Abstract
Background
Capecitabine and paclitaxel are established effective treatments, alone and combined with other cytotoxic and targeted agents, for metastatic breast cancer (MBC). Paclitaxel polyglumex (a macromolecular conjugate of paclitaxel bound to poly-L-glutamic acid) has potential advantages over conventional paclitaxel, including little alopecia, short infusion time with no premedication, enhanced tumor permeability/retention effect, and improved tolerability. We therefore examined tolerability & efficacy of paclitaxel polyglumex with capecitabine in patients with MBC.
Patients and Methods
This was a single stage phase 2 study, with interim analysis conducted with endpoints of tumor response, adverse events (toxicities), time to progression & overall survival. The main eligibility criteria were: age >18, no prior MBC chemotherapy, ECOG performance score <2, disease measurable by RECIST criteria, no HER2 overexpression or amplification, no brain metastases or peripheral sensory neuropathy. Treatment consisted of paclitaxel polyglumex 135 mg/m2 by intravenous infusion on day 1 + capecitabine 825 mg/m2 orally twice daily days 1 - 14, repeated on a 3-week cycle. Forty one (41) evaluable patients were required to test null hypothesis that complete and partial tumor response rate (CR + PR) was at most 40% against the alternative of at least 60%. Paclitaxel polyglumex + capecitabine would be considered promising in this population if ≥21 responses were observed among first 41 evaluable patients.
Results
48 patients were enrolled between April 2006 - April 2007; all patients were evaluable. The median cycles administered was 6. Eighteen (18) patients (38%; 95% CI: 24-53%) had a confirmed tumor response (2 CR, 16 PR) by RECIST criteria. Fifteen (15; 38%, 95% CI: 23%-53%) responses occurred in first 41 patients, falling short of prespecified goal of 21 responses. Median duration of tumor response was 13.2 months. Three of the responders were progression free at last follow-up with a median follow-up of 43 months. Median progression-free survival was 5.1 months (95% CI: 4.0-7.6 months). Six-month progression free survival was 42% (95% CI: 30-58%). Median dose level administered = 135 mg/m2 paclitaxel polyglumex, 825 mg/m2 capecitabine for cycles 1-7. Most common severe (grade 3/4) toxicities (at least possibly related to study drug) were: leukopenia 9 (19%), neutropenia 8 (17%), neuro-sensory 4 (8%), skin reaction-hand/foot 4 (8%), dyspnea 2 (4%). Forrty-six% (22/47) of patients experienced a grade ≥3 toxicity and 8% (4/48) experienced a grade ≥4 toxicity. No alopecia was reported.
Conclusions
Although the trial failed to reach goal of 21 confirmed tumor responses among the first 41 evaluable patients, paclitaxel polyglumex and capecitabine is well tolerated and effective in MBC.
INTRODUCTION
Despite overall advances in the care of breast cancer and declining mortality rates in the United States, metastatic breast cancer (MBC) remains a significant problem; approximately 85 - 90% of patients who develop MBC can be expected to die within 5 years. Initial treatments for MBC with single agent endocrine therapy or chemotherapy regimens yield response rates of 20 - 40%, with median time to progression for those whose disease responds to first-line chemotherapy being 3 - 8 months.
Combination therapies have the potential to target different mechanisms driving progression of malignancy, resulting in improved treatment efficacy. Studies to date have demonstrated that combining a taxane with another agent (capecitabine, gemcitabine, or trastuzumab) in MBC therapy can lead to improved treatment response, longer time to tumor progression, and longer survival compared to use of the taxane alone. Further studies to optimize combination therapy, while ameliorating adverse events, are critically important to creating additional progress in treatment of MBC.
Paclitaxel polyglumex is a conjugate of paclitaxel to a polyglutamate polymer that has a molecular weight within the ideal range to exploit the enhanced permeability and retention (EPR) effect in tumor tissues described by Maeda and others.1-5 The EPR effect results from increased permeability of abnormal capillaries and the lack of lymphatic vessels in solid tumors, which together promote accumulation of these large molecules in tumor tissues.6-8 Paclitaxel polyglumex is metabolized to deliver intracellular paclitaxel, which induces mitotic arrest and apoptosis in proliferating cells by targeting tubulin, a component of the mitotic spindle.9,10 Because poly-L-glutamic acid links to the 2′ hydroxyl of paclitaxel, a site crucial for tubulin binding, paclitaxel polyglumex is inactive until metabolized.5
In phase 1 and 2 clinical trials of paclitaxel polyglumex (combined in some studies with other cytotoxic agents), disease responses have been observed in a variety of solid tumors, including gastric, non-small cell lung, ovarian, breast, prostate, esophageal and colorectal cancer, mesothelioma, and schwannoma.11-16 In these studies, the adverse event profile for paclitaxel polyglumex was similar to that observed with conventional paclitaxel except that the frequency of adverse events was lower.
