serum alanine aminotransferase to aspartate aminotransferase ratio and degree of fatty liver in morbidly obese patients. Enzyme. 1986;36:266–269. doi: 10.1159/000469304. [DOI] [PubMed] [Google Scholar]
- 4.Chang Y, Ryu S, Sung E, Jang Y. Higher concentrations of alanine aminotransferase within the reference interval predict nonalcoholic fatty liver disease. Clin Chem. 2007;53:686–692. doi: 10.1373/clinchem.2006.081257. [DOI] [PubMed] [Google Scholar]
- 5.Westerbacka J, Corner A, Tiikkainen M, Tamminen M, Vehkavaara S, Hakkinen AM, Fredriksson J, Yki-Jarvinen H. Women and men have similar amounts of liver and intra-abdominal fat, despite more subcutaneous fat in women: implications for sex differences in markers of cardiovascular risk. Diabetologia. 2004;47:1360–1369. doi: 10.1007/s00125-004-1460-1. [DOI] [PubMed] [Google Scholar]
- 6.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 7.Meltzer AA, Everhart JE. Association between diabetes and elevated serum alanine aminotransferase activity among Mexican Americans. Am J Epidemiol. 1997;146:565–571. doi: 10.1093/oxfordjournals.aje.a009315. [DOI] [PubMed] [Google Scholar]
- 8.Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, Omatsu T, Nakajima T, Sarui H, Shimazaki M, Kato T, Okuda J, Ida K. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722–728. doi: 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]
- 9.Fraser A, Harris R, Sattar N, Ebrahim S, Davey Smith G, Lawlor DA. Alanine aminotransferase, gamma-glutamyltransferase, and incident diabetes: the British Women’s Heart andHealth Study and meta-analysis. Diabetes Care. 2009;32:741–750. doi: 10.2337/dc08-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henry RJ, Chiamori N, Golub OJ, Berkman S. Revised spectrophotometric methods for the determination of glutamic-oxalacetic transaminase, glutamic-pyruvic transaminase, and lactic acid dehydrogenase. Am J Clin Pathol. 1960;34:381–398. doi: 10.1093/ajcp/34.4_ts.381. [DOI] [PubMed] [Google Scholar]
- 11.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 12.Hsieh MH, Ho CK, Hou NJ, Hsieh MY, Lin WY, Yang JF, Chiu CC, Huang JF, Chang NC, Wang CL, Dai CY, Chuang WL, Yu ML. Abnormal liver function test results are related to metabolic syndrome and BMI in Taiwanese adults without chronic hepatitis B or C. Int J Obes (Lond) 2009;33:1309–1317. doi: 10.1038/ijo.2009.172. [DOI] [PubMed] [Google Scholar]
- 13.Kotronen A, Westerbacka J, Bergholm R, Pietilainen KH, Yki-Jarvinen H. Liver fat in the metabolic syndrome. J Clin Endocrinol Metab. 2007;92:3490–3497. doi: 10.1210/jc.2007-0482. [DOI] [PubMed] [Google Scholar]
- 14.Kim HJ, Lee KE, Kim DJ, Kim SK, Ahn CW, Lim SK, Kim KR, Lee HC, Huh KB, Cha BS. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch Intern Med. 2004;164:2169–2175. doi: 10.1001/archinte.164.19.2169. [DOI] [PubMed] [Google Scholar]
- 15.Targher G, Bertolini L, Rodella S, Lippi G, Franchini M, Zoppini G, Muggeo M, Day CP. NASH predicts plasma inflammatory biomarkers independently of visceral fat in men. Obesity (Silver Spring) 2008;16:1394–1399. doi: 10.1038/oby.2008.64. [DOI] [PubMed] [Google Scholar]
- 16.Speliotes EK, Massaro JM, Hoffmann U, Vasan RS, Meigs JB, Sahani DV, Hirschhorn JN, O’Donnell CJ, Fox CS. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham Heart Study. Hepatology. 2010;51:1979–1987. doi: 10.1002/hep.23593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karmen A, Wroblewski F, Ladue JS. Transaminase activity in human blood. J Clin Invest. 1955;34:126–131. doi: 10.1172/JCI103055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piton A, Poynard T, Imbert-Bismut F, Khalil L, Delattre J, Pelissier E, Sansonetti N, Opolon P. Factors associated with serum alanine transaminase activity in healthy subjects: consequences for the definition of normal values, for selection of blood donors, and for patients with chronic hepatitis C. MULTIVIRC Group. Hepatology. 1998;27:1213–1219. doi: 10.1002/hep.510270505. [DOI] [PubMed] [Google Scholar]
