Abstract
The topology of the plasma membrane Na+/Ca2+ exchanger of cardiac muscle, NCX1, is uncertain. Biochemical analyses have indicated the presence of 9 transmembrane segments (TMSs) whereas the recent crystal structure of a prokaryotic homologue has 10 TMSs. The discrepancy is towards the C-terminus of the proteins where the prokaryotic homologue has an additional TMS8. To resolve this apparent disagreement, we re-assessed the topology of the C-terminal TMSs of NCX1. We examined the ability of internal or external cysteine residues in the N-terminal portion of NCX1 to crosslink with cysteine residues, of uncertain orientation, in the C-terminal portion of the protein. The results strongly support a model of NCX1 with 10 TMSs as found in the prokaryotic homologue.
Keywords: Na+/Ca2+ exchange, Membrane proteins, Topology, Crosslinking
1. Introduction
The plasma membrane Na+/Ca2+ exchanger (NCX) is important in the regulation of Ca2+ homeostasis in many cell types. The role of NCX is especially pertinent in cardiac myocytes where NCX is responsible for a substantial efflux of Ca2+ on a beat-to-beat basis. There are three mammalian NCX gene products and several splice variants though the exchanger that has been examined in most detail is that present in cardiac muscle (NCX1). Much functional data has accrued on structure/function relationships of NCX1. This exchanger has been modeled to have 9 transmembrane segments (TMSs) separated by a large intracellular regulatory loop [1, 2]. Each group of TMSs contains a region of intramolecular homology referred to as an α repeat. The two α repeats face opposite sides of the membrane and are important in the transport mechanism [3, 4].
The two most detailed topological determinations [1, 2] used similar approaches. In both cases, investigators examined effects of the application of sulfhydryl agents on NCX transport function. The sulfhydryl reagents were applied either intra- or extracellularly to transporters engineered to have single cysteine residues located at strategic positions throughout the protein. The resultant 9 TMS model is consistent with two studies using immunological approaches that demonstrated that the CO2H-terminus of the protein was located intracellularly [1, 5]. Nevertheless, the determination of the topology of polytopic membrane proteins is notoriously difficult and is subject to a variety of artifacts.
Recently, Liao et al. [6] reported on the crystal structure of a Na+/Ca2+ exchanger (NCX_Mj) from Methanococcus jannaschii, an archaebacterium. This exchanger has sequence homology to NCX1 only in the critical α repeat segments. There is no sequence similarity outside of these limited regions. The structure revealed the presence of 10 α-helical TMSs rather than the 9 TMSs proposed for NCX1. It is certainly possible that these two NCXs could have a different number of TMSs. However, there is also a strong precedent for prokaryotic and eukaryotic homologues of membrane proteins having similar secondary structures. Thus, we reexamined the topology of NCX1 using a crosslinking approach to specifically investigate the region of discrepancy.
2. Experimental Procedures
2.1 Construction of exchanger cysteine mutants
Single cysteine mutants were introduced into a cysteine-less NCX1 by the QuikChange site-directed mutagenesis method (Stratagene) [2, 7, 8]. Mutations were generated in 300–500 base pair cassettes and sequenced. Full-length exchangers with double mutations were constructed by the subcloning of two mutated cassettes.
2.2 Expression of the NCX1 cysteine mutants in Insect High Five cells
The lepidopteran insect cell expression system BTI-TN-5B1-4 (High Five, Invitrogen) was used for transient transfection of NCX1 cysteine mutants. The insect cells were especially convenient as little NCX1 protein aggregated as sometimes occurred, for example, with mammalian HEK cells. High Five cells were cultured at 27°C in Express Five SFM (Invitrogen) supplemented with glutamine (20 mM) and penicillin-streptomycin (1%). NCX1 cDNAs were subcloned into the pIE1/153A (V4-) triple expression vector (Cytostore) and cells were transfected using Cellfectin reagent (Invitrogen). 24 h post-transfection, Na+ gradient-dependent 45Ca2+ uptake into intact High Five cells was measured [9, 10].