For the reasons discussed above, the North Central Cancer Treatment Group conducted a phase 2 clinical trial employing paclitaxel polyglumex in combination with capecitabine for patients who had not received chemotherapy previously as treatment for their MBC. We hypothesized that this combination would have meaningful clinical value in treatment of MBC if objectively defined disease responses could be demonstrated in at least 60% of eligible patients with MBC with acceptable treatment-associated toxicity.
PATIENTS and METHODS
Eligibility
Men and women with histologic or cytologic confirmation of the breast cancer with clinical evidence of metastatic disease, and with or without previous endocrine treatment for metastatic disease, were eligible for this study if they met the following criteria: ≥ 18 years of age; never received prior chemotherapy for metastatic disease; prior anthracycline and/or taxane in the neoadjuvant or adjuvant setting allowed if completed ≥ 6 months prior to registration; availability of diagnostic tissue and operative and pathology reports from breast cancer diagnosis and/or diagnosis of MBC; Eastern Cooperative Oncology Group performance score (ECOG PS) of 0 or 1; life expectancy ≥ 3 months; no evidence of HER2 overexpression or amplification by immunohistochemical (IHC) evaluation or fluorescence in situ hybridization (FISH), respectively; and, hemoglobin ≥ 8.0 g/dL, absolute neutrophil count ≥ 1500 cells/cu.mm, platelet count ≥ 100,000 cells/cu.mm, total bilirubin ≤ 1.5 × upper limit of normal, creatinine clearance ≥ 30 mL/min (calculated according to Cockroft and Gault). Serum transaminase and calcium were required to be within normal range.
Contraindications to enrollment into the study included the following: HIV-seropositive individuals receiving combination anti-retroviral therapy; pregnant women, nursing women, or women of child-bearing potential or their sexual partners who were unwilling to employ adequate contraception; concurrent use of endocrine therapy for MBC; if the only evidence of MBC was bone metastases or other non-measurable disease; concurrent treatment in a different clinical study in which investigational procedures were performed or investigational therapies were administered; pre-existing neuropathy of NCI CTCAE v3.0 grade > 0; major surgery, chemotherapy, or immunologic therapy ≤ 4 weeks prior to registration; radiotherapy ≤ 4 weeks prior to registration, unless such treatment was for a non-target lesion only; known brain metastasis ; neo-adjuvant and/or adjuvant therapy completed ≤ 6 months prior to registration; stage III or IV invasive, non-breast malignancies ≤ 5 years prior to registration; history of allergy or hypersensitivity to capecitabine, paclitaxel, or prior unanticipated severe reaction to fluorapyrimidine therapy, known hypersensitivity to 5-fluorouracil or known DPD deficiency; known, existing uncontrolled coagulopathy; requirement for concurrent use of allopurinol, metronidazole or the antiviral agent sorivudine (or chemically-related analogues such as brivudine); treatment with cimetidine ≤ 2 weeks prior to registration; current or recent use (≤2 weeks prior to registration) of aspirin, anticoagulants or thrombolytic agents; uncontrolled intercurrent illness including, but not limited to, ongoing or active infection, symptomatic congestive heart failure, unstable angina pectoris, cardiac arrhythmia, uncontrolled hypertension, psychiatric illness/social situations that would limit compliance with study requirements; lack of physical integrity of the upper gastrointestinal tract, clinically significant malabsorption syndrome or inability to take oral medication; any significant medical condition that would make treatment or follow-up with study procedures difficult or problematic in the opinion of the treating oncologist. The study protocol was approved by institutional review boards at all institutions where the study was carried out. All patients were enrolled only after their informed consent for participation was obtained in the manner required by the institutional review board.