- 19.Kunde SS, Lazenby AJ, Clements RH, Abrams GA. Spectrum of NAFLD and diagnostic implications of the proposed new normal range for serum ALT in obese women. Hepatology. 2005;42:650–656. doi: 10.1002/hep.20818. [DOI] [PubMed] [Google Scholar]
- 20.Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, Vianello L, Zanuso F, Mozzi F, Milani S, Conte D, Colombo M, Sirchia G. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 21.Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, Sterling RK, Shiffman ML, Stravitz RT, Sanyal AJ. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37:1286–1292. doi: 10.1053/jhep.2003.50229. [DOI] [PubMed] [Google Scholar]
- 22.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 23.Parekh S, Anania FA. Abnormal lipid and glucose metabolism in obesity: implications for nonalcoholic fatty liver disease. Gastroenterology. 2007;132:2191–2207. doi: 10.1053/j.gastro.2007.03.055. [DOI] [PubMed] [Google Scholar]
- 24.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams LA, Lindor KD. Nonalcoholic fatty liver disease. Ann Epidemiol. 2007;17:863–869. doi: 10.1016/j.annepidem.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 26.Goessling W, Massaro JM, Vasan RS, D’Agostino RB, Sr, Ellison RC, Fox CS. Aminotransferase levels and 20-year risk of metabolic syndrome, diabetes, and cardiovascular disease. Gastroenterology. 2008;135:1935–1944. 1944, e1931. doi: 10.1053/j.gastro.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, Okunade A, Klein S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A. 2009;106:15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Fox CS, Hickson D, Bidulescu A, Carr JJ, Taylor HA. Fatty liver, abdominal visceral fat, and cardiometabolic risk factors: the Jackson Heart Study. Arterioscler Thromb Vasc Biol. 2011;31:2715–2722. doi: 10.1161/ATVBAHA.111.234062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adiels M, Taskinen MR, Boren J. Fatty liver, insulin resistance, and dyslipidemia. Curr Diab Rep. 2008;8:60–64. doi: 10.1007/s11892-008-0011-4. [DOI] [PubMed] [Google Scholar]
- 30.Day CP. From fat to inflammation. Gastroenterology. 2006;130:207–210. doi: 10.1053/j.gastro.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 31.Yoneda M, Mawatari H, Fujita K, et al. High-sensitivity C-reactive protein is an independent clinical feature of nonalcoholic steatohepatitis (NASH) and also of the severity of fibrosis in NASH. J Gastroenterol. 2007;42:573–582. doi: 10.1007/s00535-007-2060-x. [DOI] [PubMed] [Google Scholar]
- 32.Pagano C, Soardo G, Esposito W, Fallo F, Basan L, Donnini D, Federspil G, Sechi LA, Vettor R. Plasma adiponectin is decreased in nonalcoholic fatty liver disease. Eur J Endocrinol. 2005;152:113–118. doi: 10.1530/eje.1.01821. [DOI] [PubMed] [Google Scholar]
- 33.Nishida M, Funahashi T, Shimomura I. Pathophysiological significance of adiponectin. Med Mol Morphol. 2007;40:55–67. doi: 10.1007/s00795-007-0366-7. [DOI] [PubMed] [Google Scholar]
- 34.Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24:29–33. doi: 10.1161/01.ATV.0000099786.99623.EF. [DOI] [PubMed] [Google Scholar]
- 35.Kojima H, Sakurai S, Uemura M, Takekawa T, Morimoto H, Tamagawa Y, Fukui H. Difference and similarity between non-alcoholic steatohepatitis and alcoholic liver disease. Alcohol Clin Exp Res. 2005;29:259S–263S. doi: 10.1097/01.alc.0000191776.37626.30. [DOI] [PubMed] [Google Scholar]
- 36.Salvaggio A, Periti M, Miano L, Tavanelli M, Marzorati D. Body mass index and liver enzyme activity in serum. Clin Chem. 1991;37:720–723. [PubMed] [Google Scholar]
- 37.Kew MC. Serum aminotransferase concentration as evidence of hepatocellular damage. Lancet. 2000;355:591–592. doi: 10.1016/S0140-6736(99)00219-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table. Sex-Specific Multivariable-Adjusted* Regressions for ALT<40 U/L with Cardiometabolic Risk Factors