2.3 Crosslinking in intact cells
Crosslinking was carried out as described previously [7]. Briefly, intact cells were rinsed twice and crosslinking was carried out at room temperature or 4°C by addition of oxidative reagent (CuPhe), MTS crosslinker 1,3-propanediyl bismethanethiosulfonate (3M; Toronto Research Chemicals) or maleimide crosslinker 1,8-bismaleimideimidodiethyleneglycol ((PEG)2; Pierce) to the intact cells in situ or suspension. Final concentrations were 1 mM CuSO4/3 mM phenanthroline, or 0.5 mM 3M or PEG2. Reactions were terminated after 20 min by addition of NEM (10 mM). Cells were lysed with 1% Triton X-100 plus protease inhibitors (complete, EDTA-free, Roche). Aliquots were analyzed by 7.5% SDS-PAGE in the absence of reducing reagents, and immunoblots used NCX1 antibody R3F1. Immunoblot bands were quantified using NIH ImageJ software. All experiments were performed at least three times and representative data are shown as mean ± SE.
3. Results
3.1 Strategy
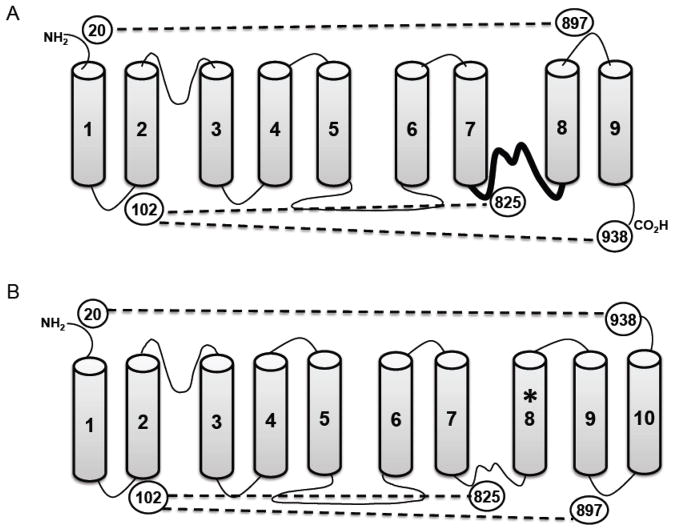
Shown in Fig. 1 are the topologies of NCX1 with 9 TMSs, as previously proposed, and with 10 TMSs using the structure of NCX_Mj as a template. The additional TMS, derived by mimicking the archaebacterial structure, is TMS8 as marked by the asterisk (Fig. 1B). In our earlier structure, this TMS was modeled to be a portion of an intracellular reentrant loop, shown as the thicker line between TMSs 7 and 8 in Fig. 1A. The presence of the additional TMS8 reverses the orientation of the last two TMSs of the previous model creating TMSs 9 and 10. The C-terminus then becomes extracellular rather than intracellular.
Fig. 1.
Schematics of the alternative topologies of NCX1. Panel A shows the currently accepted 9 TMS arrangement of NCX1, and panel B shows the 10 TMS topology as predicted by the crystal structure of the archaebacterial NCX_Mj. Circled numbers indicate locations of cysteines used in this study. Dotted lines indicate the predicted crosslinks with each model. The heavy line between TMSs 7 and 8 in panel A is the region that becomes the new TMS8 in panel B (designated with an asterisk). Note that TMSs 8 and 9 in the nine TMS model reverse orientation and become TMSs 9 and 10 in the ten TMS model.
We devised a crosslinking approach to resolve the validity of the two models. We have previously established that crosslinking of the N-terminal half of the exchanger with the C-terminal half resulted in an easily detected shift in the mobility of the protein on SDS-PAGE [11]. We have taken advantage of this mobility shift in helix packing studies [7–9]. We chose two amino acid residues near the N-terminus in which we had confidence in their extracellular or intracellular localization. These were Cys20 on the extracellular side of TMS1 and Leu102 on the intracellular side of TMS2. There is no disagreement on the topology of NCX1 in this region. For example, a residue near to Cys20 undergoes N-glycosylation [12] firmly establishing that this stretch of hydrophilic amino acids is extracellular. Likewise, the intracellular location of Leu102 has been verified in three studies [1, 2, 13] and is also consistent with the crystal structure of NCX_Mj [6].