Treatment Schedule
Treatment followed a 21 day cycle schedule. The dose levels and routine administration of study treatments were as follows: paclitaxel polyglumex 135 mg/m2 was given intravenously on day 1 and capecitabine 825 mg/m2 was given orally twice daily on days 1 - 14. Strict rules for dosage modification in response to observed toxicities were followed for the first two cycles of each participant’s treatment, until individual treatment tolerance was ascertained. Thereafter, these modifications were regarded as guidelines to produce mild-to-moderate, but not debilitating, side effects. If multiple adverse events were seen, the subsequent dose administered was based on greatest reduction required in the guidelines for any single adverse event observed. The specific dosage modification rules used in this trial are provided in Supplemental Digital Content 1, Appendix: DOSAGE MODIFICATIONS BASED ON INTERVAL ADVERSE EVENT (CYCLE 1 ONLY).
Use of hematopoietic growth factors (i.e., G- or GM-CSF or pegylated G-CSF) was permitted according to institutional guidelines and the investigator’s discretion to prevent or to treat febrile neutropenia. Erythropoietin use was allowed at investigator’s discretion.
Response and Toxicity Criteria
Disease response was assessed using RECIST criteria.17 Assessment of response was performed using an appropriate imaging modality following every second cycle of therapy. A subsequent scan was obtained ≥ 6 weeks following initial documentation of either complete response (CR) or partial response (PR). If this subsequent scan demonstrated the same objective response, then the patient was said to have a confirmed response.
Toxicities were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) v3.0.
Statistical Design and Analysis
The primary end point being evaluated to test the study hypothesis was the proportion of confirmed responses (CR or PR). In order to qualify as a confirmed response, the patient’s disease must have responded to qualify as at least a PR on consecutive evaluations at least 6 weeks apart. The study used a single stage phase II clinical trial design, based on a Simon design18, to test the null hypothesis that the true confirmed response rate is at most 40% versus the alternative that it is at least 60%. The design had a significance level of 0.10 and 90% power. The regimen would be declared ineffective if ≤ 20 of the first 41 evaluable patients achieved confirmed responses.18
All analyses were based on the intent-to-treat principle. Toxicity data were summarized for all patients receiving at least one dose of treatment. The distributions of overall survival time (OS, time from study entry to death), progression-free survival time (PFS, time from study entry to disease progression or death), and duration of response (DOR, time from first documentation of response until progression or death) were estimated using the Kaplan-Meier method. Simple descriptive statistics were used to summarize the adverse event profile and baseline characteristics.
RESULTS
Patient Population
Forty-eight (48) women were enrolled in the study from April 2006 until April 2007. The median age at study entry was 56.5 (range: 34-82). Two-thirds of the women had baseline ECOG PS of 0. Sixty-five and 44% of patients’ cancers had estrogen receptor (ER) and progesterone receptor (PR) expression, respectively, at baseline, and 77% had visceral disease at baseline. Eighty-one percent had at least one previous surgery, the most common type being axillary lymph node dissection (63%) and mastectomy (58%), and 65% had previous radiation. The most common metastases were bone (60%) and liver (52%). A complete listing of patient characteristics at study entry are displayed in Table 1.
Follow-Up
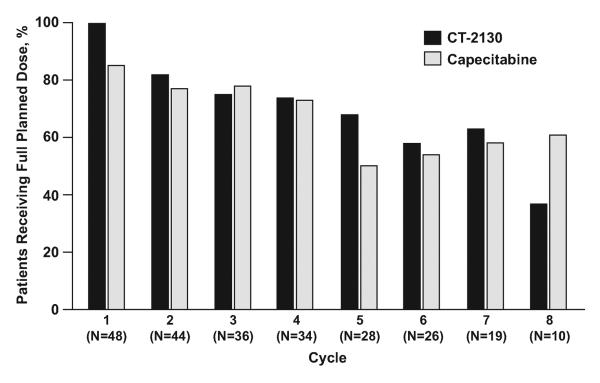
All 48 women have completed study therapy. The median number of treatment cycles administered was 6 (range: 1-32). The reasons for study discontinuation were: disease progression (31), patient refusal of further treatment (9), adverse event (7), and other (1 – patient achieved a CR and wanted a break from treatment). Eight (17%) women remain alive with a median follow-up time of 41 months (range: 14-46 months). The dosing experience of participants (relative to planned maximum dosing) is displayed in Figure 1.
Fig. 1.
Efficacy
Fifteen (15; 37%; 95% CI, 22-53%) of the first 41 evaluable women had confirmed responses, including 2 with complete responses. As this failed to surpass the pre-determined threshold, this regimen was to be considered to have failed to achieve the objective stated in the hypothesis for this patient population. Overall, 18 (38%; 95% CI, 24-53%) of 48 study participants had confirmed responses, including 2 complete responders (Table 2).
Of the 48 patients receiving study therapy, 40 have died. The median survival (as estimated by the method of Kaplan-Meier) was 20.5 months (95% CI: 16.3-28.8 months). The median progression free survival was 5.1 months (95% CI: 4.0-7.6 months). The median duration of response (time from first documentation of response until progression or death) among the 18 women with confirmed responses was 13.2 months (95% CI: 6.2-29.1 months). Six (6)-month OS was 88% (95% CI: 79-97%); Kaplan-Meier curves for OS, PFS, and DOR can be seen in Figure 2
Fig. 2.
Univariate Cox Proportional Hazards models were performed to test for relationships between time to event distributions (OS and PFS) and relevant baseline variables (age, number of metastatic sites). Patients’ status was denoted as being either above or below median value for each of the variables. No statistically significant relationships were identified in these tests of null hypothesis of hazard ratio = 1 (i.e., independence). Small sample sizes likely precluded any opportunity to identify meaningful relationships in these post-hoc analyses.
Toxicity and Tolerability
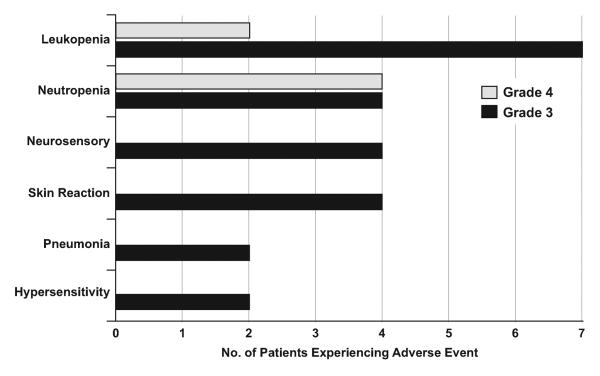
Toxicity was defined as an adverse event considered possibly, probably, or definitely related to treatment. Overall, treatment was well-tolerated in this study. Of the 48 patients who received at least one dose of treatment, 10 (21%) experienced at least 1 severe (grade 3+) hematologic toxicity and 15 (31%) experienced at least 1 severe non-hematologic toxicity. The most common severe toxicities experienced were leukopenia and neutropenia (19 and 17%, respectively). Non-hematologic toxicity was more varied, as the most common events were skin reaction and peripheral sensory neuropathy (both 8%). Overall, 46% (22/48) of patients experienced a toxicity of grade 3 or greater and 8% (4/48) experienced a toxicity of grade 4 or greater. No alopecia was reported. The frequency of severe toxicities is displayed in Figure 3.
Fig. 3.
Paclitaxel polyglumex was well tolerated with 27 of 343 (8%) administered cycles of therapy adjusted due to adverse events as follows: neurologic (17), hematologic (5), constitutional symptoms (3), metabolic/laboratory (1) and chest pain (1). Capecitabine was also well tolerated with 23 of 343 (7%) administered cycles of therapy adjusted due to adverse events as follows: dermatitis (14), hematologic (6), GI intolerance (2), and constitutional symptoms (1). Figure 1 displays treatment tolerability manifested by the percentage of patients able to receive full planned dose of therapy.
DISCUSSION
Although this study failed to reach its pre-specified efficacy goal (PR + CR ≥ 20 in the first 41 patients treated), the combination of paclitaxel polyglumex in combination with capecitabine did demonstrate significant clinical efficacy for patients who had not received chemotherapy previously as treatment for their MBC (PR + CR = 18 in 48 patients treated [38%]), with acceptable toxicity. The fact that 40% of patients enrolled in the trial received ≥ 8 cycles of study therapy attests to the tolerability of the regimen.
Several other groups of investigators have studied taxane/capecitabine combinations in MBC. These studies have demonstrated outcomes similar to those obtained in our study with respect to disease response and survival. However, more troublesome toxicities were noted with these other taxane/capecitabine combinations than were noted with the paclitaxel polyglumex/capecitabine combination in our study.