Starting with a cysteine-less exchanger [2], we either re-introduced Cys20 or mutated Leu102 to cysteine. Then, an additional cysteine was introduced by mutations at specific locations in the C-terminal half of the exchanger. These second cysteines were located at positions 825, 897, and 938 (Fig. 1). Cys825 was chosen as a positive control. In both the 9 and 10 TMS models, both Cys102 and Cys825 are intracellular and should crosslink. We did not repeat previous experiments in which the extracellular Cys20 was able to crosslink with Cys792 in the extracellular loop between TMSs 6 and 7 [12]. We verified that all constructs were functionally active by using a 45Ca2+-uptake assay as described previously [8]. Constructs retained between 15 and 47% transport activity as compared to the wild type exchanger.
It was predicted that the ability of Cys20 and Cys102 to crosslink with cysteines at either 897 or 938 would be opposite depending on whether NCX1 was in a 9 or 10 TMS configuration. Thus, the experiments were devised to determine a consistent and coherent model. A unique aspect of our approach, in contrast to previous studies [1, 2, 7], is that we mostly focus on amino acid positions that are clearly not intramembrane. This should eliminate possible misinterpretations due to uncertainty regarding the exact boundaries of TMSs.
3.2 Crosslinking
Typical results are shown in Fig. 2. High Five insect cells in suspension were incubated with a crosslinking reagent for 20 min at 20°C. Three crosslinkers were used in each experiment: copper phenanthroline (CuPhe), 1,3-propanediyl bismethanethiosulfonate (3M), and 1,8-bismaleimideimidodiethyleneglycol (PEG2). These reagents crosslink adjacent cysteines (CuPhe) or have spacer arms of 6.5 (3M) or 14.7 Å (PEG2), respectively. The reagents also have different hydrophobicities, but all should be sufficiently membrane-permeable to have ready access to both sides of the membrane over a 20 min period.
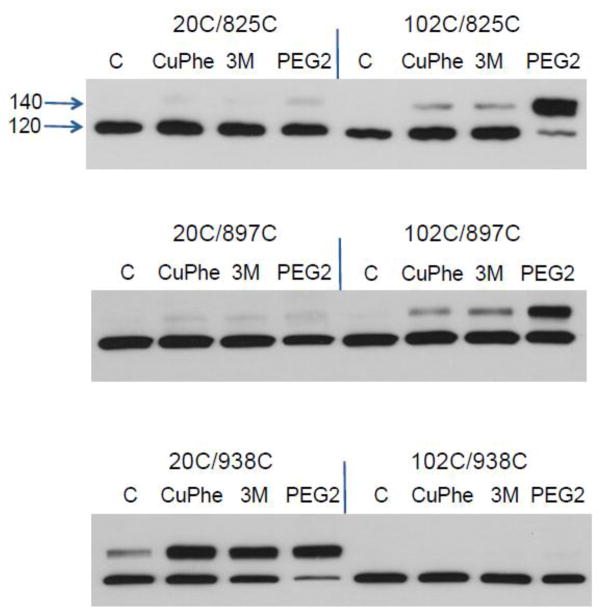
Fig. 2.
Effect of crosslinkers on NCX1 mobility. All exchangers were engineered to have only two cysteine residues. One cysteine was in the N-terminal half and the other in the C-terminal portion of the protein; for example, the notation 20C/825C indicates the use of an NCX1 with cysteines located at positions 20 and 825 (see Fig. 1). Shown are immunoblots for NCX1 protein of cellular homogenates. Suspended cells were incubated with CuPhe (1 mM), 3M (0.5 mM), or PEG2 (1 mM) for 20 min at 20° C. Disulfide bond formation results in a shift in the apparent mobility of NCX1 from 120 to 140 kDa.
The top panel of Fig. 2 is a positive control to test the efficacy of our approach. Residue 825 should be located near the intracellular surface of the plasma membrane and should crosslink with residue 102, and not residue 20, in either model (Fig. 1). This is indeed the case. Crosslinking is especially effective with PEG2 as the crosslinking reagent suggesting that residues 102 and 825 are separated by several angstroms in the three dimensional structure. The amount of protein crosslinked with PEG2 was 75.5 ± 9.6%.