The combination of capecitabine (1250 mg/m2 twice daily for 2 weeks followed by a 1-week rest period) and docetaxel (75 mg/m2 day 1) in a 21-day cycle resulted in significant improvements in time to disease progression, response rate, and overall survival compared with docetaxel 100 mg/m2 monotherapy day 1, repeated every 21 days in women with MBC previously treated with anthracycline.19 In comparison to docetaxel alone, docetaxel/capecitabine resulted in a 35% reduction in risk of disease progression (p=0.001, median time to progression: 186 days vs. 128 days), a superior response rate (32% vs. 22%, p=0.025), an extension in survival (p=0.013, median overall survival: 442 days vs. 352 days) and improvement in one-year survival rate (57% vs. 47%). However, adverse events were a significant problem with docetaxel/capecitabine at the doses administered in this study. All patients developed alopecia. Moreover, greater than 70% of patients receiving the combination therapy reported grade 3 and 4 predominant adverse events of diarrhea, stomatitis, and hand-and-foot syndrome, leading to 65% of study patients in the combination arm requiring dose reduction and 26% of study patients withdrawing prematurely due to adverse events or intercurrent illness. These adverse events led to a median delivered dose intensity of capecitabine of 2000 mg/m2 per day in the combination arm, and a recommendation of 25% dose reduction in capecitabine starting dose by the investigators. Studies have now demonstrated that the efficacy of the regimen was not compromised in the patients receiving the lower dose compared to the intended dose. Doses of capecitabine in combination studies ranging from 1500-2000 mg/m2 daily for 14 days are now widely used (both in clinical practice and ongoing clinical trials), to maintain the therapeutic ratio of this agent.
In another study the combination of paclitaxel and capecitabine also led to significant anti-tumor activity, with modest adverse events. In this multi-center phase 2 trial, paclitaxel 175 mg/m2 was administered every 21 days with capecitabine at a dose of 825 mg/m2 twice daily for 14 days of the 21 day cycle as initial chemotherapy for MBC.20 The objective response rate was 51%, with a 15% complete response rate. In addition, 19% of patients had disease stabilization for 6 months or more for a clinical benefit rate of 70%. Adverse events were modest, with neutropenia (15%), alopecia (13%), and hand-and-foot syndrome (11%) as the only grade 3 or 4 adverse events occurring in 10% or more of patients. Only two events were of grade 4 severity. As described, this regimen was associated with some degree of alopecia in 66% of patients.
The combination of nab-paclitaxel and capecitabine has also been studied in a phase 2 clinical trial.21 Overall response rate was 61% with CR in 4%, PR in 57%, and 7 patients with sustained (≥ 24 weeks) stable disease for a clinical benefit rate of 76.1%. The median PFS was 10.6 months, and the median overall survival was 19.9 months. The most common adverse events (AEs) that were ≥ grade 3 were pain, hand-foot syndrome, and neutropenia.
In summary, we have demonstrated that the combination of paclitaxel polyglumex and capecitabine is tolerable and effective as initial treatment for MBC, although the combination failed to meet the pre-specified efficacy endpoint in this study. This combination appears to have similar efficacy, and comparable or superior tolerability as was noted in other studies of taxane/capecitabine combinations. Given the tolerability and efficacy of the combination demonstrated in this study, additional studies of paclitaxel polyglumex and capecitabine on other schedules or in combination with other cytotoxic agents or targeted therapies for MBC would appear warranted. Randomized studies comparing the various taxane/capecitabine combinations, including paclitaxel polyglumex + capecitabine, should also be considered.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health grant CA25224 (PI: JC Buckner) for the North Central Cancer Treatment Group, and through a grant from Cell Therapeutics, Incorporated.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gerlowski LE, Jain RK. Microvascular permeability of normal and neoplastic tissues. Microvasc Res. 1986;31:288–305. doi: 10.1016/0026-2862(86)90018-x. [DOI] [PubMed] [Google Scholar]