Next, we tested the ability of cysteines at both positions 20 and 102 to form disulfide bonds with uncertain membrane locations. A cysteine at position 897 readily crosslinked with the internal Cys102 but not with the external Cys20 (Fig. 2, middle panel). Again, the PEG2 reagent was most effective in catalyzing disulfide bond formation (41.9 ± 14.0% yield). We obtained the opposite result with the C-terminal residue at position 938 (Fig. 2, bottom panel). Cysteines at positions 20 and 938 readily formed disulfide bonds with all three crosslinkers. The amount of NCX1 protein involved in crosslinking was 51.2 ± 6.2, 60.5 ± 5.3, and 73.7 ± 5.1% for CuPhe, 3M, and PEG2, respectively. The ability of CuPhe to induce disulfide bond formation suggests that the N- and C-termini have ready accessibility to one another. The data indicate the presence of an additional TMS 8 and an extracellular C-terminus. The results are clearly consistent with the 10 TMS model (Fig. 1B).
All experiments were performed three times. Virtually identical results were obtained when incubations of the suspended cells were carried out at 0°C, rather than at 20°C. Likewise, very similar results were observed if crosslinking reagents were incubated with the High Five cells while still attached to culture dishes rather than to suspended cells.
4. Discussion
In the absence of a crystal structure, it is critical to at least determine the secondary structure of a Na+/Ca2+ exchanger from a higher organism. Knowledge of the topology, for example, is necessary to better interpret structure/function studies. Initial biochemical analyses indicate that NCX1 has 9 TMSs [1, 2]. However, the recent structure of an archaebacterial exchanger, NCX_Mj, shows 10 TMSs. This would suggest that mammalian NCXs might also have 10 TMSs. It should also be noted that two biochemical studies have indicated that a homologous Na+/Ca2+-K+ exchanger, NCKX2, has 10TMSs [14, 15]. Determinations of the topologies of membrane proteins are fraught with difficulties and we decided to re-examine the membrane topology of NCX1 using an intramolecular crosslinking approach.
The previous approaches to assess NCX1 topology result in a secondary structure that is remarkably similar to that of NCX_Mj for the initial 7 TMSs. Discrepancies between the two topologies exist only near the C-terminal portions of the proteins. NCX1 is modeled to have 2 additional TMSs while NCX_Mj has 3 additional TMSs. An extra TMS8 inserted into NCX1, to mimic NCX_Mj, would reverse the orientation of the final 2 TMSs and would move the C-terminus into the interior of the cell (see Fig. 1). We re-examined the topology of only this portion of NCX1.
The results are remarkably unequivocal and can only be fit by the 10 TMS model. This incorporates an additional TMS8 and an extracellular C-terminus. The extracellular Cys20 crosslinks readily with the C-terminal C938, and the intracellular C102 crosslinks with C897 now modeled to be located in an intracellular loop beween TMSs 9 and 10 (see Figs. 1 and 2).
We tentatively assign 10 TMSs to NCX1 though further analysis would certainly be welcome. We are not aware of our technique having been used by others and the approach may be generally useful in topological analysis.
Acknowledgments
This research was supported by National Institutes of Health grant HL49101.
Abbreviations
- PEG2
1,8-bismaleimideimidodiethyleneglycol
- CuPhe
copper phenanthroline
- NCX
Na+/Ca2+ exchanger
- 3M
1,3-propanediyl bismethanethiosulfonate
- TMS
transmembrane segment
Footnotes
Disclosures
None declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Iwamoto T, Nakamura TY, Pan Y, Uehara A, Imanaga I, Shigekawa M. Unique topology of the internal repeats in the cardiac Na+/Ca2+ exchanger. FEBS Lett. 1999;446:264–8. doi: 10.1016/s0014-5793(99)00218-5. [DOI] [PubMed] [Google Scholar]
- 2.Nicoll DA, Ottolia M, Lu L, Lu Y, Philipson KD. A new topological model of the cardiac sarcolemmal Na+-Ca2+ exchanger. J Biol Chem. 1999;274:910–17. doi: 10.1074/jbc.274.2.910. [DOI] [PubMed] [Google Scholar]
- 3.Nicoll DA, Hryshko LV, Matsuoka S, Frank JS, Philipson KD. Mutation of amino acid residues in the putative transmembrane segments of the cardiac sarcolemmal Na+-Ca2+ exchanger. J Biol Chem. 1996;271:13385–91. doi: 10.1074/jbc.271.23.13385. [DOI] [PubMed] [Google Scholar]
- 4.Ottolia M, Nicoll DA, Philipson KD. Mutational analysis of the α-1 repeat of the cardiac Na+-Ca2+ exchanger. J Biol Chem. 2005;280:1061–9. doi: 10.1074/jbc.M411899200. [DOI] [PubMed] [Google Scholar]
- 5.Cook O, Low W, Rahamimoff H. Membrane topology of the rat brain Na+-Ca2+ exchanger. Biochim Biophys Acta. 1998;1371:40–52. doi: 10.1016/s0005-2736(97)00272-1. [DOI] [PubMed] [Google Scholar]
- 6.Liao J, Li H, Zeng W, Sauer DB, Belmares R, Jiang Y. Structural insight into the ion-exchange mechanism of the sodium/calcium exchanger. Science. 2012;335:686–90. doi: 10.1126/science.1215759. [DOI] [PubMed] [Google Scholar]
- 7.Ren X, Nicoll DA, Xu L, Qu Z, Philipson KD. Transmembrane segment packing of the Na+/Ca2+ exchanger investigated with chemical cross-linkers. Biochemistry. 2010;49:8585–91. doi: 10.1021/bi101173c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren X, Nicoll DA, Philipson KD. Helix packing of the cardiac Na+-Ca2+ exchanger: proximity of transmembrane segments 1, 2, and 6. J Biol Chem. 2006;281:22808–14. doi: 10.1074/jbc.M604753200. [DOI] [PubMed] [Google Scholar]
- 9.Qiu Z, Nicoll DA, Philipson KD. Helix packing of functionally important regions of the cardiac Na+-Ca2+ exchanger. J Biol Chem. 2001;276:194–9. doi: 10.1074/jbc.M005571200. [DOI] [PubMed] [Google Scholar]
- 10.Ren X, Nicoll DA, Galang G, Philipson KD. Intermolecular cross-linking of Na+-Ca2+ exchanger proteins: evidence for dimer formation. Biochemistry. 2008;47:6081–7. doi: 10.1021/bi800177t. [DOI] [PubMed] [Google Scholar]
- 11.Santacruz-Toloza L, Ottolia M, Nicoll DA, Philipson KD. Functional analysis of a disulfide bond in the cardiac Na+-Ca2+ exchanger. J Biol Chem. 2000;275:182–8. doi: 10.1074/jbc.275.1.182. [DOI] [PubMed] [Google Scholar]
- 12.Hryshko LV, Nicoll DA, Weiss JN, Philipson KD. Biosynthesis and initial processing of the cardiac sarcolemmal Na+-Ca2+ exchanger. Biochim Biophys Acta. 1993;1151:35–42. doi: 10.1016/0005-2736(93)90068-b. [DOI] [PubMed] [Google Scholar]
- 13.Doering AE, Nicoll DA, Lu Y, Lu L, Weiss JN, Philipson KD. Topology of a functionally important region of the cardiac Na+/Ca2+ exchanger. J Biol Chem. 1998;273:778–83. doi: 10.1074/jbc.273.2.778. [DOI] [PubMed] [Google Scholar]
- 14.Cai X, Zhang K, Lytton J. A novel topology and redox regulation of the rat brain K+-dependent Na+/Ca2+ exchanger, NCKX2. J Biol Chem. 2002;277:48923–30. doi: 10.1074/jbc.M208818200. [DOI] [PubMed] [Google Scholar]
- 15.Kinjo TG, Szerencsei RT, Winkfein RJ, Kang K, Schnetkamp PP. Topology of the retinal cone NCKX2 Na/Ca-K exchanger. Biochemistry. 2003;42:2485–91. doi: 10.1021/bi0270788. [DOI] [PubMed] [Google Scholar]