- 2.Maeda H, Matsumura Y. Tumoritropic and lymphotropic principles of macromolecular drugs. Crit Rev Ther Drug Carrier Syst. 1989;6:193–210. [PubMed] [Google Scholar]
- 3.Putnam D, Kopecek J. Polymer conjugates with anticancer activity. Adv Polym Sci. 1995;122:55–123. [Google Scholar]
- 4.Vasey PA, Kaye SB, Morrison R, et al. Phase I clinical and pharmacokinetic study of PK1 [N-(2-hydroxylpropyl)-methacrylamide copolymer doxorubicin]: first member of a new class of chemotherapeutic agents-drug-polymer conjugates. Clin Cancer Res. 1999;5:83–94. [PubMed] [Google Scholar]
- 5.Singer JW, Baker B, De Vries P, et al. Poly-(L)-glutamic acid-paclitaxel (CT-2103) [XYOTAX], a biodegradable polymeric drug conjugate: characterization, preclinical pharmacology, and preliminary clinical data. Adv Exp Med Biol. 2003;519:81–99. doi: 10.1007/0-306-47932-X_6. [DOI] [PubMed] [Google Scholar]
- 6.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 7.Greish K, Fang J, Inutsuka T, Nagamitsu A, Maeda H. Macromolecular therapeutics: advantages and prospects with special emphasis on solid tumour targeting. Clin Pharmacokinet. 2003;42:1089–1105. doi: 10.2165/00003088-200342130-00002. [DOI] [PubMed] [Google Scholar]
- 8.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 9.Manfredi JJ, Parness J, Horwitz SB. Taxol binds to cellular microtubules. J Cell Biol. 1982;94:688–696. doi: 10.1083/jcb.94.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhalla KN. Microtubule-targeted anticancer agents and apoptosis. Oncogene. 2003;22:9075–9086. doi: 10.1038/sj.onc.1207233. [DOI] [PubMed] [Google Scholar]
- 11.Paz-Ares L, Ross H, O’Brien M, et al. Phase III trial comparing paclitaxel poliglumex vs docetaxel in the second-line treatment of non-small-cell lung cancer. Br J Cancer. 2008;98:1608–1613. doi: 10.1038/sj.bjc.6604372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan MA, Darcy KM, Rose PG, et al. Paclitaxel poliglumex and carboplatin as first-line therapy in ovarian, peritoneal or fallopian tube cancer: a phase I and feasibility trial of the Gynecologic Oncology Group. Gynecol Oncol. 2008;110:329–335. doi: 10.1016/j.ygyno.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verschraegen CF, Skubitz K, Daud A, Kudelka AP, Rabinowitz I, Allievi C, Eisenfeld A, Singer JW, Oldham FB. A phase I and pharmacokinetic study of paclitaxel poliglumex and cisplatin in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2009;63:903–910. doi: 10.1007/s00280-008-0813-8. [DOI] [PubMed] [Google Scholar]
- 14.Mita M, Mita A, Sarantopoulos J, Takimoto CH, et al. Phase I study of paclitaxel poliglumex administered weekly for patients with advanced solid malignancies. Cancer Chemother Pharmacol. 2009;64:287–295. doi: 10.1007/s00280-008-0869-5. [DOI] [PubMed] [Google Scholar]
- 15.Beer TM, Ryan C, Alumkal J, Ryan CW, Sun J, Eilers KM. A phase II study of paclitaxel poliglumex in combination with transdermal estradiol for the treatment of metastatic castration-resistant prostate cancer after docetaxel chemotherapy. Anticancer Drugs. 2010;21:433–438. doi: 10.1097/CAD.0b013e3283355211. [DOI] [PubMed] [Google Scholar]
- 16.Dipetrillo T, Suntharalingam M, Ng T, et al. Neoadjuvant paclitaxel poliglumex, cisplatin, and radiation for esophageal cancer: a phase 2 trial. Am J Clin Oncol. 2012;35:64–67. doi: 10.1097/COC.0b013e318201a126. [DOI] [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors (RECIST Guidelines) J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.Simon R. Optimal two-stage designs for phase II clinical trials. Controlled Clinical Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 19.O’Shaughnessy J, Miles D, Vukelja S, et al. Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: phase III trial results. J Clin Oncol. 2002;20:2812–2823. doi: 10.1200/JCO.2002.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Gradishar WJ, Meza LA, Bipinkumar A, et al. Capecitabine plus paclitaxel as front-line therapy for metastatic breast cancer: a multicenter phase II study. J Clin Oncol. 2004;22:2321–2327. doi: 10.1200/JCO.2004.12.128. [DOI] [PubMed] [Google Scholar]
- 21.Schwartzberg LS, Arena FP, Mintzer DM, Epperson AL, Walker MS. Phase II multicenter trial of albumin-bound paclitaxel and capecitabine in first-line treatment of patients with metastatic breast cancer. Clin Breast Cancer. 2012;12:87–93. doi: 10.1016/j.clbc.2011.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.